Clinical Significance and Prognostic Effect of Serum 25-hydroxyvitamin D Concentrations in Critical and Severe Hand, Foot and Mouth Disease
Abstract
:1. Introduction
2. Methods and Materials
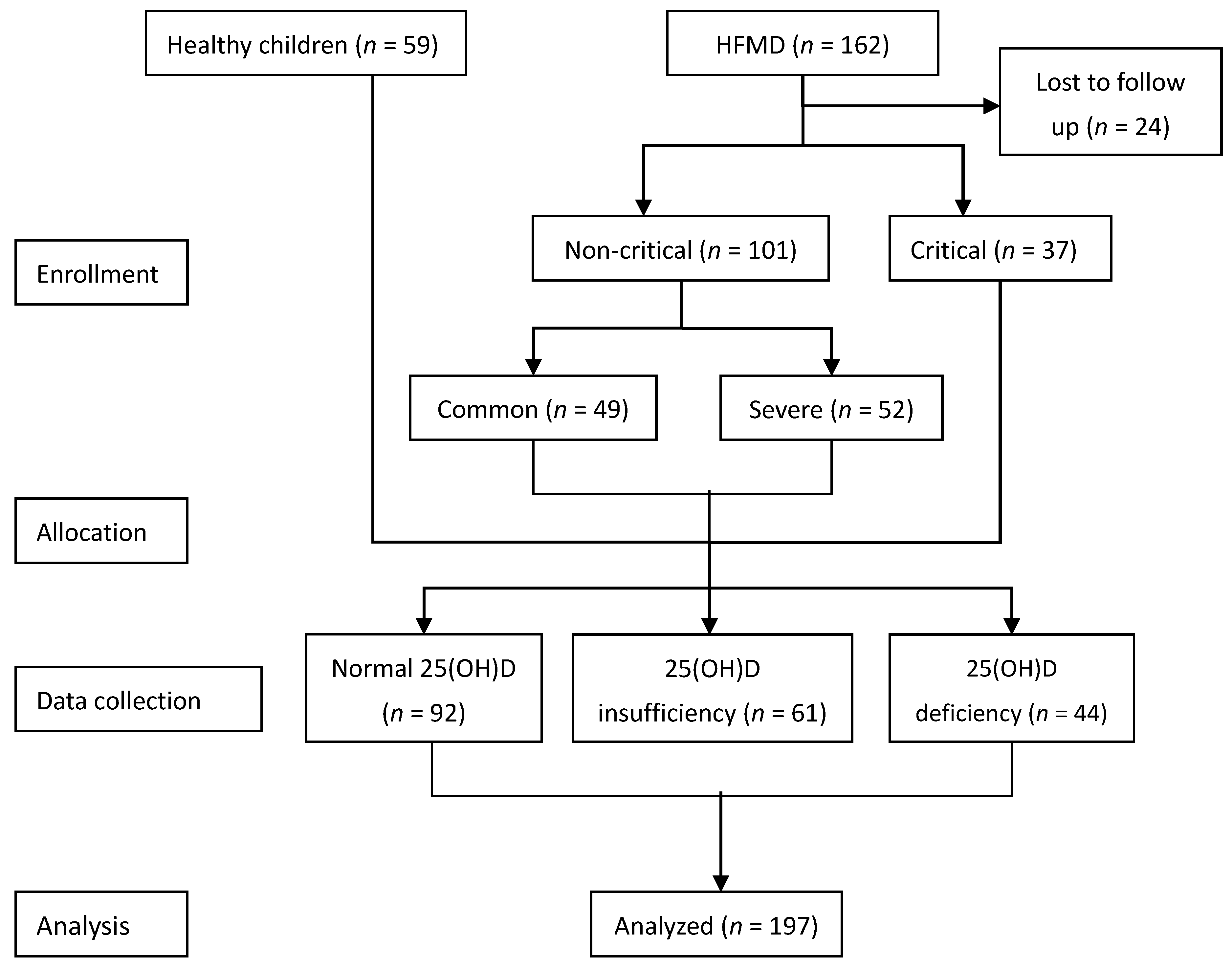
2.1. Study Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Measurement Method and Range of Reference Values
2.5. Statistical Methods
3. Results
3.1. General Condition of the Selected Subjects
3.2. Comparison of 25(OH)D Concentrations and the Incidence of 25(OH)D Deficiency and Insufficiency among Groups
3.3. Comparison of 25(OH)D Concentrations and Prognostic Relationships between Subgroups in the Critical and Severe HFMD Group
3.4. Comparison of Death and Survival in Children with Critical and Severe HFMD
3.5. Logistic Regression Analysis of the Prognostic Effect of 25(OH)D in Children with Critical and Severe HFMD
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koh, W.M.; Bogich, T.; Siegel, K.; Jin, J.; Chong, E.Y.; Tan, C.Y.; Chen, M.I.; Horby, P.; Cook, A.R. The Epidemiology of Hand, Foot and Mouth Disease in Asia: A Systematic Review and Analysis. Pediatr. Infect. Dis. J. 2016, 35, e285. [Google Scholar] [CrossRef] [PubMed]
- Clinical Experts Group of the Ministry of Health for Hand, Foot and Mouth Disease. Experts consensus on rescue and treatment of severe cases with enterovirus 71 (EV71) infection. Chin. J. Pediatr. 2011, 9, 675–678. (In Chinese) [Google Scholar]
- Wang, P.; Zhao, H.; You, F.; Zhou, H.; Goggins, W.B. Seasonal modeling of hand, foot, and mouth disease as a function of meteorological variations in Chongqing, China. Int. J. Biometeorol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.F.; Zheng, J.; Liu, J. Child Healthcare Department, Baiyun District Maternal and Child Health Care Hospital. Analysis on 25-hydroxy vitamin D level of 1309 children of 0–7 year-old. Chin. J. Women Child. Health 2015, 6, 26–29. (In Chinese) [Google Scholar]
- Paul, L.; Eisman, J.A.; Center, J.R. Vitamin D deficiency in critically ill patients. N. Engl. J. Med. 2009, 360, 1912–1914. [Google Scholar]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of the People’s Republic of China. Diagnosis and treatment guideline on hand-foot-mouth disease (2010 Edition). Int. J. Respir. 2010, 24, 5. (In Chinese) [Google Scholar]
- Sharma, B.; Agarwal, D.; Baijal, S.; Negi, T.; Choudhuri, G.; Saraswat, V. Effect of endoscopic sphincterotomy on gall bladder bile lithogenicity and motility. Gut 1998, 42, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chang, I.; Chen, W.; Huang, Y.; Chen, G.; Shih, S.; Juang, J.; Shih, H.; Hsiung, C.A.; Lin, T.; et al. HLA-A33 is associated with susceptibility to enterovirus 71 infection. Pediatrics 2008, 122, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Vukić, M.; Neme, A.; Seuter, S.; Saksa, N.; de Mello, V.D.F.; Nurmi, T.; Uusitupa, M.; Tuomainen, T.; Virtanen, J.K.; Carlberg, C. Relevance of Vitamin D Receptor Target Genes for Monitoring the Vitamin D Responsiveness of Primary Human Cells. PLoS ONE 2015, 10, e0124339. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.; Zimmerman, L.H.; McNorton, K.; Dolman, M.; Tyburski, J.; Baylor, A.; Wilson, R. Effects of vitamin D deficiency in critically ill surgical patients. Am. J. Surg. 2012, 203, 379–382. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.D.; Menon, K.; Lawson, M.L.; Williams, K.; Doherty, D.R. 1,25-Dihydroxyvitamin D Levels in Pediatric Intensive Care Units: Risk Factors and Association With Clinical Course. J. Clin. Endocrinol. Metab. 2015, 100, 2942–2945. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.D.; Doherty, D.R.; Lawson, M.L.; Al-Dirbashi, O.Y.; Chakraborty, P.; Ramsay, T.; Menon, K. The relationship between vitamin D status and adrenal insufficiency in critically ill children. J. Clin. Endocrinol. Metab. 2013, 98, E877–E881. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Nair, P.; Eisman, J.A.; Center, J.R. Vitamin D deficiency in the intensive care unit: An invisible accomplice to morbidity and mortality? Intensive Care Med. 2009, 35, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Venkatram, S.; Chilimuri, S.; Adrish, M.; Salako, A.; Patel, M.; Diaz-Fuentes, G. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Critical Care 2011, 15, R292. [Google Scholar] [CrossRef] [PubMed]
- Kempker, J.A.; Tangpricha, V.; Ziegler, T.R.; Martin, G.S. Vitamin D in sepsis: From basic science to clinical impact. Crit. Care 2012, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Takuhiro, M.; Litonjua, A.A.; Braun, A.B.; Gibbons, F.K.; Edward, G.; Christopher, K.B. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit. Care Med. 2014, 42, 97–107. [Google Scholar]
- Zhang, P.P.; Li, Y.T.; Li, X.F.; Li, Z.B.; Chen, Z.G. Analysis of 25-hydroxy vitamin D levels in children aged 0 to 14 years old in Guangzhou City. Chin. J. Child Health Care 2014, 8, 856–859. (In Chinese) [Google Scholar]
- Wang, S.; Shen, G.; Jiang, S.; Xu, H.; Li, M.; Wang, Z.; Zhang, S.; Yu, Y. Nutrient Status of Vitamin D among Chinese Children. Nutrients 2017, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Raj, D.; Warsi, S.; Chowdhary, S. Vitamin D Deficiency and Critical Illness. Indian J. Pediatr. 2015, 82, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Paul, L. Vitamin D metabolism and deficiency in critical illness. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 769–781. [Google Scholar]
- Ponnarmeni, S.; Kumar Angurana, S.; Singhi, S.; Bansal, A.; Dayal, D.; Kaur, R.; Patial, A.; Verma Attri, S. Vitamin D deficiency in critically ill children with sepsis. Paediatr. Int. Child Health 2016, 36, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Annemieke, V.; Geert, C.; Roger, B.; Chantal, M. Vitamin D: A pleiotropic hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar]
- Watkins, R.R.; Lemonovich, T.L.; Salata, R.A. An update on the association of vitamin D deficiency with common infectious diseases. Can. J. Physiol. Pharmacol. 2015, 93, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Miragliotta, G.; Miragliotta, L. Vitamin D and infectious diseases. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 267–271. [Google Scholar] [CrossRef] [PubMed]
| Group | Healthy Control | Non-Critical HFMD | Critical HFMD | * p | |||||
|---|---|---|---|---|---|---|---|---|---|
| Common | Severe | ||||||||
| Gender (n) | Male | Female | Male | Female | Male | Female | Male | Female | 0.83 |
| 31 | 28 | 23 | 26 | 29 | 23 | 20 | 17 | ||
| Age (month) | 29 ± 13 | 30 ± 11 | 29 ± 13 | 26 ± 11 | 0.55 | ||||
| BMI | 16.5 ± 1.8 | 16.7 ± 2.3 | 15.9 ± 1.9 | 15.9 ± 2.1 | 0.15 | ||||
| Healthy Control | Common HFMD | Severe HFMD | Critical HFMD | |
|---|---|---|---|---|
| n | 59 | 49 | 52 | 37 |
| 25(OH)D (ng/mL) | 28.1 ± 6.6 | 29.5 ± 8.1 | 31.9 ± 9.7 | 20.0 ± 8.4 |
| 25(OH)D > 30 ng/mL, n (%) | 30(51) | 23(47) | 33(64) | 6(16) |
| 25(OH)D 20–29.9 ng/mL, n (%) | 21(36) | 16(33) | 11(21) | 13(35) |
| 25(OH)D ≤ 20 ng/mL, n (%) | 8(13) | 10(20) | 8(15) | 18(49) |
| * p | <0.001 | 0.004 | <0.001 |
| Group | Normal 25(OH)D (n = 33) | 25(OH)D Insufficiency (n = 25) | 25(OH)D Deficiency (n = 31) | * p |
|---|---|---|---|---|
| Age (month) | 31 ± 12 | 25 ± 9 | 27 ± 13 | 0.95 |
| BMI | 16.1 ± 1.7 | 15.7 ± 1.9 | 16 ± 12 | 0.93 |
| 25(OH)D (ng/mL) | 36 ± 6.2 | 24.8 ± 3.0 | 13.6 ± 3.6 | <0.001 |
| LAC (mmol/L) | 2.6 ± 2.8 | 4.3 ± 3.4 | 7.2 ± 4.3 | 0.004 |
| LDH (U/L) | 600 ± 600 | 500 ± 200 | 1300 ± 900 | <0.001 |
| CK-MB (μg/L) | 3.4 ± 2.1 | 3.4 ± 1.7 | 10.9 ± 10.2 | 0.001 |
| DD (mg/L) | 1.7 ± 2.0 | 2.1 ± 1.4 | 4.8 ± 4.9 | 0.02 |
| Ca++ (mmol/L) | 1.0 ± 0.3 | 1.2 ± 0.2 | 1.0 ± 0.3 | 0.06 |
| PCIS | 85 ± 11 | 72 ± 8 | 66 ± 11 | 0.02 |
| Brainstem encephalitis (case) | 3 | 6 | 16 | 0.04 |
| Neurogenic pulmonary edema (case) | 2 | 5 | 16 | 0.02 |
| Circulatory failure (case) | 2 | 4 | 15 | 0.01 |
| Death (case) | 2 | 4 | 13 | 0.04 |
| Prognostic Group | Survival (n = 70) | Death (n = 19) | p |
|---|---|---|---|
| Age (month) | 28 ± 12 | 27 ± 12 | 0.79 |
| BMI | 15.7 ± 1.9 | 16.4 ± 2.2 | 0.19 |
| 25(OH)D (ng/mL) | 30.0 ± 9.7 | 15.6 ± 6.7 | <0.001 |
| LAC (mmol/L) | 3.3 ± 3.0 | 9.5 ± 3.5 | <0.001 |
| LDH (U/L) | 500 (300–700) | 1700 (500–2600) | 0.01 |
| CK-MB (μg/L) | 2.6 (1.4–4.0) | 14 (4–21) | <0.001 |
| DD (mg/L) | 1.7 ± 1.7 | 7.2 ± 5.0 | <0.001 |
| Ca++ (mmol/L) | 1.06 ± 0.3 | 1.11 ± 0.3 | 0.60 |
| PCIS | 79 ± 11 | 60 ± 9 | <0.001 |
| Indicator | Regression Coefficient (β) | p | OR | 95% Confidence Interval |
|---|---|---|---|---|
| Age | −0.38 | 0.06 | 0.68 | 0.46–1.02 |
| BMI | −0.78 | 0.19 | 0.46 | 0.14–1.46 |
| 25(OH)D * | 0.71 | 0.045 | 2.03 | 1.02–4.06 |
| LDH | 0.01 | 0.06 | 1.01 | 1.00–1.01 |
| LAC | −0.74 | 0.07 | 0.48 | 0.22–1.07 |
| CK-MB | 0.24 | 0.30 | 1.27 | 0.81–1.99 |
| DD * | −1.22 | 0.04 | 0.30 | 0.09–0.93 |
| Ca++ | −4.48 | 0.15 | 0.01 | 0.00–4.69 |
| PCIS * | 0.39 | 0.03 | 1.48 | 1.04–2.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, H.-X.; Liu, C.-J.; Li, J.; Chen, S.-J.; Xu, F. Clinical Significance and Prognostic Effect of Serum 25-hydroxyvitamin D Concentrations in Critical and Severe Hand, Foot and Mouth Disease. Nutrients 2017, 9, 478. https://doi.org/10.3390/nu9050478
Dang H-X, Liu C-J, Li J, Chen S-J, Xu F. Clinical Significance and Prognostic Effect of Serum 25-hydroxyvitamin D Concentrations in Critical and Severe Hand, Foot and Mouth Disease. Nutrients. 2017; 9(5):478. https://doi.org/10.3390/nu9050478
Chicago/Turabian StyleDang, Hong-Xing, Cheng-Jun Liu, Jing Li, Shi-Jiao Chen, and Feng Xu. 2017. "Clinical Significance and Prognostic Effect of Serum 25-hydroxyvitamin D Concentrations in Critical and Severe Hand, Foot and Mouth Disease" Nutrients 9, no. 5: 478. https://doi.org/10.3390/nu9050478
APA StyleDang, H.-X., Liu, C.-J., Li, J., Chen, S.-J., & Xu, F. (2017). Clinical Significance and Prognostic Effect of Serum 25-hydroxyvitamin D Concentrations in Critical and Severe Hand, Foot and Mouth Disease. Nutrients, 9(5), 478. https://doi.org/10.3390/nu9050478




