The Effects of Magnesium Supplementation on Subjective Anxiety and Stress—A Systematic Review
Abstract
:1. Introduction
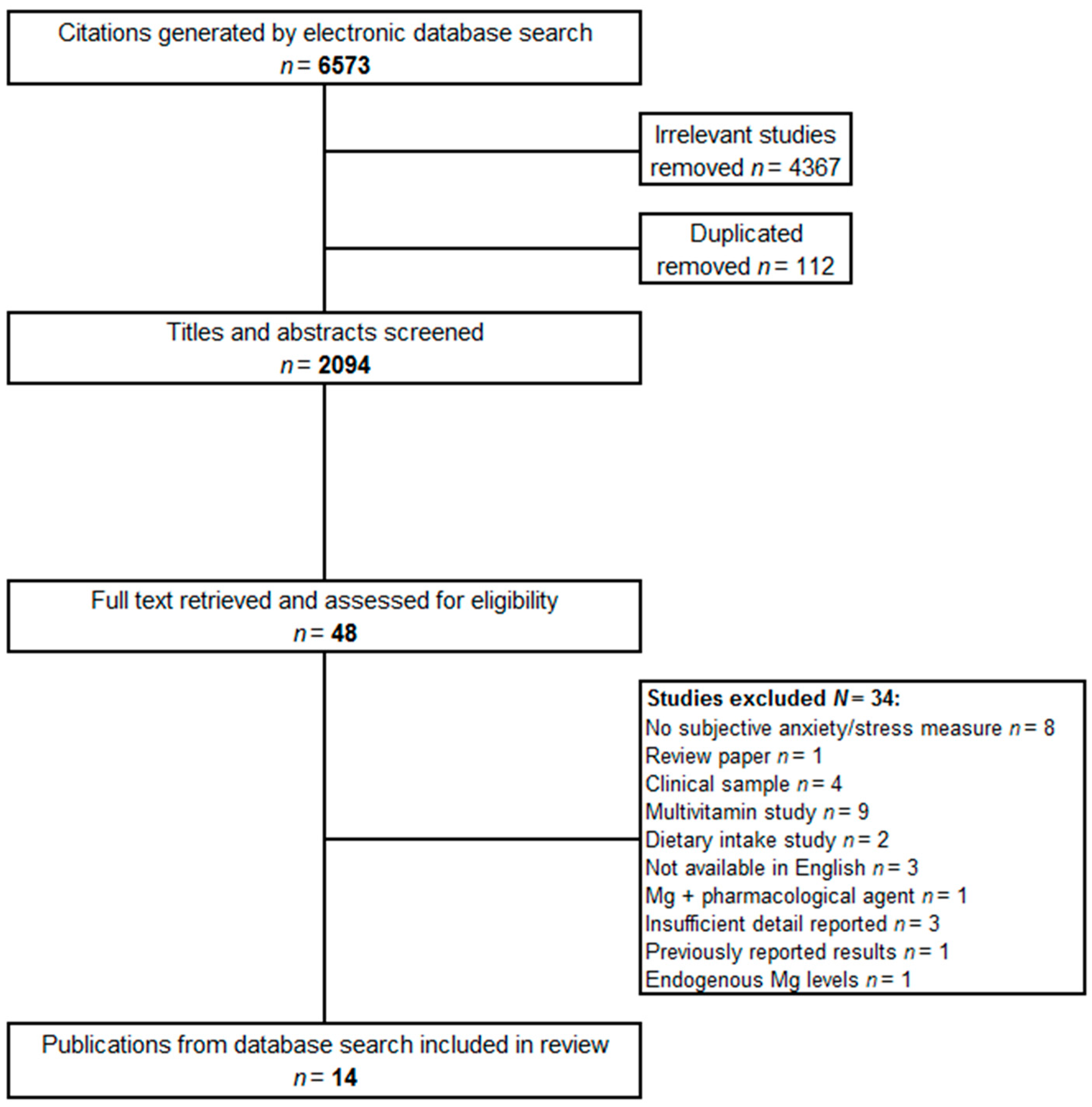
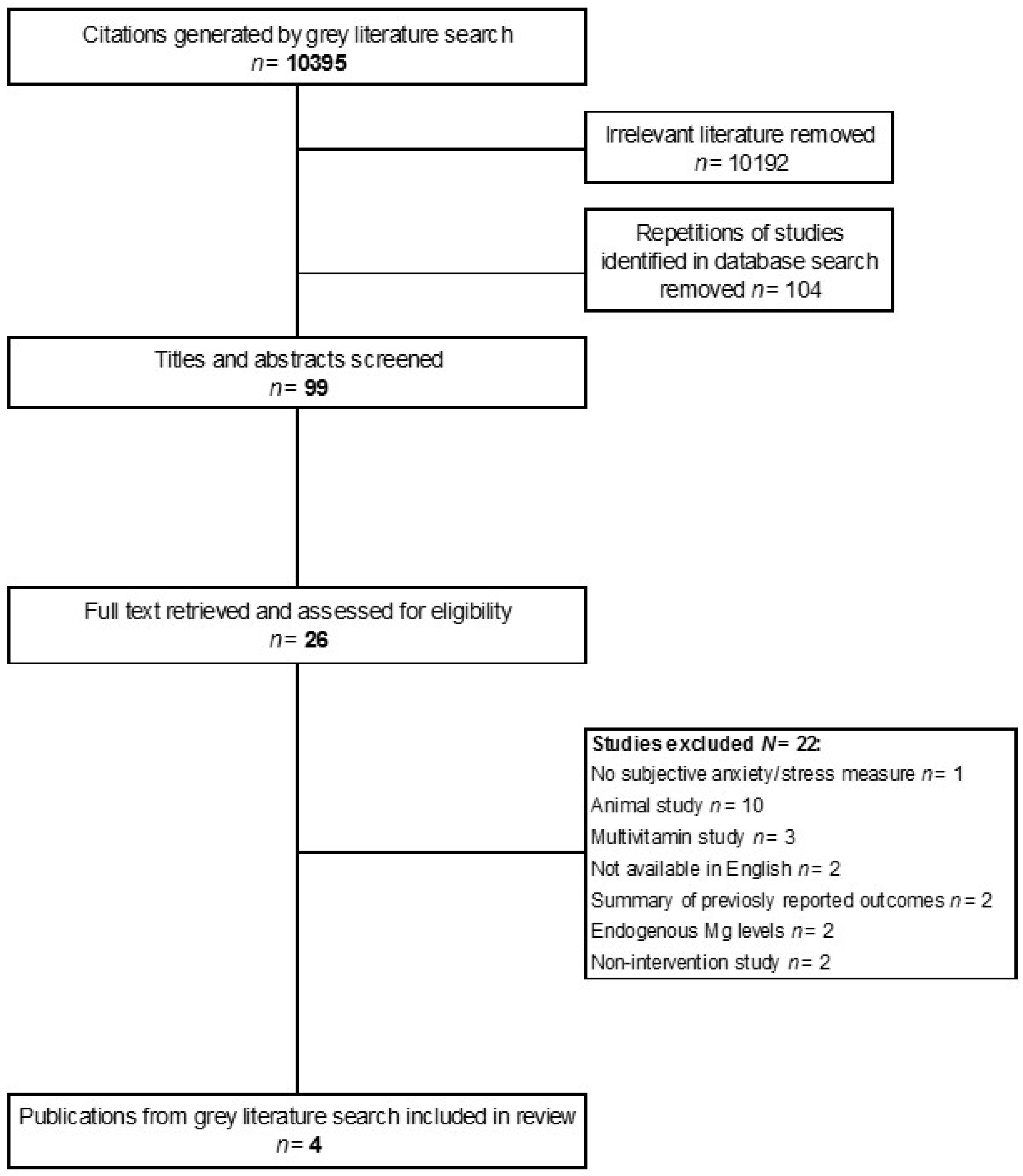
2. Materials and Methods
2.1. Selection of Studies
2.2. Literature Search
2.3. Data Extraction
3. Results and Discussion
3.1. Mild Anxiety
Summary of Effects of Mg in Anxious Samples
3.2. Premenstrual Syndrome
Summary of Effects of Mg in PMS Samples
3.3. Postpartum Anxiety
Summary of Effects of Mg in Postpartum
3.4. Mild Hypertension
Summary of Effects of Mg in Hypertensive Samples
3.5. Moderating Variables
3.5.1. Dosage and Differential Bioavailability of Mg Forms
3.5.2. Duration of Intake
3.5.3. Mg Status
4. Conclusions and Research Recommendations
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 242, 47–66. [Google Scholar]
- Topf, J.M.; Murray, P.T. Hypomagnesemia and hypermagnesemia. Rev. Endocr. Metab. Disord. 2003, 42, 195–206. [Google Scholar] [CrossRef]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E.; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 243, 166–171. [Google Scholar]
- Ford, E.S.; Mokdad, A.H. Dietary magnesium intake in a national sample of US adults. J. Nutr. 2003, 1339, 2879–2882. [Google Scholar]
- Dolega-Cieszkowski, J.H.; Bobyn, J.P.; Whiting, S.J. Dietary intakes of Canadians in the 1990s using population-weighted data derived from the provincial nutrition surveys. Appl. Physiol. Nutr. Metab. 2006, 316, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Preziosi, P.; Durlach, V.; Valeix, P.; Ribas, L.; Bouzid, D.; Favier, A.; Hercberg, S. Dietary magnesium intake in a French adult population. Magnes. Res. 1997, 104, 321–328. [Google Scholar]
- Song, Y.; Sesso, H.D.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S. Dietary magnesium intake and risk of incident hypertension among middle-aged and older US women in a 10-year follow-up study. Am. J. Cardiol. 2006, 9812, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Q.; Manson, J.E.; Cook, N.R.; Albert, C.M.; Buring, J.E.; Liu, S. Dietary magnesium intake and risk of cardiovascular disease among women. Am. J. Cardiol. 2005, 968, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Q.; Manson, J.E.; Buring, J.E.; Liu, S. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care 2004, 271, 59–65. [Google Scholar] [CrossRef]
- Derom, M.L.; Sayón-Orea, C.; Martínez-Ortega, J.M.; Martínez-González, M.A. Magnesium and depression: A systematic review. Nutr. Neurosci. 2013, 165, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Eby, G.A., III; Eby, K.L. Magnesium for treatment-resistant depression: A review and hypothesis. Med. Hypotheses 2010, 744, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Murck, H. Magnesium and affective disorders. Nutr. Neurosci. 2002, 56, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Hallak, M.; Berman, R.F.; Irtenkauf, S.M.; Evans, M.I.; Cotton, D.B. Peripheral Magnesium-Sulfate Enters the Brain and Increases the Threshold for Hippocampal Seizures in Rats. Am. J. Obstet. Gynecol. 1992, 1676, 1605–1610. [Google Scholar] [CrossRef]
- Cotton, D.B.; Hallak, M.; Janusz, C.; Irtenkauf, S.M.; Berman, R.F. Central Anticonvulsant Effects of Magnesium-Sulfate on N-Methyl-d-Aspartate Induced Seizures. Am. J. Obstet. Gynecol. 1993, 1683, 974–978. [Google Scholar] [CrossRef]
- Murck, H.; Steiger, A. Mg2+ reduces ACTH secretion and enhances spindle power without changing delta power during sleep in men—Possible therapeutic implications. Psychopharmacology 1998, 1373, 247–252. [Google Scholar] [CrossRef]
- Glynn, P.; Cooper, D.M.F.; Schulster, D. Modulation of Response of Bovine Adrenocortical Adenylate-Cyclase to Corticotropin. Biochem. J. 1977, 1682, 277–282. [Google Scholar] [CrossRef]
- Ueda, K.; Okamura, N.; Hirai, M.; Tanigawara, Y.; Saeki, T.; Kioka, N.; Komano, T.; Hori, R. Human p-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J. Biol. Chem. 1992, 26734, 24248–24252. [Google Scholar]
- Karssen, A.M.; Meijer, O.C.; van der Sandt, I.C.; Lucassen, P.J.; de Lange, E.C.; de Boer, A.G.; de Kloet, E.R. Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology 2001, 1426, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Tsuruo, T. Characterization of the atpase activity of the mr 170,000 to 180,000 membrane glycoprotein (p-glycoprotein) associated with multidrug resistance in k562/adm cells. Cancer Res. 1988, 4817, 4926–4932. [Google Scholar]
- Muroyama, A.; Inaka, M.; Matsushima, H.; Sugino, H.; Marunaka, Y.; Mitsumoto, Y. Enhanced susceptibility to MPTP neurotoxicity in magnesium-deficient C57BL/6N mice. Neurosci. Res. 2009, 631, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Singewald, N.; Sinner, C.; Hetzenauer, A.; Sartori, S.B.; Murck, H. Magnesium-deficient diet alters depression- and anxiety-related behavior in mice—Influence of desipramine and Hypericum perforatum extract. Neuropharmacology 2004, 478, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.A.; Iezhitsa, I.N.; Kharitonova, M.V.; Kravchenko, M.S. Depression-like and anxiety-related behaviour of rats fed with magnesium-deficient diet. Zhurnal Vysshei Nervnoi Deyatelnosti Imeni I P Pavlova 2008, 584, 476–485. [Google Scholar]
- Whittle, N.; Li, L.; Chen, W.Q.; Yang, J.W.; Sartori, S.B.; Lubec, G.; Singewald, N. Changes in brain protein expression are linked to magnesium restriction-induced depression-like behavior. Amino Acids 2011, 404, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Overland, S.; Stewart, R.; Tell, G.S.; Bjelland, I.; Mykletun, A. Association between magnesium intake and depression and anxiety in community-dwelling adults: The Hordaland Health Study. Aust. N. Z. J. Psychiatry 2009, 431, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, N.; Mori, M. An analysis of hypermagnesemia and hypomagnesemia. Jpn. J. Med. 1990, 294, 368–372. [Google Scholar] [CrossRef]
- Banki, C.M.; Vojnik, M.; Papp, Z.; Balla, K.Z.; Arató, M. Cerebrospinal-fluid magnesium and calcium related to amine metabolites, diagnosis, and suicide attempts. Biol. Psychiatry 1985, 202, 163–171. [Google Scholar] [CrossRef]
- Bjorum, N. Electrolytes in blood in endogenous depression. Acta Psychiatr. Scand. 1972, 481, 59–68. [Google Scholar] [CrossRef]
- Cade, J.F. A Significant Elevation of Plasma Magnesium Levels in Schizophrenia and Depressive States. Med. J. Aust. 1964, 1, 195–196. [Google Scholar] [PubMed]
- Widmer, J.; Bovier, P.; Karege, F.; Raffin, Y.; Hilleret, H.; Gaillard, J.M.; Tissot, R. Evolution of Blood Magnesium, Sodium and Potassium in Depressed-Patients Followed for 3 Months. Neuropsychobiology 1992, 264, 173–179. [Google Scholar] [CrossRef]
- Poleszak, E.; Szewczyk, B.; Kedzierska, E.; Wlaź, P.; Pilc, A.; Nowak, G. Antidepressant- and anxiolytic-like activity of magnesium in mice. Pharmacol. Biochem. Behav. 2004, 78, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.C.; Lobato, K.R.; Binfaré, R.W.; Ferreira, P.K.; Rosa, A.O.; Santos, A.R.; Rodrigues, A.L. Evidence for the involvement of the monoaminergic system in the antidepressant-like effect of magnesium. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 332, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Fromm, L.; Heath, D.L.; Vink, R.; Nimmo, A.J. Magnesium attenuates post-traumatic depression/anxiety following diffuse traumatic brain injury in rats. J. Am. Coll. Nutr. 2004, 23, 529S–533S. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Rodriguez, L.; Rodriguez-Moran, M.; Guerrero-Romero, F. Depressive symptoms and hypomagnesemia in older diabetic subjects. Arch. Med. Res. 2007, 387, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Eby, G.A.; Eby, K.L. Rapid recovery from major depression using magnesium treatment. Med. Hypotheses 2006, 672, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Enya, M.; Kanoh, Y.; Mune, T.; Ishizawa, M.; Sarui, H.; Yamamoto, M.; Takeda, N.; Yasuda, K.; Yasujima, M.; Tsutaya, S.; et al. Depressive state and paresthesia dramatically improved by intravenous MgSO4 in Gitelman‘s syndrome. Intern. Med. 2004, 435, 410–414. [Google Scholar] [CrossRef]
- Bhudia, S.K.; Cosgrove, D.M.; Naugle, R.I.; Rajeswaran, J.; Lam, B.K.; Walton, E.; Petrich, J.; Palumbo, R.C.; Gillinov, A.M.; Apperson-Hansen, C.; et al. Magnesium as a neuroprotectant in cardiac surgery: A randomized clinical trial. J. Thorac. Cardiovasc. Surg. 2006, 1314, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Pavlinac, D.; Langer, R.; Lenhard, L.; Deftos, L. Magnesium in Affective-Disorders. Biol. Psychiatry 1979, 144, 657–661. [Google Scholar]
- Chouinard, G.; Beauclair, L.; Geiser, R.; Etienne, P. A Pilot-Study of Magnesium Aspartate Hydrochloride (Magnesiocard) as a Mood Stabilizer for Rapid Cycling Bipolar Affective-Disorder Patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 1990, 142, 171–180. [Google Scholar] [CrossRef]
- Cox, I.M.; Campbell, M.J.; Dowson, D. Red-Blood-Cell Magnesium and Chronic Fatigue Syndrome. Lancet 1991, 3378744, 757–760. [Google Scholar] [CrossRef]
- Arzoz-Fabregas, M.; Ibarz-Servio, L.; Fernández-Castro, J.; Valiente-Malmagro, M.; Roca-Antonio, J.; Edo-Izquierdo, S.; Buisan-Rueda, O. Chronic stress and calcium oxalate stone disease: Influence on blood cortisol and urine composition. Urology 2013, 826, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Aguilar-Gaxiola, S.; Alonso, J.; Chatterji, S.; Lee, S.; Ormel, J.; Ustün, T.B.; Wang, P.S. The global burden of mental disorders: An update from the WHO World Mental Health (WMH) surveys. Epidemiol. Psichiatr. Soc. 2009, 181, 23–33. [Google Scholar] [CrossRef]
- Sartori, S.B.; Whittle, N.; Hetzenauer, A.; Singewald, N. Magnesium deficiency induces anxiety and HPA axis dysregulation: Modulation by therapeutic drug treatment. Neuropharmacology 2012, 621, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Pyndt Jørgensen, B.; Winther, G.; Kihl, P.; Nielsen, D.S.; Wegener, G.; Hansen, A.K.; Sørensen, D.B. Dietary magnesium deficiency affects gut microbiota and anxiety-like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015, 2705, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Laarakker, M.C.; van Lith, H.A.; Ohl, F. Behavioral characterization of A/J and C57BL/6J mice using a multidimensional test: Association between blood plasma and brain magnesium-ion concentration with anxiety. Physiol. Behav. 2011, 1022, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Iezhitsa, I.N.; Spasov, A.A.; Kharitonova, M.V.; Kravchenko, M.S. Effect of magnesium chloride on psychomotor activity, emotional status, and acute behavioural responses to clonidine, d-amphetamine, arecoline, nicotine, apomorphine, and L-5-hydroxytryptophan. Nutr. Neurosci. 2011, 141, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Grases, G.; Pérez-Castelló, J.A.; Sanchis, P.; Casero, A.; Perelló, J.; Isern, B.; Rigo, E.; Grases, F. Anxiety and stress among science students. Study of calcium and magnesium alterations. Magnes. Res. 2006, 192, 102–106. [Google Scholar]
- Mocci, F.; Canalis, P.; Tomasi, P.A.; Casu, F.; Pettinato, S. The effect of noise on serum and urinary magnesium and catecholamines in humans. Occup. Med. 2001, 511, 56–61. [Google Scholar] [CrossRef]
- Takase, B.; Akima, T.; Uehata, A.; Ohsuzu, F.; Kurita, A. Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clin. Cardiol. 2004, 274, 223–227. [Google Scholar] [CrossRef]
- Held, K.; Antonijevic, I.A.; Künzel, H.; Uhr, M.; Wetter, T.C.; Golly, I.C.; Steiger, A.; Murck, H. Oral Mg2+ supplementation reverses age-related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry 2002, 354, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.L.; Shekhar, A. Panic-Prone State Induced in Rats with GABA Dysfunction in the Dorsomedial Hypothalamus Is Mediated by NMDA Receptors. J. Neurosci. 2006, 2626, 7093–7104. [Google Scholar] [CrossRef] [PubMed]
- Coan, E.J.; Collingridge, G.L. Magnesium ions block an N-methyl-d-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci. Lett. 1985, 53, 21–26. [Google Scholar] [CrossRef]
- Masugi, M.; Yokoi, M.; Shigemoto, R.; Muguruma, K.; Watanabe, Y.; Sansig, G.; van der Putten, H.; Nakanishi, S. Metabotropic glutamate receptor subtype 7 ablation causes deficit in fear response and conditioned taste aversion. J. Neurosci. 1999, 193, 955–963. [Google Scholar]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Papadopol, V.; Nechifor, M. Magnesium in Neuroses and Neuroticism. In Magnesium and the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Lydiard, R.B. The Role of GABA in Anxiety Disorders. J. Clin. Psychiatry 2003, 643, 21–27. [Google Scholar]
- Lakhan, S.E.; Vieira, K.F. Nutritional and herbal supplements for anxiety and anxiety-related disorders: Systematic review. Nutr. J. 2010, 91, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Boyle, N.B. Call for unpublished findings. Magnes. Res. 2016, 29, 34. [Google Scholar]
- Cazaubiel, J.M.; Desor, D. Evaluation of the anti-stress effects of a fermented milk containing milk protein hydrolysate on healthy human subjects sensitive to the stress of everyday life. Proprietary data cited in Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies, No. 1924/20061. Eur. Food Saf. Auth. J Unpublished work. 2008, 1–10. [Google Scholar]
- Rouillon, F.; Lejoyeux, M.; Martineau, C. A Double-blind Controlled Study of PCR 7060 vs. Buspirone in the Treatment of Generalised Anxiety Disorder Conference Abstract. In Proceedings of the 8th European College of Neuropsychopharmacology Congress, Venice, Italy, 30 September–4 October 1995. [Google Scholar]
- Caillard, V. Sanofi Internal report MAB6-26. Paris, France, Unpublished work. 1992. [Google Scholar]
- Caillard, V. Sanofi Internal report MAB6-32. Paris, France, Unpublished work. 1995. [Google Scholar]
- Boyle, N.B.; Lawton, C.; Dye, L. The effects of magnesium supplementation on subjective anxiety. Magnes. Res. 2016, 293, 120–125. [Google Scholar]
- Khine, K.; Rosenstein, D.L.; Elin, R.J.; Niemela, J.E.; Schmidt, P.J.; Rubinow, D.R. Magnesium (Mg) Retention and Mood Effects After Intravenous Mg Infusion in Premenstrual Dysphoric Disorder. Biol. Psychiatry 2006, 594, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.F.; De Souza, M.C.; Marakis, G.; Robinson, P.A.; Morris, A.P.; Bolland, K.M. Unexpected benefit of sorbitol placebo in Mg intervention study of premenstrual symptoms: Implications for choice of placebo in RCTs. Med. Hypotheses 2002, 583, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 30–35. [Google Scholar] [CrossRef]
- Moos, R.H. The development of a menstrual distress questionnaire. Psychosom. Med. 1968, 306, 853–867. [Google Scholar] [CrossRef]
- De Souza, M.C.; Walker, A.F.; Robinson, P.A.; Bolland, K. A synergistic effect of a daily supplement for 1 month of 200 mg magnesium plus 50 mg vitamin B6 for the relief of anxiety-related premenstrual symptoms: A randomized, double-blind, crossover study. J. Womens Health Gend.-Based Med. 2000, 92, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Fard, F.E.; Mirghafourvand, M.; Charandabi, S.M.; Farshbaf-Khalili, A.; Javadzadeh, Y.; Asgharian, H. Effects of zinc and magnesium supplements on postpartum depression and anxiety: A randomized controlled clinical trial. Women Health 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.F.; Marakis, G.; Morris, A.P.; Robinson, P.A. Promising hypotensive effect of hawthorn extract: A randomized double-blind pilot study of mild, essential hypertension. Phytother. Res. 2002, 161, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Borrello, G.; Mastroroberto, P.; Curcio, F.; Lucia Mazza, M. The effects of magnesium oxide on mild essential hypertension and quality of life. Curr. Ther. Res. 1996, 5710, 767–774. [Google Scholar] [CrossRef]
- Spielberger, C.D. State-Trait Anxiety Inventory, 2nd ed.; Bibliography Consulting Psychologists Press: Palo Alto, CA, USA, 1989. [Google Scholar]
- Hanus, M.; Lafon, J.; Mathieu, M. Double-blind, randomised, placebo-controlled study to evaluate the efficacy and safety of a fixed combination containing two plant extracts (Crataegus oxyacantha and Eschscholtzia californica) and magnesium in mild-to-moderate anxiety disorders. Curr. Med. Res. Opin. 2004, 201, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 676, 361–370. [Google Scholar] [CrossRef]
- Bourgeois, M. Rôle du Magne-B6 dans les Manifestations Anxueuses en Pratique Medicale Courante Psychiatr. Pract. Med. 1987, 39, 18–22. [Google Scholar]
- Scharbach, H. Anxiété et Magné B6. La Vie Médicale 1988, 17, 867. [Google Scholar]
- Gendle, M.H.; O’Hara, K.P. Oral Magnesium Supplementation and Test Anxiety in University Undergraduates. J. Artic. Support Null Hypothesis 2015, 11, 21–30. [Google Scholar]
- Driscoll, R. Westside Test Anxiety Scale Validation; Education Resources Information Center: Washington, DC, USA, 2007. [Google Scholar]
- Muthayya, S.; Thomas, T.; Srinivasan, K.; Rao, K.; Kurpad, A.V.; van Klinken, J.W.; Owen, G.; de Bruin, E.A. Consumption of a mid-morning snack improves memory but not attention in school children. Physiol. Behav. 2007, 901, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, E.; Cueto, S.; Jacoby, E.R. Fasting and cognition in well- and undernourished schoolchildren: A review of three experimental studies. Am. J. Clin. Nutr. 1998, 674, 779S–784S. [Google Scholar]
- Markus, C.R.; Olivier, B.; Panhuysen, G.E.; Van Der Gugten, J.; Alles, M.S.; Tuiten, A.; Westenberg, H.G.; Fekkes, D.; Koppeschaar, H.F.; de Haan, E.E. The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am. J. Clin. Nutr. 2000, 716, 1536–1544. [Google Scholar]
- Schatzberg, A.F.; Nemeroff, C.B. The American Psychiatric Publishing Textbook of Psychopharmacology; APA Press: Washington, DC, USA, 2009. [Google Scholar]
- Campbell, D.T.; Kenny, D.A. Primer on Regression Artifacts; Guildford Press: New York, NY, USA, 1999. [Google Scholar]
- Sramek, J.J.; Tansman, M.; Suri, A.; Hornig-Rohan, M.; Amsterdam, J.D.; Stahl, S.M.; Weisler, R.H.; Cutler, N.R. Efficacy of buspirone in generalized anxiety disorder with coexisting mild depressive symptoms. J. Clin. Psychiatry 1996, 577, 287–291. [Google Scholar]
- Taylor, D.P. Buspirone, a new approach to the treatment of anxiety. FASEB J. 1988, 29, 2445–2452. [Google Scholar]
- Quaranta, S.; Buscaglia, M.A.; Meroni, M.G.; Colombo, E.; Cella, S. Pilot study of the efficacy and safety of a modified-release magnesium 250 mg tablet (Sincromag) for the treatment of premenstrual syndrome. Clin. Drug Investig. 2007, 271, 51–58. [Google Scholar] [CrossRef]
- Canning, S.; Dye, L.; Waterman, M.; Orsi, N.; Ayres, J.; Simpson, N. A randomised, double-blind, placebo-controlled trial to test the efficacy of Hypericum perforatum (St. John’s Wort) for the treatment of premenstrual syndrome. CNS Drugs 2009, 54, 506–515. [Google Scholar]
- Fathizadeh, N.; Ebrahimi, E.; Valiani, M.; Tavakoli, N.; Yar, M.H. Evaluating the effect of magnesium and magnesium plus vitamin B6 supplement on the severity of premenstrual syndrome. Iran J. Nurs. Midwifery Res. 2010, 15, 401–405. [Google Scholar] [PubMed]
- Facchinetti, F.; Borella, P.; Sances, G.; Fioroni, L.; Nappi, R.E.; Genazzani, A.R. Oral magnesium successfully relieves premenstrual mood changes. Obstet. Gynecol. 1991, 782, 177–181. [Google Scholar]
- Walker, A.F.; De Souza, M.C.; Vickers, M.F.; Abeyasekera, S.; Collins, M.L.; Trinca, L.A. Magnesium supplementation alleviates premenstrual symptoms of fluid retention. J. Womens Health 1998, 79, 1157–1165. [Google Scholar] [CrossRef]
- Steiner, M.; Haskett, R.F.; Carroll, B.J. Premenstrual tension syndrome: The development of research diagnostic criteria and new rating scales. Acta Psychiatr. Scand. 1980, 62, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M. Premenstrual syndrome: An update on definitions, diagnosis and management. Adv. Psychiatr. Treat. 2001, 7, 469–477. [Google Scholar] [CrossRef]
- Canning, S.; Waterman, M.; Dye, L. Dietary supplements and herbal remedies for premenstrual syndrome (PMS): A systematic research review of the evidence for their efficacy. J. Reprod. Infant Psychol. 2006, 244, 363–378. [Google Scholar] [CrossRef]
- Firoz, M.; Graber, M. Bioavailability of US commercial magnesium preparations. Magnes. Res. 2001, 144, 257–262. [Google Scholar]
- Lindberg, J.S.; Zobitz, M.M.; Poindexter, J.R.; Pak, C.Y. Magnesium bioavailability from magnesium citrate and magnesium oxide. J. Am. Coll. Nutr. 1990, 91, 48–55. [Google Scholar] [CrossRef]
- Walker, A.F.; Marakis, G.; Christie, S.; Byng, M. Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study. Magnes. Res. 2003, 163, 183–191. [Google Scholar]
| Author | Study Design | Condition | Sample (N) | Sex | Age (year) | Treatment (s) | Control | Duration | Outcome Measure | Results | Effect Summary |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bourgeois et al. [74] | RCT | Mild anxiety (Hamilton Anxiety Scale score 10–30) | N = 81 (n = 27 per condition) | 20M:61F | 18–65 | (i) Mg 300 mg as lactate + vit B6 750 mg; (ii) Lorazepam 3 mg; (iii) (i) + (ii) combined | Lorazepam 3 mg (positive verum) | 6 weeks | Hamilton Anxiety Scale | Reduced anxiety scores in all treatments. No significant differences between treatments. | x * |
| Scharbach [75] | RCT | Mild anxiety (Hamilton Anxiety Scale score 15–30) | N = 133 (Treatments (i) n = 44; (ii) n = 46; (ii) n = 43) | 32M:109F | 18–65 | (i) Mg 300 mg as lactate + vit B6 750 mg; (ii) Lorazepam 2 mg; (iii) (i) + (ii) combined | Lorazepam 2 mg (positive verum) | 6 weeks | Hamilton Anxiety Scale | Reduced anxiety scores in all treatments. No significant differences between treatments. | x * |
| Caillard [60] | RCT | Mild anxiety/general anxiety disorder (Hamilton Anxiety Scale score 15–30 & general anxiety disorder (DSM III criteria)) | N = 93 | 25M:68F | x = 41 (SD = 12; 18–65) | Mg 192 mg as lactate + vit B6 20 mg | Placebo | 6 weeks | Hamilton Anxiety Scale | Significant change from baseline (Total score) between groups at Day 21 (Mg + vit B6: x = 12.1 (SD = 6.0); placebo: x = 15.5 (SD = 5.8)) vs. Day 0 (Mg + vit B6: x = 21.0 (SD = 4.5); placebo: x = 22.6 (SD = 4.4); p < .03). No significant differences between Day 0 & Day 42. | + |
| Rouillon et al., [59] | RCT | Mild anxiety/general anxiety disorder Hamilton Anxiety Scale score 15–30 & general anxiety disorder (DSM III-R criteria)) | N = 99 (Mg n = 51; Buspirone n = 48) | 38M:61F | x = 37.7 (SD = 10.7; 19–65) | Mg 192 mg as lactate + vit B6 20 mg | Buspirone 40 mg (positive verum) | 6 weeks | Hamilton Anxiety Scale | Decrease in anxiety scores in both treatment groups across intake. No significant difference between the efficacy of Mg + vit B6 & Buspirone. | x * |
| Caillard [61] | RCT | Symptoms of functional impairment associated with anxiety or a somatic disorder (Hamilton Anxiety Scale 1; Raskin depression scale < 7; COVI anxiety scale = 7) | N = 103 | 26M:77F | x = 37 (18–65) | Mg 192 mg as lactate + vit B6 20 mg | Placebo | 6 weeks | Hamilton Anxiety Scale (somatic score) | Significantly lower somatic anxiety rating after treatment at Day 21 (x = 8.4 (SD = 3.8); p = 0.004) & Day 42 (x = 6.5 (SD = 3.0); p = 0.02) vs. placebo (Day 21: x = 9.9 (SD = 2.9); Day 42: x = 7.8 (SD = 3.6)). | + |
| Hanus et al. [72] | RCT | Mild anxiety/general anxiety order (Hamilton Anxiety Scale score 16–28 & somatic score ≥ 50% total score; & general anxiety disorder) DSM-III-R)) | N = 264 (Treatment n = 130; Placebo n = 134) | 26M:213F | Placebo: x = 44.5 (18–82); Treatment: x = 44.8 (19–81) | Hawthorn extract 75 mg, California poppy 20 mg + elemental Mg 75 mg (Sympathyl®) | Placebo | 12 weeks | Hamilton Anxiety Scale Self-reported anxiety (100 mm VAS) Physician global impression | Total anxiety score: Significant decrease in both conditions. Effect larger in treatment group. Mean change from baseline between Day 0 & Day 90 significantly greater in treatment group (x = −10.6 (SD = 1.2)) vs. placebo (x = −8.9 (SD = 1.2); p = 0.005). Somatic score change from baseline: Treatment (x = −6.5 (SD = 0.7)) Placebo (x = −5.7 (SD = 0.7); p = 0.054). Self-rated anxiety VAS: Mean change from baseline between Day 0 & Day 90 significantly greater in treatment group (x = −38.5) vs. placebo (x = −29.2; p = 0.005). Physician global impression: benefit > risk rating significantly greater in treatment (90%) vs. placebo (80%; p = 0.0018). | + |
| Cazaubiel & Desor [58] | RCT | Mild anxiety (Hospital Depression & Anxiety Scale (HADS) score 4–12) | N = 80 (Treatment n = 40; Placebo n = 40) | 26M:54F | Not reported | Fermented cow’s milk drink (100 mL) containing milk protein hydrolysate 222 mg + Mg 48 mg (Mg form unknown) + blackberry puree | Placebo | 4 weeks | HADS Symptom Checklist Cohen Perceived Stress Scale Vitaliano Coping scale | No significant difference between treatment & placebo on study outcome measures. Post hoc analysis on restricted data (HADS anxiety subscale score 4–8, excluding scores ≥ 9) revealed significant decrease of 31% in treatment group (n = 15) vs. placebo (n = 16) on the anxiety sub-scale of the HADS (p < 0.05). | + 2 |
| Gendle et al. [76] | RCT | Subjective anxiety (Westside Test Anxiety Scale; normal anxiety; elevated normal anxiety; high anxiety; very high anxiety) | N = 122 | 31M:91F | x = 19.3 (SD = 1.17; 18–22) | Mg 300 mg as Mg citrate | Placebo (gelatin) | 5 days | Spielberger State-Trait Anxiety Inventory | No significant difference between treatment and placebo on pre-exam anxiety rating. | x |
| Author | Study Design | Condition | Sample (N) | Age (year) | Treatment (s) | Control | Duration | Outcome Measure | Results | Effect Summary |
|---|---|---|---|---|---|---|---|---|---|---|
| Facchinetti et al. [88] | RCT Cross Placebo Cross | Premenstrual symptom complaints Moos Menstrual Distress Questionnaire (2 consecutive cycles (DSM-IIIR criteria)) | N = 28 | Placebo: x = 31.6 (SD = 5.9; 24–39); Treatment: x = 32.4 (SD = 6.2; 24–39) | Mg 360 mg as Mg pyrrolidone carboxylic acid | Placebo | 2 months baseline + 4 menstrual cycles. Treatment: Mg x 2 2 cycles; placebo: placebo x 2 cycles + Mg x 2 cycles (intake during luteal phases only) | Moos Menstrual Distress Questionnaire (8 symptom categories: pain, inability to concentrate, autonomic reactions, water retention, negative affect, arousal, total score). | Mg significantly reduced negative affect ratings in the placebo crossover group (x = 0.51 (SD = 0.45)) vs. placebo intake (x = 0.76 (SD = 0.70); p < 0.05) & in the Mg treatment group after 2 (x = 0.44 (SD = 0.47)) & 4 (x = 0.45 (SD = 0.46)) cycles vs. baseline (p < 0.02). | + |
| Walker et al. [89] | R-Cross | Premenstrual symptom complaints Menstrual Health Questionnaire (MHQ; retrospective assessment of symptoms during last cycle) | N = 38 | 18–50 (71%–18–25; 7.9%–26–34; 13.2%–35–41; 7.9%–45–50) | Mg 200 mg as Mg oxide | Placebo (cellulose) | 4 menstrual cycles (2 cycles per treatment) | 22 item ordinal daily menstrual symptom diary (6 symptom categories: anxiety; cravings; hydration, depression, other, total) | No significant effect of treatment on anxiety related premenstrual syndrome symptoms. | x |
| De Souza et al. [67] | R-Cross | Premenstrual symptom complaints Menstrual Health Questionnaire (MHQ; retrospective assessment of previous month and baseline) | N = 44 | x = 32 | (i) Mg 200 mg; (ii) vit B6 50 mg; (iii) Mg 200 mg + vit B6 50 mg (as Mg oxide) | Placebo | 5 consecutive menstrual cycles | 30 item ordinal daily menstrual symptom diary (6 symptom categories: anxiety; cravings; hydration, depression, other, total) | No overall treatment effect. Predefined factorial treatment contrasts of adjusted mean scores showed a significant effect of Mg 200 mg + vit B6 50 mg (x = 16.3) for reducing anxiety related premenstrual symptoms vs. baseline (x = 29.3) & placebo (x = 19.8; p = 0.04) for one menstrual cycle. | + 1 |
| Walker et al. [64] | R-Cross | Premenstrual symptom complaints Menstrual Health Questionnaire (MHQ; retrospective assessment of previous month and baseline) | N = 85 | x = 35 | (i) Mg 200 mg; (ii) Mg 350 mg; (iii) Mg 500 mg (all as Mg oxide) | Placebo (sorbitol 1305 mg) | 2 menstrual cycles per condition | 20 item ordinal daily menstrual symptom diary (6 symptom categories: anxiety; cravings; hydration, depression, other, total) | Significant reduction in anxiety-related premenstrual symptoms after 2 months placebo (sorbitol) intake (x = 1.7 (SD = 2)) vs. 200 mg (x = 3.6 (SD = 2)), 350 mg (x = 2.8 (SD = 2)) & 500 mg (x = 3.2 (SD = 2)) Mg treatments. | x |
| Khine et al. [63] | P Post-hoc R-Cross | Premenstrual complaints / Premenstrual Dysphoric Disorder (PMDD) Daily premenstrual symptoms VAS (3 months) & retrospective DSM-IV criteria for PMDD | N = 31 (PMDD n = 17; Placebo n = 14) | Control: x = 28.6 (SD = 6.4; 20–43); PMDD: x = 37.4 (SD = 4.4; 20–43) | Mg sulphate intravenous infusion 0.1mmol/kg body mass (4 h) | Premenstrual complaint-free controls | 24 h post infusion | Spielberger State-Trait Anxiety Inventory Premenstrual Tension Scale (Subjective & Objective) 100 mm premenstrual symptom VAS | No significant mood changes in controls. Evidence of improved VAS mood ratings in initial 6 PMDD women after Mg infusion resulted in post hoc initiated RCT-cross with remaining 10 PMDD women receiving Mg & placebo infusion. Mg infusion subsequently demonstrated to have no mood improvement effects above placebo. | x |
| Quaranta et al. [85] | NR-Cross | Premenstrual symptom complaints Moos Modified Menstrual Distress Questionnaire (baseline score ≥ 25) | N = 38 | x = 32.6 (SD = 8.0; 18–45) | Mg 250 mg (Mg form unknown) | None | 3 menstrual cycles | Moos Modified Premenstrual Distress Questionnaire (including symptom categories: nervous tension, mood swings, irritability, anxiety). Monthly subjective PMS symptom diary | Moos Modified Menstrual Distress Questionnaire: Total score: Significant reduction after 3 months (x = 19.7 (SD = 7.6)) vs. screening visit (x = 30.5 (SD = 4.5); p < 0.001). Monthly subjective PMS symptom diary: Total score: Significant reduction at month 1 (x = 23.3 (SD = 10.6)), month 2 (x = 19.6 (SD = 7.8)), & month 3 (x = 17.9 (SD = 7.3)) with treatment vs. baseline months 1 (x = 31.8 (SD = 6.4)) & 2 (x = 31.3 (SD = 8.4); p < 0.001). PMS anxiety subscale: Significant decrease in anxiety subscale ratings at month 1 (x = 6.3), month 2 (x = 5.3), & month 3 (x = 5.0) with treatment vs. baseline (x = 8.4; p < 0.001). | + |
| Fathizadeh et al. [87] | RCT | Premenstrual symptom complaints Daily premenstrual symptoms record (2 months) | N= 116 (Treatments (i) n = 38; (ii) n = 41; Placebo n = 37) | Placebo: x = 28.03; Treatment (i): x = 28.71; Treatment; (ii): x = 30.02 (all 15–45) | (i) Mg 250 mg; (ii) Mg 250 mg + vit B6 40 mg (Mg form unknown) | Placebo | 2 months | Daily menstrual symptom diary (6 symptom categories: anxiety, cravings, hydration, depression, somatic, total) | Significant reduction in total PMS symptoms in all conditions. Mg + vit B6 resulted in greatest reduction (p < 0.05). Significant main effect of treatments on change from baseline anxiety ratings (Mg + vit B6: x = −22.61 (SD= 20.36); Mg: x = −12.14 (SD = 26.14); placebo: x = 0 (SD = 20.41); p < 0.001). However, no between treatment planned contrasts or post-hoc tests reported. | +? |
| Author | Study Design | Condition | Sample (N) | Sex | Age (year) | Treatment (s) | Control | Duration | Outcome Measure | Results | Effect Summary |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fard et al. [68] | RCT | Postpartum ≤48 h | N = 95 (Treatments: (i) n = 31; (ii) n = 31; Placebo: n = 33; | F | Treatments: (i) x = 29.4 (SD = 5.4); (ii) x = 26.4 (SD = 4.8); Placebo x = 27.6 (SD = 5.1) | (i) Zinc sulphate 27 mg (11 mg elemental zinc); (ii) Mg sulphate 320 mg (64.6 elemental Mg) | Placebo (lactose, starch, cellulose, Mg stearate) | 8 weeks | Spielberger State-Trait Anxiety Inventory | No significant differences between treatments | x |
| Author | Study Design | Condition | Sample (N) | Sex | Age (year) | Treatment (s) | Control | Duration | Outcome Measure | Results | Effect Summary |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Borrello et al. [70] | RCT | Mild hypertension (Diastolic BP > 90 mmHg or Systolic BP > 140 mmHg) | N = 83 (Treatment n = 42; Placebo n = 41) | 30M:53F | Placebo: x = 49; Treatment: x = 51 | Mg oxide 200 mg | Placebo | 12 weeks | 44 item Quality of Life Likert questionnaire (subscales: emotional behaviour & concerns about the future) | Significantly higher total quality of life rating after 12 weeks treatment (x = 67.58 (SD = 5)) vs. baseline (x = 73.58 (SD = 6)) & placebo (x = 73.23 (SD = 8); p < 0.05). | + |
| Walker et al. [64] | RCT | Mild hypertension (Diastolic BP 85–100 mmHg) | N = 36 (9 per condition) | 18M:18F | Placebo: x = 49; Treatment (i): x = 53.2; Treatment (ii): x = 53; Treatment (iii): x = 48.8 | (i) Mg amino acid chelate (600 mg elemental Mg/day); (ii) Hawthorn extract 500 mg; (iii) (i) + (ii) combined | Placebo (cellulose) | 10 weeks | Subjective well-being questionnaire (subscales: vitality, anxiety & depression) | No significant effects on subjective well-being. | x |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyle, N.B.; Lawton, C.; Dye, L. The Effects of Magnesium Supplementation on Subjective Anxiety and Stress—A Systematic Review. Nutrients 2017, 9, 429. https://doi.org/10.3390/nu9050429
Boyle NB, Lawton C, Dye L. The Effects of Magnesium Supplementation on Subjective Anxiety and Stress—A Systematic Review. Nutrients. 2017; 9(5):429. https://doi.org/10.3390/nu9050429
Chicago/Turabian StyleBoyle, Neil Bernard, Clare Lawton, and Louise Dye. 2017. "The Effects of Magnesium Supplementation on Subjective Anxiety and Stress—A Systematic Review" Nutrients 9, no. 5: 429. https://doi.org/10.3390/nu9050429
APA StyleBoyle, N. B., Lawton, C., & Dye, L. (2017). The Effects of Magnesium Supplementation on Subjective Anxiety and Stress—A Systematic Review. Nutrients, 9(5), 429. https://doi.org/10.3390/nu9050429





