Application of an In Vivo Hepatic Triacylglycerol Production Method in the Setting of a High-Fat Diet in Mice
Abstract
:1. Introduction
2. Materials and Methods
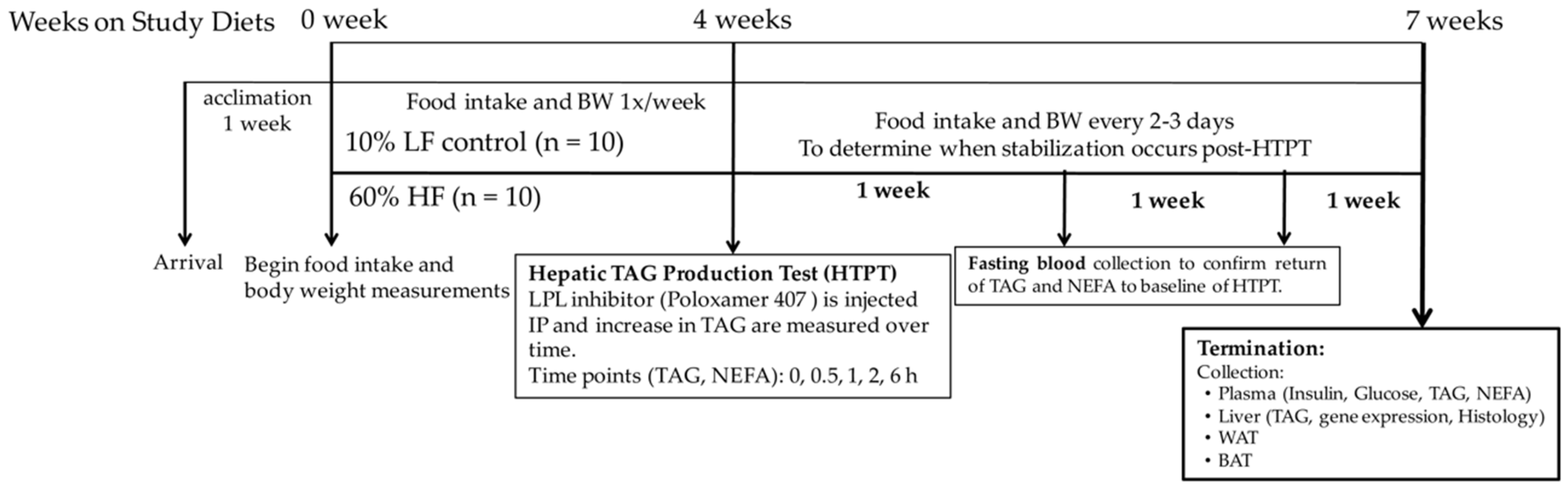
2.1. Study Design
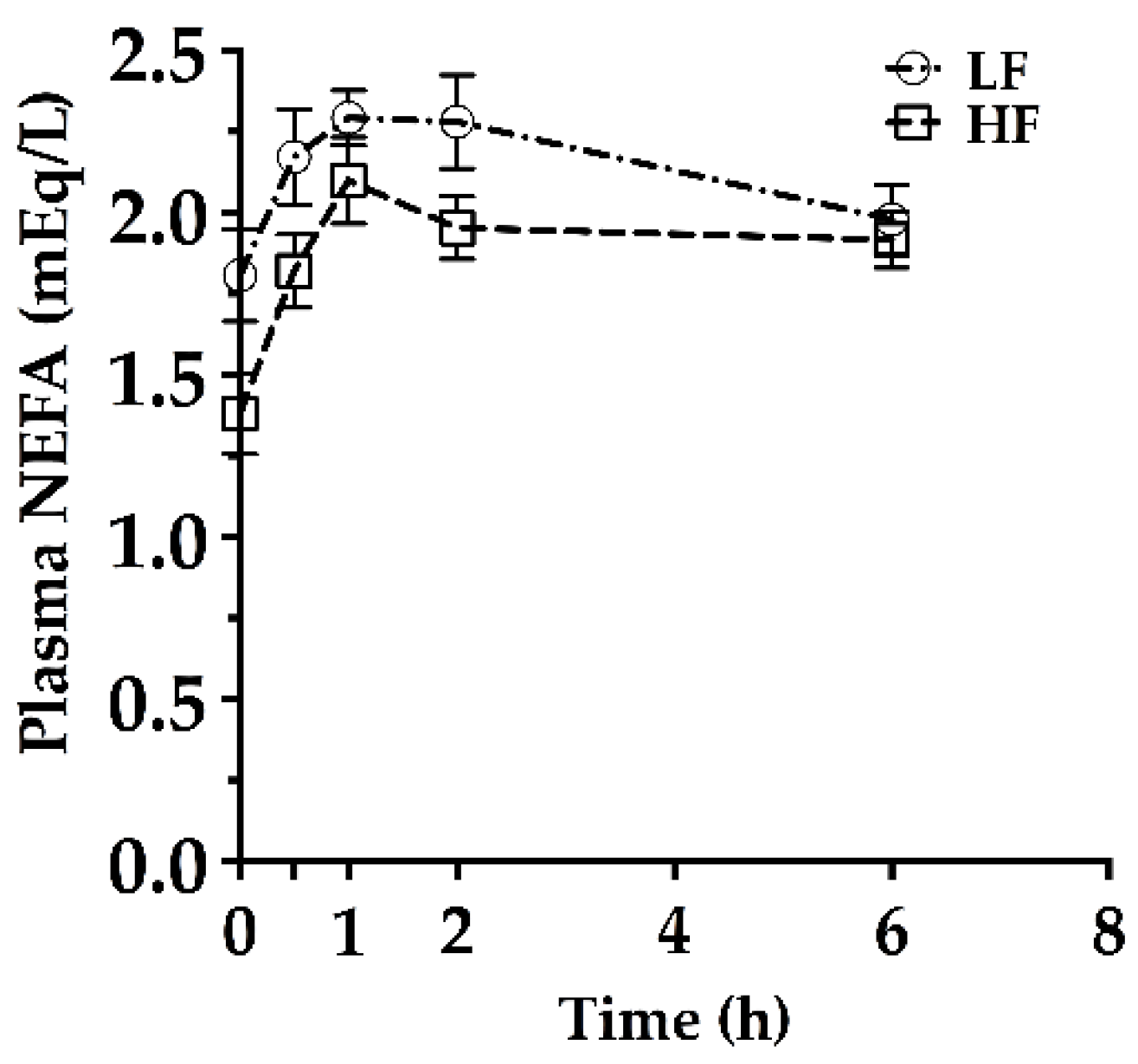
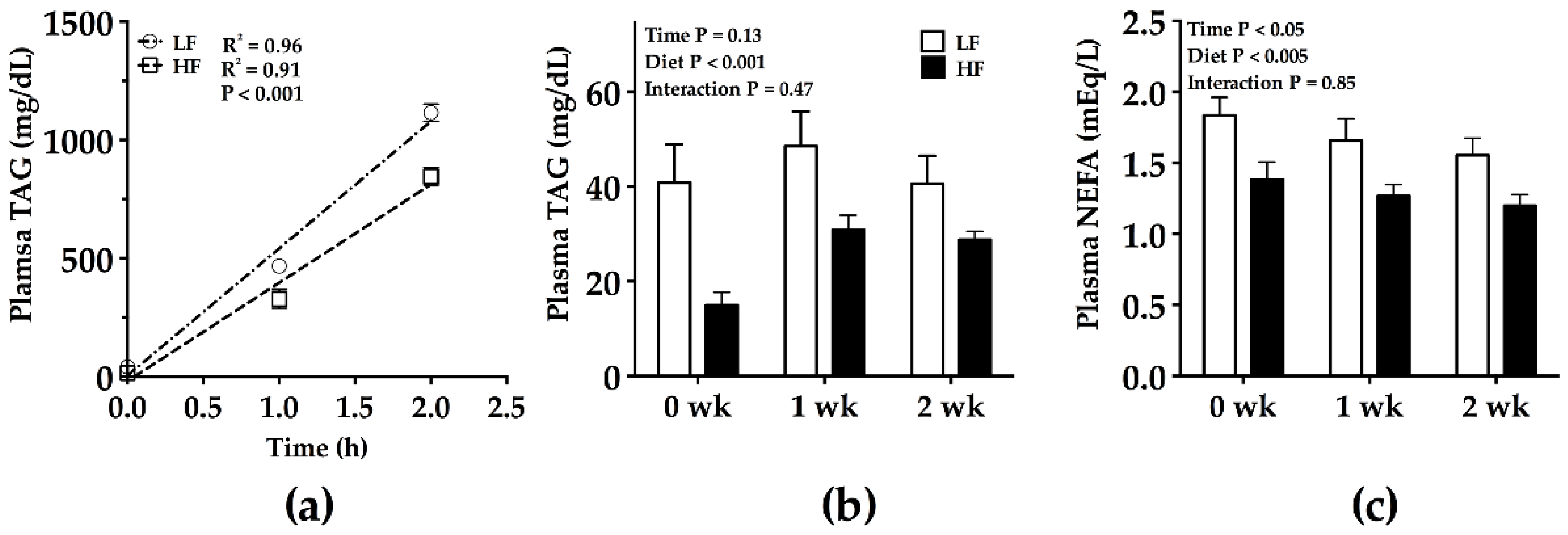
2.2. Hepatic Triacylglycerol Production Test (HTPT)
2.3. Blood Analyte Measurements
2.4. Liver TAG Determination
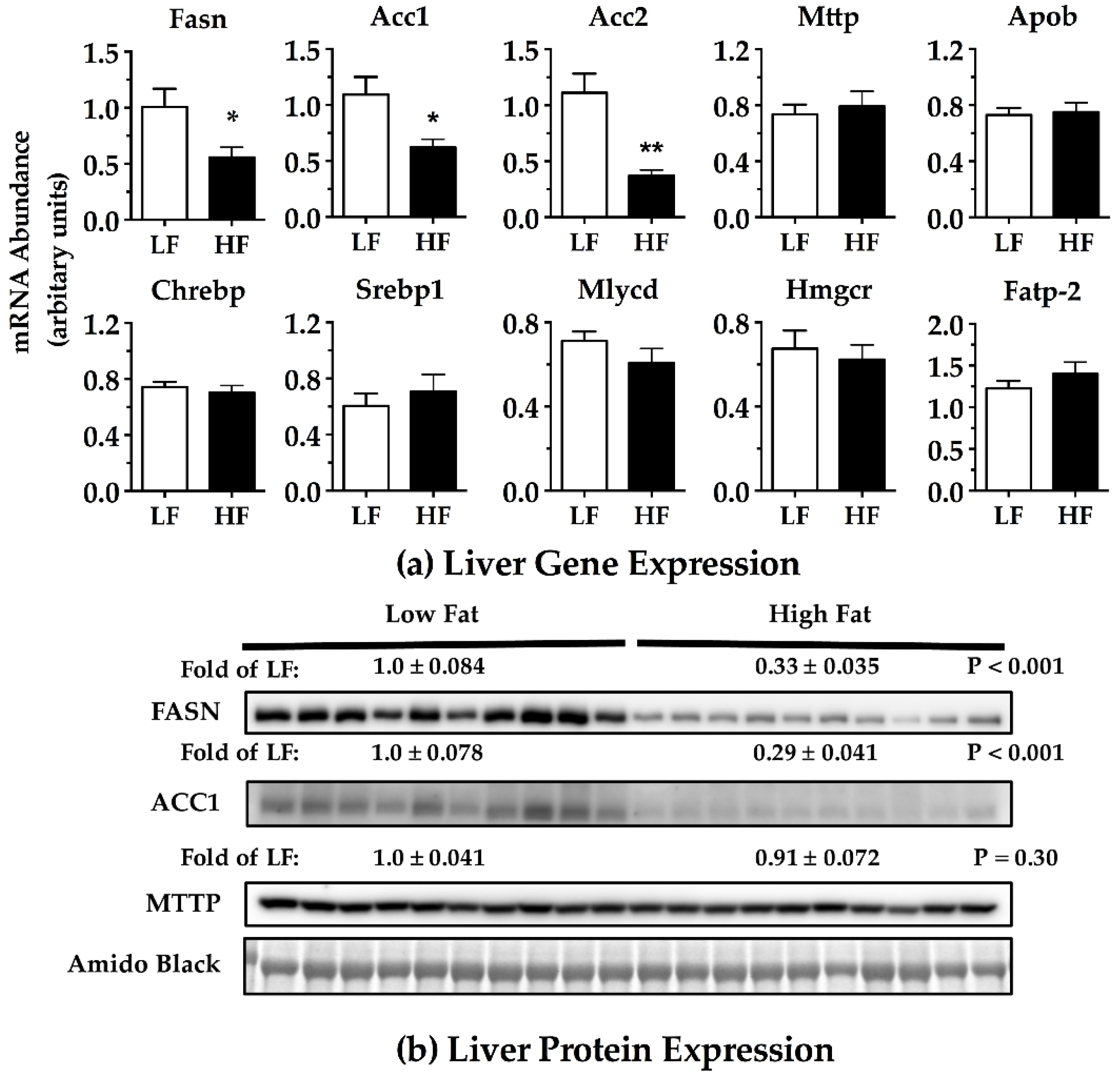
2.5. Total RNA Isolation and Gene Expression
2.6. Immunoblotting
2.7. Histology
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verges, B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Surwit, R.S.; Kuhn, C.M.; Cochrane, C.; McCubbin, J.A.; Feinglos, M.N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988, 37, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.P.; Dunn, T.N.; Oort, P.J.; Grino, M.; Adams, S.H. Inflammatory phenotyping identifies CD11d as a gene markedly induced in white adipose tissue in obese rodents and women. J. Nutr. 2011, 141, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.P.; Dunn, T.N.; Drayton, J.B.; Oort, P.J.; Adams, S.H. A high calcium diet containing nonfat dry milk reduces weight gain and associated adipose tissue inflammation in diet-induced obese mice when compared to high calcium alone. Nutr. Metab. (Lond.) 2012, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; Habibi, J.; Ford, D.A.; Nistala, R.; Lastra, G.; Manrique, C.; Dunham, M.M.; Ford, K.D.; Thyfault, J.P.; Parks, E.J.; et al. Dipeptidyl peptidase-4 inhibition ameliorates Western diet-induced hepatic steatosis and insulin resistance through hepatic lipid remodeling and modulation of hepatic mitochondrial function. Diabetes 2015, 64, 1988–2001. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Terauchi, Y. Lessons from mouse models of high-fat diet-induced NAFLD. Int. J. Mol. Sci. 2013, 14, 21240–21257. [Google Scholar] [CrossRef] [PubMed]
- Pramfalk, C.; Pavlides, M.; Banerjee, R.; McNeil, C.A.; Neubauer, S.; Karpe, F.; Hodson, L. Fasting Plasma insulin concentrations are associated with changes in hepatic fatty acid synthesis and partitioning prior to changes in liver fat content in healthy adults. Diabetes 2016, 65, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.S.; Cromley, D.A.; McCoy, M.G.; Rader, D.J.; Billheimer, J.T. Determining hepatic triglyceride production in mice: Comparison of poloxamer 407 with Triton WR-1339. J. Lipid Res. 2005, 46, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Wout, Z.G.; Pec, E.A.; Maggiore, J.A.; Williams, R.H.; Palicharla, P.; Johnston, T.P. Poloxamer 407-mediated changes in plasma cholesterol and triglycerides following intraperitoneal injection to rats. J. Parenter. Sci. Technol. 1992, 46, 192–200. [Google Scholar] [PubMed]
- Palmer, W.K.; Emeson, E.E.; Johnston, T.P. Poloxamer 407-induced atherogenesis in the C57BL/6 mouse. Atherosclerosis 1998, 136, 115–123. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Slone Standley, G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Baumgardner, J.N.; Shankar, K.; Hennings, L.; Badger, T.M.; Ronis, M.J. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G27–G38. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.W.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 2016, 91, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.D.; Lauritsen, M.; Nielsen, L.B. Hepatic expression of microsomal triglyceride transfer protein and in vivo secretion of triglyceride-rich lipoproteins are increased in obese diabetic mice. Diabetes 2002, 51, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Raabe, M.; Véniant, M.M.; Sullivan, M.A.; Zlot, C.H.; Björkegren, J.; Nielsen, L.B.; Wong, J.S.; Hamilton, R.L.; Young, S.G. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Investig. 1999, 103, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Hirahatake, K.M.; Meissen, J.K.; Fiehn, O.; Adams, S.H. Comparative effects of fructose and glucose on lipogenic gene expression and intermediary metabolism in HepG2 liver cells. PLoS ONE 2011, 6, e26583. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Gleason, J.A.; Dansinger, M.L. Dietary fructose and glucose differentially affect lipid and glucose homeostasis. J. Nutr. 2009, 139, 1257S–1262S. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Urstad, A.P.L.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Zammit, V.A.; Waterman, I.J.; Topping, D.; McKay, G. Insulin stimulation of hepatic triacylglycerol secretion and the etiology of insulin resistance. J. Nutr. 2001, 131, 2074–2077. [Google Scholar] [PubMed]
- Aguilera, C.M.; Gil-Campos, M.; Cañete, R.; Gil, A. Alterations in plasma and tissue lipids associated with obesity and metabolic syndrome. Clin. Sci. (Lond.) 2008, 114, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.P.; Goldberg, I.J. Inhibition of pancreatic lipase by poloxamer 407 may provide an adjunct treatment strategy for weight loss. J. Pharm. Pharmacol. 2006, 58, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Wasan, K.M.; Subramanian, R.; Kwong, M.; Goldberg, I.J.; Wright, T.; Johnston, T.P. Poloxamer 407-mediated alterations in the activities of enzymes regulating lipid metabolism in rats. J. Pharm. Pharm. Sci. 2003, 6, 189–197. [Google Scholar] [PubMed]
- Vatner, D.F.; Majumdar, S.K.; Kumashiro, N.; Petersen, M.C.; Rahimi, Y.; Gattu, A.K.; Bears, M.; Camporez, J.P.; Cline, G.W.; Jurczak, M.J.; et al. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc. Natl. Acad. Sci. USA 2015, 112, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.; Mercer, K.; Engi, B.; Pulliam, C.; Zimniak, P.; Hennings, L.; Shearn, C.; Badger, T.; Petersen, D. Global deletion of glutathione S-Transferase A4 exacerbates developmental N 1 onalcoholic steatohepatitis. Am. J. Pathol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bruckdorfer, K.R.; Khan, I.H.; Yudkin, J. Fatty acid synthetase activity in the liver and adipose tissue of rats fed with various carbohydrates. Biochem. J. 1972, 129, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.; Perdereau, D.; Foufelle, F.; Prip-Buus, C.; Ferre, P. Regulation of lipogenic enzyme gene expression by nutrients and hormones. FASEB J. 1994, 8, 36–42. [Google Scholar] [PubMed]
- Koo, H.Y.; Wallig, M.A.; Chung, B.H.; Nara, T.Y.; Cho, B.H.; Nakamura, M.T. Dietary fructose induces a wide range of genes with distinct shift in carbohydrate and lipid metabolism in fed and fasted rat liver. Biochim. Biophys. Acta 2008, 1782, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J. Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr. Rev. 2005, 63, 133–157. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Iizuka, K.; Miller, B.C.; Uyeda, K. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc. Natl. Acad. Sci. USA 2004, 101, 15597–15602. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.A.; Carvalho, F.; Pearson, M.; Horton, J.D.; Browning, J.D.; Jones, J.G.; Burgess, S.C. A high-fat diet suppresses de novo lipogenesis and desaturation but not elongation and triglyceride synthesis in mice. J. Lipid Res. 2014, 55, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Oosterveer, M.H.; van Dijk, T.H.; Tietge, U.J.; Boer, T.; Havinga, R.; Stellaard, F.; Groen, A.K.; Kuipers, F.; Reijngoud, D.J. High fat feeding induces hepatic fatty acid elongation in mice. PLoS ONE 2009, 4, e6066. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Cho, Y.R.; Kim, H.J.; Higashimori, T.; Danton, C.; Lee, M.K.; Dey, A.; Rothermel, B.; Kim, Y.B.; Kalinowski, A.; et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 2005, 54, 3530–3540. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Kowalski, G.M.; Leslie, S.J.; Risis, S.; Yang, C.; Lee-Young, R.S.; Babb, J.R.; Meikle, P.J.; Lancaster, G.I.; Henstridge, D.C.; et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 2013, 56, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.N.B.; Veidal, S.S.; Rigbolt, K.T.G.; Tølbøl, K.S.; Roth, J.D.; Jelsing, J.; Vrang, N.; Feigh, M. Obese diet-induced mouse models of nonalcoholic steatohepatitis-tracking disease by liver biopsy. World J. Hepatol. 2016, 8, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Mohammed, B.S.; Magkos, F.; Korenblat, K.M.; Patterson, B.W.; Klein, S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 2008, 134, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Linfoot, P.; Dare, D.; Aghajanian, K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr. 2003, 77, 43–50. [Google Scholar] [PubMed]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, L.C.; Hellerstein, M.; Seidman, C.; Neese, R.; Diakun, J.; Hirsch, J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J. Clin. Investig. 1996, 97, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Mills, K.T.; Yao, L.; Demanelis, K.; Eloustaz, M.; Yancy, W.S., Jr.; Kelly, T.N.; He, J.; Bazzano, L.A. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: A meta-analysis of randomized controlled clinical trials. Am. J. Epidemiol. 2012, 176, S44–S54. [Google Scholar] [CrossRef] [PubMed]


| Diet (Product Code) | ||
|---|---|---|
| Control (D12450J) | High Fat (D12492) | |
| Ingredients (g/kg) | ||
| Casein, 30-Mesh | 190 | 258 |
| l-Cysteine | 2.84 | 3.88 |
| Corn Starch | 480 | 0.00 |
| Maltodextrin 10 | 118 | 162 |
| Sucrose | 65.2 | 88.9 |
| Cellulose, BW200 | 47.4 | 64.6 |
| Soybean oil | 23.7 | 32.3 |
| Lard | 19.0 | 317 |
| Mineral Mix S10026 | 9.48 | 12.9 |
| Dicalcium Phosphate | 12.3 | 16.8 |
| Calcium Carbonate | 5.21 | 7.11 |
| Potassium Citrate, 1 H2O | 15.6 | 21.3 |
| Vitamin Mix V10001 | 9.48 | 12.9 |
| Choline Bitartrate | 1.90 | 2.58 |
| FD&C Yellow Dye #5 | 0.04 | 0.00 |
| FD&C Red Dye #40 | 0.00 | 0.00 |
| RD&C Blue Dye #1 | 0.01 | 0.07 |
| Macronutrients (% by weight) | ||
| Protein | 19.2% | 26.0% |
| Total Carbohydrate | 67.3% | 26.0% |
| Sucrose | 6.52% | 8.89% |
| Fat | 4.3% | 35.0% |
| Macronutrients (% kcal) | ||
| Protein | 20% | 20% |
| Total Carbohydrate | 70% | 20% |
| Sucrose | 6.8% | 6.8% |
| Fat | 10% | 60% |
| kcal/g | 3.85 | 5.24 |
| Gene | Gene Name | Forward | Reverse |
|---|---|---|---|
| 18S | 18S ribosomal RNA | GAGGCCCTGTAATTGGAATGAG | CGCTATTGGAGCTGGAATTACC |
| ApoB | Apolipoprotein B | ATACCACGTTTGCAAGCAGAAGCC | TGTTGAGCCGTAAGCTGTAGCAGA |
| Acc1 | Acetyl-CoA Carboxylase 1/alpha | TAACAGAATCGACACTGGCTGGCT | ATGCTGTTCCTCAGGCTCACATCT |
| Acc2/Acacb | Acetyl-CoA Carboxylase 1/beta | AGTCTTCCGTGCCTTTGTAC | TTCTGCAAACTCATCCCTCG |
| ChREBP/Mlxpl | Carbohydrate-responsive element-binding protein | CATCTCCAGCCTCGTCTTC | CTTGGTCTTAGGGTCTTCAGG |
| Fapt-2 | Fatty Acid Transport Protein 2 | AGTACATCGGTGAACTGCTTCGGT | TGCCTTCAGTGGAAGCGTAGAACT |
| Fasn | Fatty Acid Synthase | TGACCTCGTGATGAACGTGTAC | GGGTGAGGACGTTTACAAAGG |
| Hmgcr | 3-Hydroxy-3-Methylglutaryl-CoA Reductase | GCCCTCAGTTCAAATTCACAG | TTCCACAAGAGCGTCAAGAG |
| Mlycd | Malonyl-CoA Decarboxylase | CTCGGGACCTTCCTCATAAAG | CTCCTTCCCCTGCACATTC |
| Mttp | Microsomal Triglyceride Transfer Protein | TTCCCAGTAGGTTGGCTTTC | CACCTGGTTCACCCTGTTTA |
| Srebp1 | Sterol regulatory element-binding protein 1 | GGCTATTCCGTGAACATCTCCTA | ATCCAAGGGCATCTGAGAACTC |
| Low Fat | High Fat | p Values | |
|---|---|---|---|
| Cumulative Macronutrient Intake | |||
| Total Carbohydrate (g) | 79.1 ± 1.1 | 28.1 ± 0.7 | <0.001 |
| Sucrose (g) | 7.7 ± 0.1 | 9.5 ± 0.2 | <0.001 |
| Fat (g) | 5.0 ± 0.1 | 37.4 ± 0.9 | <0.001 |
| Protein (g) | 22.6 ± 0.3 | 28.1 ± 0.7 | <0.001 |
| Plasma Metabolic Markers | |||
| Insulin (pg/mL) | 376 ± 24 | 636 ± 38 | <0.001 |
| TAG (mg/dL) | 80.2 ± 8.00 | 47.4 ± 3.86 | 0.003 |
| NEFA (mEq/dL) | 0.644 ± 0.037 | 0.441 ± 0.027 | <0.001 |
| Glucose (mg/dL) | 133 ± 2.38 | 121 ± 1.55 | <0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono-Moore, K.D.; Ferguson, M.; Blackburn, M.L.; Issafras, H.; Adams, S.H. Application of an In Vivo Hepatic Triacylglycerol Production Method in the Setting of a High-Fat Diet in Mice. Nutrients 2017, 9, 16. https://doi.org/10.3390/nu9010016
Ono-Moore KD, Ferguson M, Blackburn ML, Issafras H, Adams SH. Application of an In Vivo Hepatic Triacylglycerol Production Method in the Setting of a High-Fat Diet in Mice. Nutrients. 2017; 9(1):16. https://doi.org/10.3390/nu9010016
Chicago/Turabian StyleOno-Moore, Kikumi D., Matthew Ferguson, Michael L. Blackburn, Hassan Issafras, and Sean H. Adams. 2017. "Application of an In Vivo Hepatic Triacylglycerol Production Method in the Setting of a High-Fat Diet in Mice" Nutrients 9, no. 1: 16. https://doi.org/10.3390/nu9010016
APA StyleOno-Moore, K. D., Ferguson, M., Blackburn, M. L., Issafras, H., & Adams, S. H. (2017). Application of an In Vivo Hepatic Triacylglycerol Production Method in the Setting of a High-Fat Diet in Mice. Nutrients, 9(1), 16. https://doi.org/10.3390/nu9010016





