Methionine and Choline Supply during the Periparturient Period Alter Plasma Amino Acid and One-Carbon Metabolism Profiles to Various Extents: Potential Role in Hepatic Metabolism and Antioxidant Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Animal Management
2.3. Blood Sample Collection and Analyses of Plasma AA and Their Derivatives
2.4. Liver Sample Collection and Quantitative RT-PCR (qPCR)
2.5. Statistical Analysis
3. Results
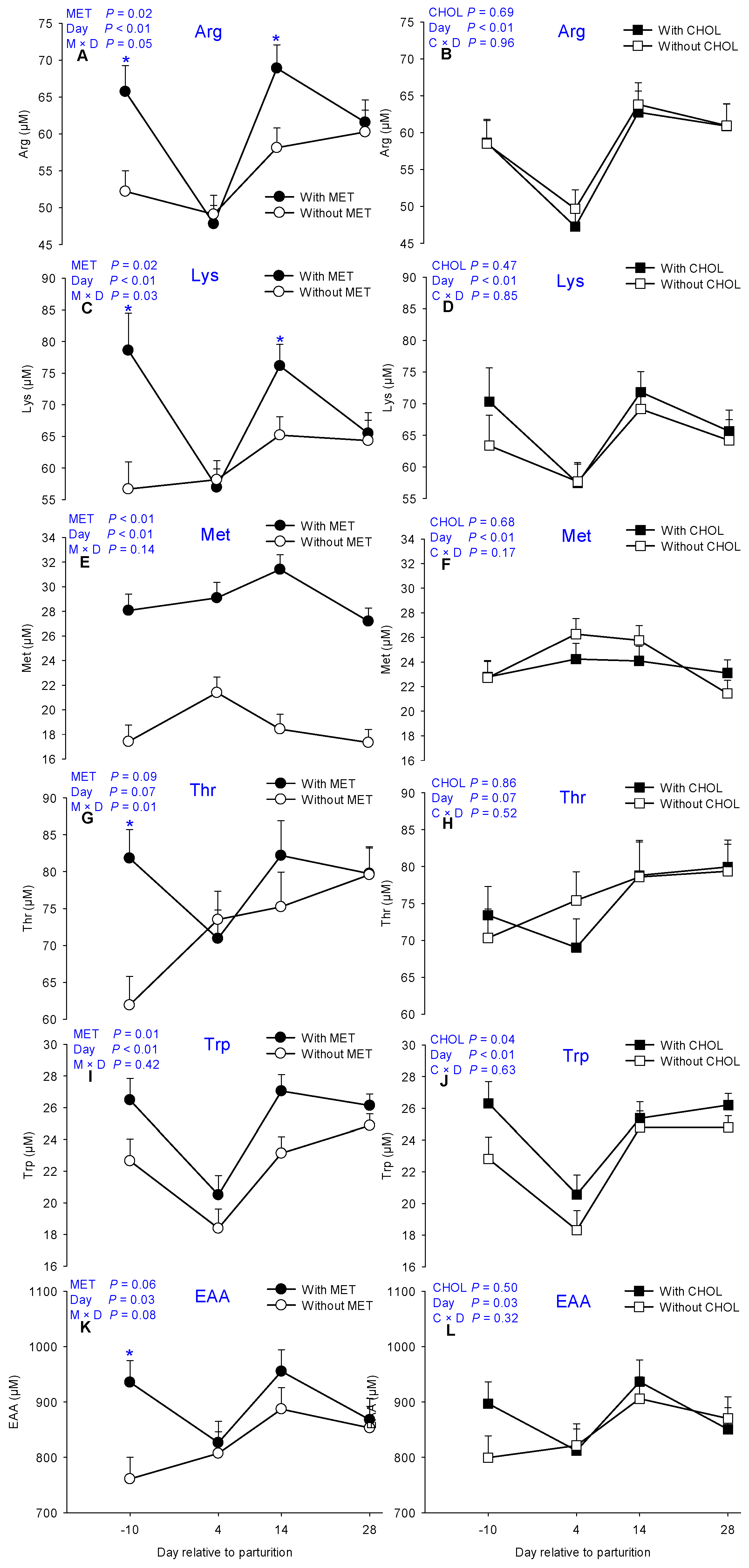
3.1. Essential AA
3.2. Non-Essential AA
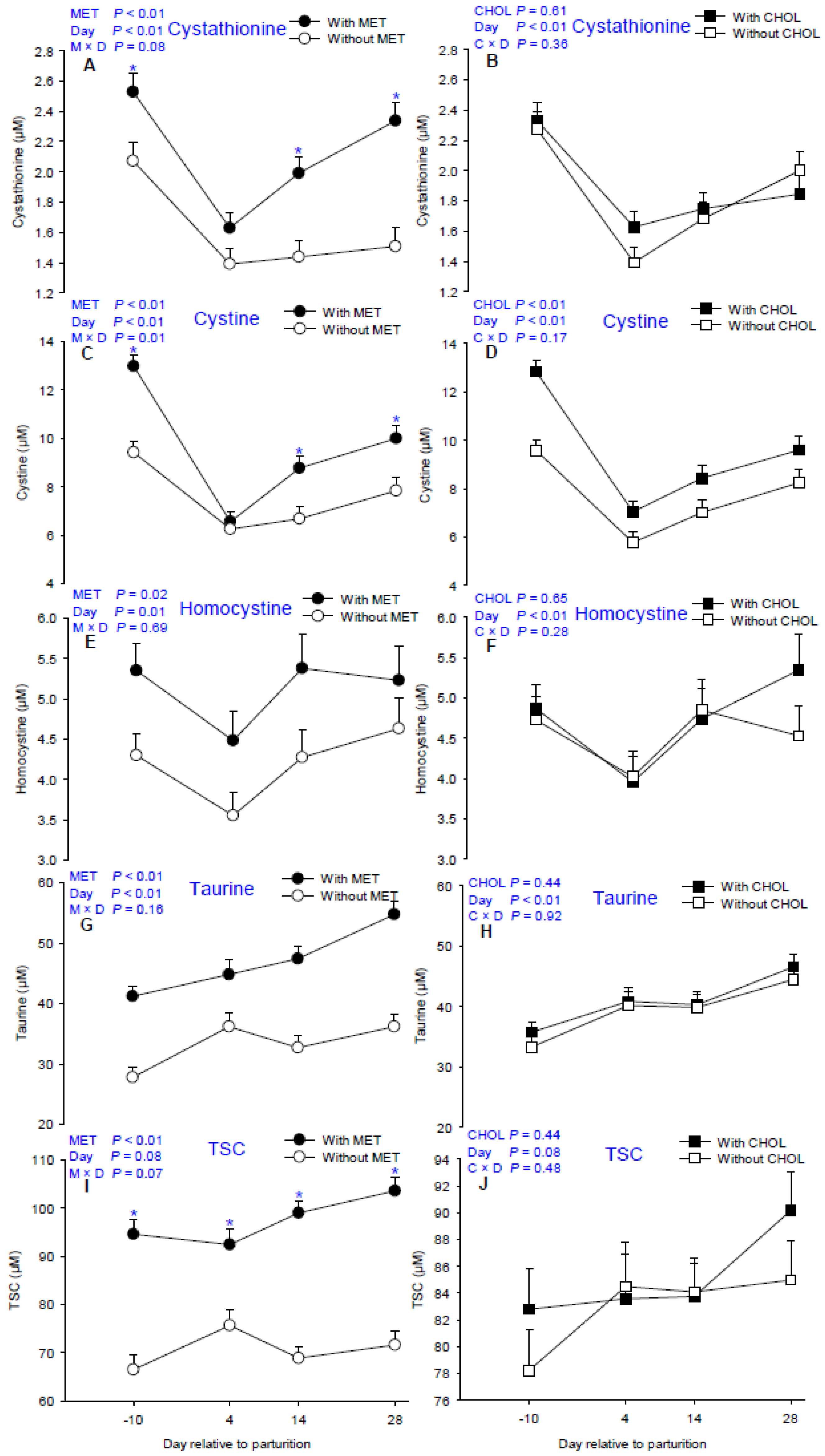
3.3. Sulfur-Containing Compounds
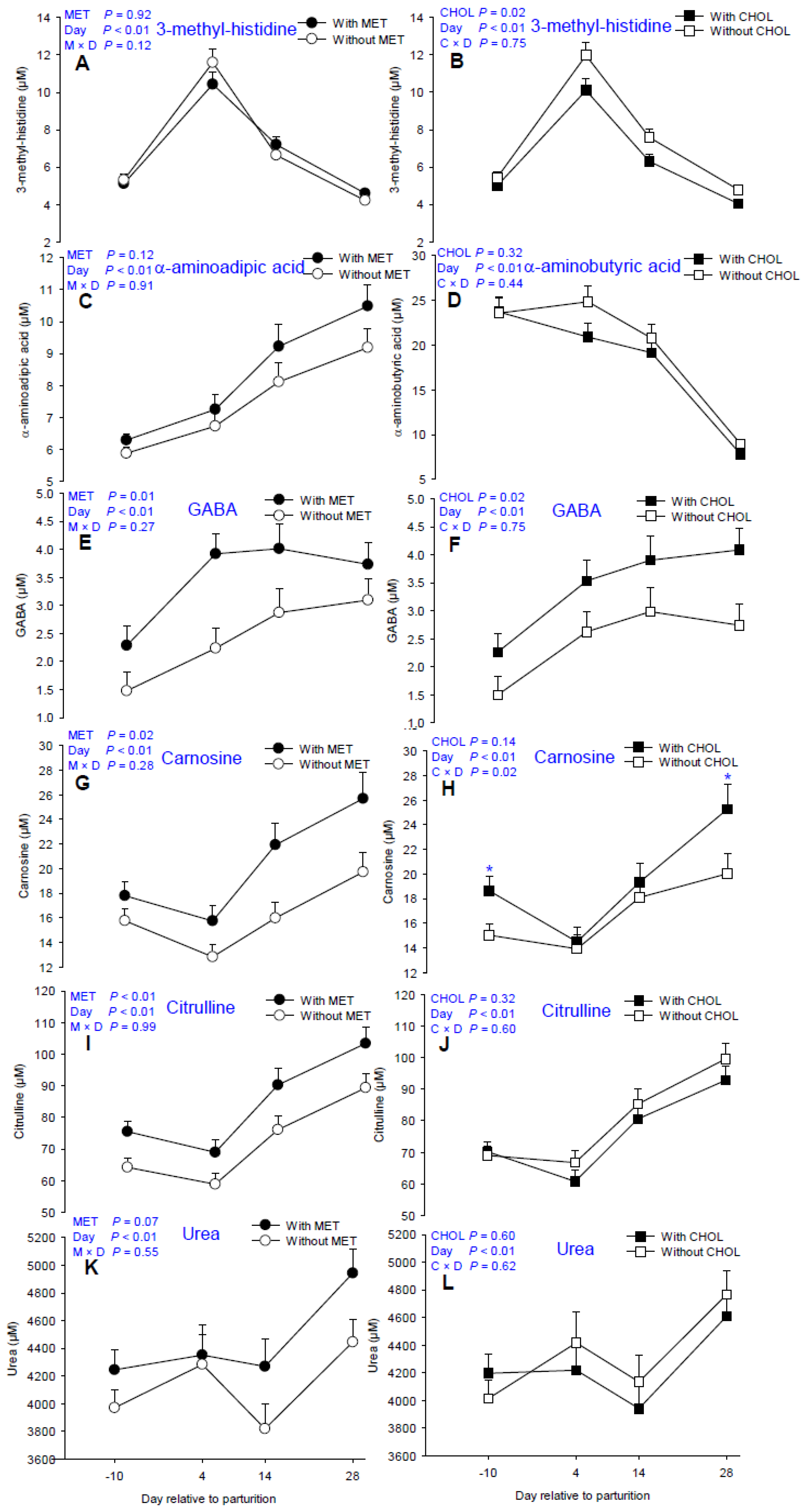
3.4. Non-Proteinogenic AA and Derivatives
3.5. Pyruvate Carboxylase and Phosphoenolpyruvate Carboxykinase 1 Expression
4. Discussion
4.1. Enhancing the Supply of MET Improved Plasma AA Profiles
4.2. Utilization of Circulating AA Close to Parturition
4.3. Sulfur-Containing Compound Pool and Metabolism
4.4. AA Derivatives
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bell, A.W.; Burhans, W.S.; Overton, T.R. Protein nutrition in late pregnancy, maternal protein reserves and lactation performance in dairy cows. Proc. Nutr. Soc. 2000, 59, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P.; Horst, R.L. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Zhou, Z.; Loor, J.J.; Piccioli-Cappelli, F.; Librandi, F.; Lobley, G.E.; Trevisi, E. Circulating amino acids during the peripartal period in cows with different liver functionality index. J. Dairy Sci. 2016, 99, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.R. Substrate utilization for hepatic gluconeogenesis in the transition dairy cow. In Proceedings of the 1998 Cornell Nutrition Conference for Feed Manufacturers, Syracuse, NY, USA, 19 October 1998; pp. 237–246.
- McNeill, D.M.; Slepetis, R.; Ehrhardt, R.A.; Smith, D.M.; Bell, A.W. Protein requirements of sheep in late pregnancy: Partitioning of nitrogen between gravid uterus and maternal tissues. J. Anim. Sci. 1997, 75, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.S.; Ji, P.; Drackley, J.K.; Luchini, D.; Loor, J.J. Supplemental smartamine m or metasmart during the transition period benefits postpartal cow performance and blood neutrophil function. J. Dairy Sci. 2013, 96, 6248–6263. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.S.; Ji, P.; Drackley, J.K.; Luchini, D.; Loor, J.J. Smartamine M and MetaSmart supplementation during the peripartal period alter hepatic expression of gene networks in 1-carbon metabolism, inflammation, oxidative stress, and the growth hormone-insulin-like growth factor 1 axis pathways. J. Dairy Sci. 2014, 97, 7451–7464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Bulgari, O.; Vailati-Riboni, M.; Trevisi, E.; Ballou, M.A.; Cardoso, F.C.; Luchini, D.N.; Loor, J.J. Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period. J. Dairy Sci. 2016, 99, 8956–8969. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Vailati-Riboni, M.; Trevisi, E.; Drackley, J.K.; Luchini, D.N.; Loor, J.J. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J. Dairy Sci. 2016, 99, 8716–8732. [Google Scholar] [CrossRef] [PubMed]
- Pisulewski, P.M.; Rulquin, H.; Peyraud, J.L.; Verite, R. Lactational and systemic responses of dairy cows to postruminal infusions of increasing amounts of methionine. J. Dairy Sci. 1996, 79, 1781–1791. [Google Scholar] [CrossRef]
- Schwab, C.G.; Bozak, C.K.; Whitehouse, N.L.; Mesbah, M.M. Amino acid limitation and flow to duodenum at four stages of lactation. 1. Sequence of lysine and methionine limitation. J. Dairy Sci. 1992, 75, 3486–3502. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [PubMed]
- Ulrey, C.L.; Liu, L.; Andrews, L.G.; Tollefsbol, T.O. The impact of metabolism on DNA methylation. Hum. Mol. Genet. 2005, 14, R139–R147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Vailati-Riboni, M.; Luchini, D.; Loor, J.J. Rumen-protected methyl donors during the transition period: Circulating plasma amino acids in response to supplemental rumen-protected methionine or choline. In Proceedings of the ASAS-ADSA-CSAS-WSASAS Joint Annual Meetings, Salt Lake City, UT, USA, 19–23 July 2016.
- Wong, E.R.; Thompson, W. Choline oxidation and labile methyl groups in normal and choline-deficient rat liver. Biochim. Biophys. Acta 1972, 260, 259–271. [Google Scholar] [CrossRef]
- Li, Z.; Vance, D.E. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Baldi, A.; Dell’Orto, V. Comparative mammalian choline metabolism with emphasis on the high-yielding dairy cow. Nutr. Res. Rev. 2002, 15, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Doepel, L.; Lapierre, H.; Kennelly, J.J. Peripartum performance and metabolism of dairy cows in response to prepartum energy and protein intake. J. Dairy Sci. 2002, 85, 2315–2334. [Google Scholar] [CrossRef]
- Meijer, G.A.L.; Vandermeulen, J.; Bakker, J.G.M.; Vanderkoelen, C.J.; Vanvuuren, A.M. Free amino-acids in plasma and muscle of high-yielding dairy-cows in early lactation. J. Dairy Sci. 1995, 78, 1131–1141. [Google Scholar] [CrossRef]
- Verbeke, R.; Roets, E.; Peeters, G. Variations in the concentrations of free amino acids in the plasma of the dairy cow at parturition. J. Dairy Res. 1972, 39, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.; Homolka, P.; Koukolova, V. Effect of dietary rumen-protected choline on milk production of dairy cows: A meta-analysis. J. Dairy Sci. 2010, 93, 3746–3754. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, A.; D’Occhio, M.J.; Al Jassim, R. The role of rumen-protected choline in hepatic function and performance of transition dairy cows. Br. J. Nutr. 2016, 116, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Vailati-Riboni, M.; Trevisi, E.; drackley, J.K.; Luchini, D.; Loor, J.J. Better postpartal performance in dairy cows supplemented with rumen-protected methionine than choline during the peripartal period. J. Dairy Sci. 2016, in press. [Google Scholar]
- Graulet, B.; Richard, C.; Robert, J.C. Methionine availability in plasma of dairy cows supplemented with methionine hydroxy analog isopropyl ester. J. Dairy Sci. 2005, 88, 3640–3649. [Google Scholar] [CrossRef]
- Benoit, S.L.A. Can Choline Spare Methioinine from Catabolism in Lactating Mice and Dairy Cows? University of Maryland: College Park, MD, USA, 2009. [Google Scholar]
- De Veth, M.J.; Artegoitia, V.M.; Campagna, S.R.; Lapierre, H.; Harte, F.; Girard, C.L. Choline absorption and evaluation of bioavailability markers when supplementing choline to lactating dairy cows. J. Dairy Sci. 2016, 99, 9732–9744. [Google Scholar] [CrossRef] [PubMed]
- Fekkes, D. State-of-the-art of high-performance liquid chromatographic analysis of amino acids in physiological samples. J. Chromatogr. B Biomed. Appl. 1996, 682, 3–22. [Google Scholar] [CrossRef]
- Deyl, Z.; Hyanek, J.; Horakova, M. Profiling of amino acids in body fluids and tissues by means of liquid chromatography. J. Chromatogr. 1986, 379, 177–250. [Google Scholar] [CrossRef]
- Dann, H.M.; Morin, D.E.; Bollero, G.A.; Murphy, M.R.; Drackley, J.K. Prepartum intake, postpartum induction of ketosis, and periparturient disorders affect the metabolic status of dairy cows. J. Dairy Sci. 2005, 88, 3249–3264. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- O’Connor, J.D.; Sniffen, C.J.; Fox, D.G.; Chalupa, W. A net carbohydrate and protein system for evaluating cattle diets: IV. Predicting amino acid adequacy. J. Anim. Sci. 1993, 71, 1298–1311. [Google Scholar] [PubMed]
- Mitchell, H.H.; Block, R.J. Some relationships between the amino acid contents of proteins and their nutritive values for the rat. J. Biol. Chem. 1946, 163, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, B.; Kennelly, J.J. Kinetics of methionine and choline and their incorporation into plasma lipids and milk components in lactating goats. J. Dairy Sci. 1984, 67, 1912–1918. [Google Scholar] [CrossRef]
- Zhou, Z.; Garrow, T.A.; Dong, X.; Luchini, D.N.; Loor, J.J. Hepatic activity and transcription of betaine-homocysteine methyltransferase, methionine synthase, and cystathionine synthase in periparturient dairy cows are altered to different extents by supply of methionine and choline. J. Nutr. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Kristensen, N.B. Precursors for liver gluconeogenesis in periparturient dairy cows. Animal 2013, 7, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Meijer, G.A.; van der Meulen, J.; Bakker, J.G.; van der Koelen, C.J.; van Vuuren, A.M. Free amino acids in plasma and muscle of high yielding dairy cows in early lactation. J. Dairy Sci. 1995, 78, 1131–1141. [Google Scholar] [CrossRef]
- Komaragiri, M.V.; Erdman, R.A. Factors affecting body tissue mobilization in early lactation dairy cows. 1. Effect of dietary protein on mobilization of body fat and protein. J. Dairy Sci. 1997, 80, 929–937. [Google Scholar] [CrossRef]
- Kuhla, B.; Nurnberg, G.; Albrecht, D.; Gors, S.; Hammon, H.M.; Metges, C.C. Involvement of skeletal muscle protein, glycogen, and fat metabolism in the adaptation on early lactation of dairy cows. J. Prot. Res. 2011, 10, 4252–4262. [Google Scholar] [CrossRef] [PubMed]
- Schalinske, K.L.; Smazal, A.L. Homocysteine imbalance: A pathological metabolic marker. Adv. Nutr. 2012, 3, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Guttormsen, A.B.; Solheim, E.; Refsum, H. Variation in plasma cystathionine and its relation to changes in plasma concentrations of homocysteine and methionine in healthy subjects during a 24-h observation period. Am. J. Clin. Nutr. 2004, 79, 76–79. [Google Scholar] [PubMed]
- Stabler, S.P.; Sekhar, J.; Allen, R.H.; O’Neill, H.C.; White, C.W. Alpha-lipoic acid induces elevated S-adenosylhomocysteine and depletes S-adenosylmethionine. Free Radic. Biol. Med. 2009, 47, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Romeu, M.; Nogues, R.; Marcas, L.; Sánchez-Martos, V.; Mulero, M.; Martinez-Vea, A.; Mallol, J.; Giralt, M. Evaluation of oxidative stress biomarkers in patients with chronic renal failure: A case control study. BMC Res. Notes 2010, 3, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Vetrani, C.; Costabile, G.; Di Marino, L.; Rivellese, A.A. Nutrition and oxidative stress: A systematic review of human studies. Int. J. Food Sci. Nutr. 2013, 64, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Saharan, S.; Mandal, P.K. The emerging role of glutathione in Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 40, 519–529. [Google Scholar]
- Garcia, R.A.; Stipanuk, M.H. The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J. Nutr. 1992, 122, 1693–1701. [Google Scholar] [PubMed]
- Hayes, K.C.; Sturman, J.A. Taurine in metabolism. Annu. Rev. Nutr. 1981, 1, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.S.; Trevisi, E.; Ji, P.; Drackley, J.K.; Luchini, D.; Bertoni, G.; Loor, J.J. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with smartamine M or MetaSmart. J. Dairy Sci. 2014, 97, 7437–7450. [Google Scholar] [CrossRef] [PubMed]
- Pocius, P.A.; Clark, J.H.; Baumrucker, C.R. Glutathione in bovine blood: Possible source of amino acids for milk protein synthesis. J. Dairy Sci. 1981, 64, 1551–1554. [Google Scholar] [CrossRef]
- Harris, C.I.; Milne, G. The urinary excretion of Nt-methyl histidine by cattle: Validation as an index of muscle protein breakdown. Br. J. Nutr. 1981, 45, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.F.; Kucukgergin, C.; Ozdemirler-Erata, G.; Kocak-Toker, N.; Uysal, M. The effect of carnosine treatment on prooxidant-antioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology 2010, 11, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.B.; Bu, D.P.; Wang, J.Q.; Sun, X.Z.; Pan, L.; Zhou, L.Y.; Liu, W. Effects of rumen-protected gamma-aminobutyric acid on performance and nutrient digestibility in heat-stressed dairy cows. J. Dairy Sci. 2014, 97, 5599–5607. [Google Scholar] [CrossRef] [PubMed]
- Higgs, S.; Barber, D.J. Effects of baclofen on feeding behaviour examined in the runway. Prog. Neuro-Psychopharmacol. 2004, 28, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Wang, C.; Liu, H.Y.; Liu, J.X.; Ferguson, J.D. Effects of rumen-protected gamma-aminobutyric acid on feed intake, lactation performance, and antioxidative status in early lactating dairy cows. J. Dairy Sci. 2013, 96, 3222–3227. [Google Scholar] [CrossRef] [PubMed]

| MET | CHOL | Day | p-Value 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA (µM) | Without | With | Without | With | SEM 1 | −10 | 4 | 14 | 28 | MET 3 | CHOL 4 | Time | M × T 5 | C × T 6 |
| Essential AA | ||||||||||||||
| Arginine | 54.75 | 60.43 | 57.99 | 57.05 | 1.75 | 58.58 ab | 48.47 c | 63.29 a | 60.92 ab | 0.02 | 0.69 | <0.01 | 0.05 | 0.96 |
| Histidine | 52.38 | 54.55 | 54.04 | 52.87 | 1.18 | 57.28 a | 53.53 b | 52.30 b | 50.92 b | 0.19 | 0.49 | <0.01 | 0.13 | 0.17 |
| Isoleucine | 95.58 | 103.61 | 100.64 | 98.40 | 4.42 | 98.18 | 95.54 | 106.87 | 97.83 | 0.19 | 0.72 | 0.18 | 0.07 | 0.36 |
| Leucine | 153.31 | 161.79 | 152.70 | 162.43 | 6.69 | 151.35 b | 153.51 b | 173.18 a | 152.92 b | 0.35 | 0.31 | 0.03 | 0.27 | 0.31 |
| Lysine | 60.99 | 68.74 | 63.47 | 66.06 | 2.45 | 66.75 ab | 57.56 c | 70.47 a | 64.91 b | 0.02 | 0.47 | <0.01 | 0.03 | 0.85 |
| Methionine | 18.65 | 28.95 | 24.04 | 23.55 | 0.83 | 22.75 c | 25.25 a | 24.92 ab | 22.27 c | <0.01 | 0.68 | <0.01 | 0.14 | 0.17 |
| Phenylalanine | 46.64 | 47.09 | 45.88 | 47.86 | 1.02 | 45.00 b | 47.64 b | 51.16 a | 43.97 b | 0.75 | 0.17 | <0.01 | 0.26 | 0.92 |
| Threonine | 72.55 | 78.67 | 75.93 | 75.30 | 2.50 | 71.87 | 72.22 | 78.70 | 79.66 | 0.09 | 0.86 | 0.07 | 0.01 | 0.52 |
| Tryptophan | 22.26 | 25.04 | 22.68 | 24.61 | 0.64 | 24.56 ab | 19.44 c | 25.08 a | 25.50 a | <0.01 | 0.04 | <0.01 | 0.42 | 0.63 |
| Valine | 226.77 | 244.54 | 229.16 | 241.99 | 9.32 | 230.11 | 223.02 | 249.41 | 240.25 | 0.17 | 0.33 | 0.08 | 0.26 | 0.31 |
| BCAA 7 | 476.69 | 510.97 | 483.71 | 503.55 | 19.87 | 480.67 | 473.17 | 530.28 | 491.93 | 0.21 | 0.48 | 0.08 | 0.19 | 0.27 |
| EAA | 827.12 | 896.20 | 849.22 | 874.1 | 25.54 | 848.26 bc | 816.78 bc | 921.2 a | 860.39 ab | 0.06 | 0.50 | 0.03 | 0.08 | 0.32 |
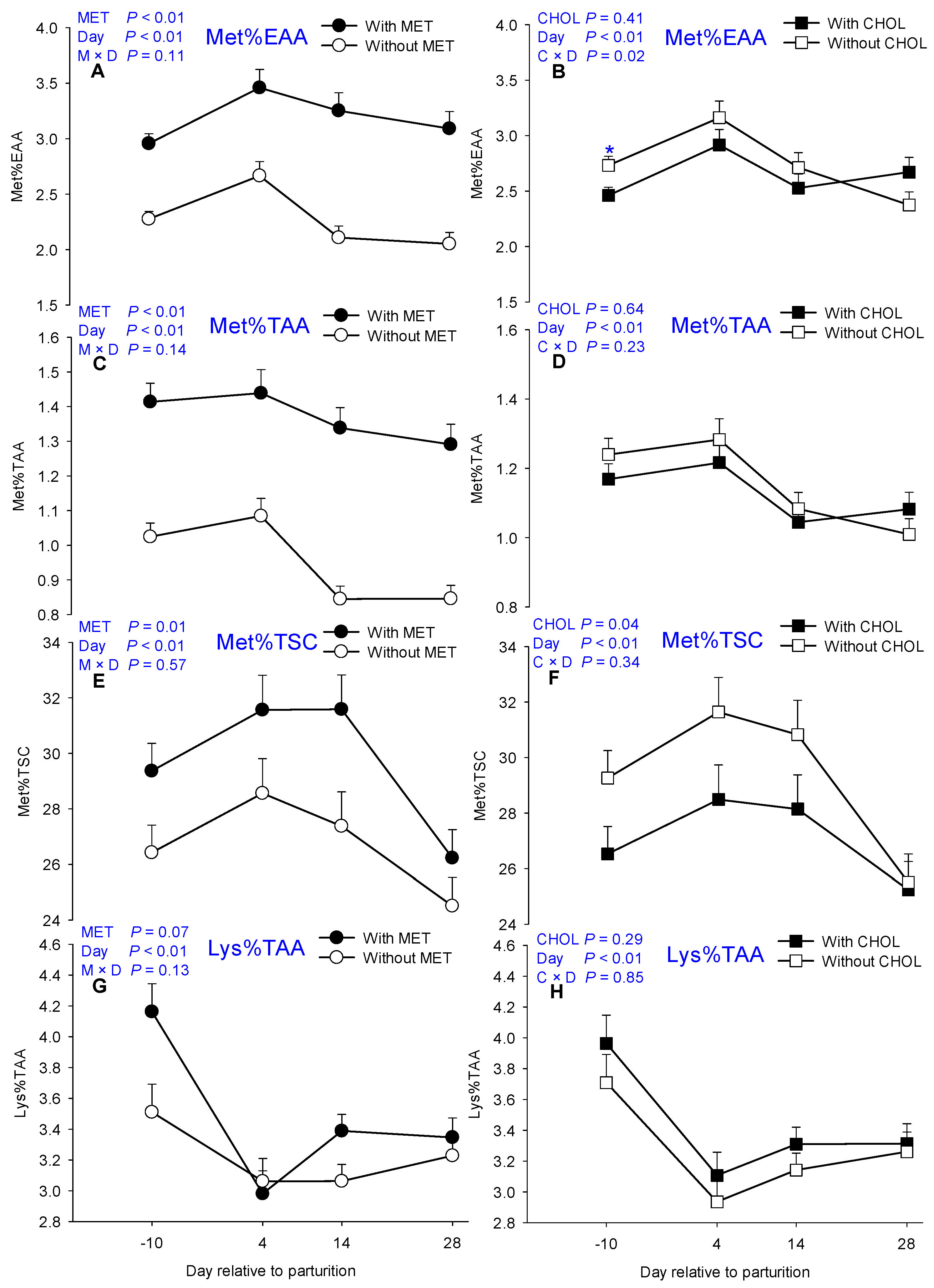
| Met%EAA | 2.26 | 3.18 | 2.73 | 2.64 | 0.09 | 2.59 b | 3.04 a | 2.62 b | 2.52 b | <0.01 | 0.41 | <0.01 | 0.11 | 0.23 |
| Non-essential AA | ||||||||||||||
| Alanine | 187.39 | 206.37 | 198.37 | 195.39 | 5.55 | 188.83 b | 175.45 c | 211.30 a | 211.95 a | 0.02 | 0.71 | <0.01 | 0.05 | 0.32 |
| Asparagine | 34.90 | 39.63 | 37.78 | 36.61 | 1.48 | 27.82 c | 34.08 b | 45.67 a | 44.19 a | 0.02 | 0.56 | <0.01 | 0.22 | 0.42 |
| Aspartate | 4.21 | 4.79 | 4.24 | 4.76 | 0.21 | 4.91 a | 3.78 b | 4.80 a | 4.57 a | 0.04 | 0.07 | <0.01 | 0.59 | 0.14 |
| Glutamate | 35.93 | 38.34 | 36.44 | 37.81 | 1.04 | 44.24 a | 32.17 d | 37.84 b | 35.24 c | 0.10 | 0.36 | <0.01 | 0.54 | 0.38 |
| Glutamine | 246.22 | 259.53 | 257.92 | 247.82 | 6.18 | 278.17 a | 252.84 b | 242.52 b | 237.96 b | 0.15 | 0.25 | <0.01 | 0.21 | 0.29 |
| Glycine * | 426.83 | 406.23 | 427.39 | 405.67 | 15.10 | 236.99 c | 456.97 b | 536.19 a | 435.98 b | 0.35 | 0.31 | <0.01 | 0.92 | 0.22 |
| Proline | 72.30 | 78.24 | 74.03 | 76.50 | 2.02 | 59.19 c | 67.52 b | 90.21 a | 84.15 a | 0.04 | 0.39 | <0.01 | 0.35 | 0.74 |
| Serine | 86.62 | 88.92 | 88.67 | 86.87 | 2.28 | 73.88 c | 90.85 b | 100.78 a | 85.57 b | 0.48 | 0.58 | <0.01 | 0.16 | 0.49 |
| Tyrosine | 40.82 | 43.39 | 40.78 | 43.43 | 1.53 | 42.03 a | 36.26 b | 46.32 a | 43.80 a | 0.24 | 0.24 | <0.01 | 0.08 | 0.94 |
| NEAA 8 | 1114.59 | 1151.58 | 1153.58 | 1112.59 | 24.79 | 946.13 c | 1136.83 b | 1301.27 a | 1177.00 b | 0.29 | 0.24 | <0.01 | 0.36 | 0.23 |
| TAA 9 | 1926.95 | 2052.97 | 1995.87 | 1982.22 | 41.32 | 1781.41 d | 1947.89 bc | 2215.47 a | 2035.97 b | 0.03 | 0.82 | <0.01 | 0.10 | 0.53 |
| Met%TAA | 0.94 | 1.37 | 1.15 | 1.13 | 0.04 | 1.20 a | 1.25 a | 1.06 b | 1.05 b | <0.01 | 0.64 | <0.01 | 0.14 | 0.23 |
| Lys%TAA | 3.22 | 3.47 | 3.26 | 3.42 | 0.10 | 3.84 a | 3.02 c | 3.23 bc | 3.29 b | 0.08 | 0.28 | <0.01 | 0.13 | 0.85 |
| MET | CHOL | Day | p-Value 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter (µM) | Without | With | Without | With | SEM 1 | −10 | 4 | 14 | 28 | MET 3 | CHOL 4 | Time | M × T 5 | C × T 6 |
| AA and derivatives # | ||||||||||||||
| 1-methyl histidine | 15.36 | 16.56 | 16.79 | 15.15 | 0.57 | 12.82 c | 12.58 c | 18.38 b | 21.85 a | 0.12 | 0.04 | <0.01 | 0.36 | 0.50 |
| 3-methyl histidine | 6.44 | 6.48 | 6.97 | 5.99 | 0.29 | 5.21 c | 11.00 a | 6.91 b | 4.40 d | 0.92 | 0.02 | <0.01 | 0.12 | 0.75 |
| α-aminoadipic acid | 7.37 | 8.15 | 8.03 | 7.48 | 0.36 | 6.09 d | 6.99 c | 8.65 b | 9.81 a | 0.12 | 0.27 | <0.01 | 0.91 | 0.30 |
| α-aminobutyric acid | 15.64 | 19.78 | 18.13 | 17.07 | 0.81 | 8.94 c | 23.60 a | 22.77 a | 19.93 b | <0.01 | 0.32 | <0.01 | 0.61 | 0.44 |
| β-alanine | 9.06 | 8.87 | 8.81 | 9.11 | 0.32 | 8.97 | 8.69 | 9.24 | 8.94 | 0.68 | 0.50 | 0.54 | 0.96 | 0.92 |
| γ-aminobutyric acid | 2.42 | 3.49 | 2.46 | 3.45 | 0.29 | 1.88 b | 3.08 a | 3.44 a | 3.41 a | 0.01 | 0.02 | <0.01 | 0.27 | 0.75 |
| Carnosine * | 15.88 | 19.92 | 16.60 | 19.07 | 1.29 | 16.74 c | 14.20 d | 18.71 b | 22.51 a | 0.02 | 0.14 | <0.01 | 0.30 | 0.02 |
| Citrulline | 71.19 | 83.47 | 79.08 | 75.14 | 2.92 | 69.61 c | 63.69 c | 82.86 b | 96.11 a | <0.01 | 0.30 | <0.01 | 0.99 | 0.60 |
| Glutathione | 4.68 | 4.73 | 4.84 | 4.57 | 0.27 | 4.60 b | 6.11 a | 4.35 bc | 3.76 c | 0.89 | 0.50 | <0.01 | 0.50 | 0.74 |
| Hydroxylysine | 0.32 | 0.28 | 0.30 | 0.29 | 0.04 | 0.33 | 0.29 | 0.33 | 0.25 | 0.37 | 0.79 | 0.21 | 0.61 | 0.70 |
| Hydroxyproline | 15.19 | 15.36 | 15.42 | 15.13 | 0.48 | 12.80 c | 18.10 a | 16.31 b | 13.89 c | 0.81 | 0.67 | <0.01 | 0.94 | 0.12 |
| Ornithine | 29.14 | 32.00 | 30.48 | 30.60 | 1.35 | 39.37 a | 22.11 d | 30.04 c | 33.25 b | 0.13 | 0.95 | <0.01 | 0.12 | 0.45 |
| Phosphoserine | 6.22 | 6.18 | 6.07 | 6.33 | 0.18 | 5.53 c | 5.91 bc | 6.72 ab | 6.74 a | 0.88 | 0.30 | <0.01 | 0.71 | 0.91 |
| Sarcosine | 10.65 | 11.43 | 11.50 | 10.58 | 0.56 | 8.32 b | 11.31 a | 12.03 a | 12.50 a | 0.33 | 0.27 | <0.01 | 0.76 | 0.37 |
| Urea | 4121.63 | 4442.94 | 4323.55 | 4235.16 | 123.89 | 4104.53 bc | 4317.26 b | 4036.81 c | 4687.49 a | 0.07 | 0.60 | <0.01 | 0.55 | 0.62 |
| Sulfur-containing compounds | ||||||||||||||
| Cystathionine | 1.60 | 2.12 | 1.84 | 1.89 | 0.07 | 2.30 | 1.51 | 1.71 | 1.92 | <0.01 | 0.61 | <0.01 | 0.08 | 0.36 |
| Cystine | 7.56 | 9.58 | 7.65 | 9.48 | 34.5 | 11.20 a | 6.41 d | 7.73 c | 8.92 b | <0.01 | 0.02 | <0.01 | 0.01 | 0.17 |
| Homocystine | 4.17 | 5.10 | 4.52 | 4.70 | 0.30 | 4.80 a | 3.99 b | 4.80 a | 4.92 a | 0.02 | 0.65 | 0.01 | 0.69 | 0.28 |
| Taurine | 33.23 | 47.04 | 39.43 | 40.84 | 1.28 | 34.51 | 40.49 | 40.06 | 45.48 | <0.01 | 0.44 | <0.01 | 0.16 | 0.92 |
| TSC 7 | 70.65 | 97.37 | 82.47 | 85.95 | 1.93 | 80.52 | 84.03 | 83.93 | 87.57 | <0.01 | 0.44 | 0.08 | 0.07 | 0.48 |
| Met%TSC | 28.44 | 31.4 | 31.09 | 28.74 | 0.72 | 29.65 b | 32.35 ab | 31.15 b | 26.51 c | 0.01 | 0.03 | <0.01 | 0.74 | 0.24 |
| MET | CHOL | Day | p-Value 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genes | Without | With | Without | With | SEM 1 | −10 | 7 | 20 | 30 | MET 3 | CHOL 4 | Time | M × T 5 | C × T 6 |
| PC | 2.12 | 2.02 | 2.09 | 2.05 | 0.07 | 1.49 c | 2.88 a | 2.23 b | 1.93 d | 0.33 | 0.70 | <0.01 | 0.28 | 0.34 |
| PCK1 | 2.27 | 2.43 | 2.39 | 2.31 | 0.10 | 1.57 c | 2.62 ab | 2.74 a | 2.69 ab | 0.27 | 0.55 | <0.01 | 0.48 | 0.46 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Vailati-Riboni, M.; Luchini, D.N.; Loor, J.J. Methionine and Choline Supply during the Periparturient Period Alter Plasma Amino Acid and One-Carbon Metabolism Profiles to Various Extents: Potential Role in Hepatic Metabolism and Antioxidant Status. Nutrients 2017, 9, 10. https://doi.org/10.3390/nu9010010
Zhou Z, Vailati-Riboni M, Luchini DN, Loor JJ. Methionine and Choline Supply during the Periparturient Period Alter Plasma Amino Acid and One-Carbon Metabolism Profiles to Various Extents: Potential Role in Hepatic Metabolism and Antioxidant Status. Nutrients. 2017; 9(1):10. https://doi.org/10.3390/nu9010010
Chicago/Turabian StyleZhou, Zheng, Mario Vailati-Riboni, Daniel N. Luchini, and Juan J. Loor. 2017. "Methionine and Choline Supply during the Periparturient Period Alter Plasma Amino Acid and One-Carbon Metabolism Profiles to Various Extents: Potential Role in Hepatic Metabolism and Antioxidant Status" Nutrients 9, no. 1: 10. https://doi.org/10.3390/nu9010010
APA StyleZhou, Z., Vailati-Riboni, M., Luchini, D. N., & Loor, J. J. (2017). Methionine and Choline Supply during the Periparturient Period Alter Plasma Amino Acid and One-Carbon Metabolism Profiles to Various Extents: Potential Role in Hepatic Metabolism and Antioxidant Status. Nutrients, 9(1), 10. https://doi.org/10.3390/nu9010010






