The Extract of Aster Koraiensis Prevents Retinal Pericyte Apoptosis in Diabetic Rats and Its Active Compound, Chlorogenic Acid Inhibits AGE Formation and AGE/RAGE Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of AKE
2.2. Isolation of 3,5-di-O-Caffeoylguinic Acid and Chlorogenic Acid
2.3. High Performance Liquid Chromatography Analysis
2.4. AGE Formation Assay
2.5. AGE/RAGE Binding Activity Assay
2.6. Animals and Induction of Diabetes
2.7. Trypsin-Digested Vessel Preparation
2.8. Terminal Deoxynucleotidyl Transferase Mediated dUTP Nick End Labeling (TUNEL) Staining
2.9. Immunohistochemical Staining
2.10. In Situ Hybridization
2.11. Statistical Analysis
3. Results
3.1. Standardization of AKE
3.2. Inhibitory Effects of 3,5-di-O-Caffeoylquinic Acid and Chlorogenic Acid on AGE Formation in Vitro
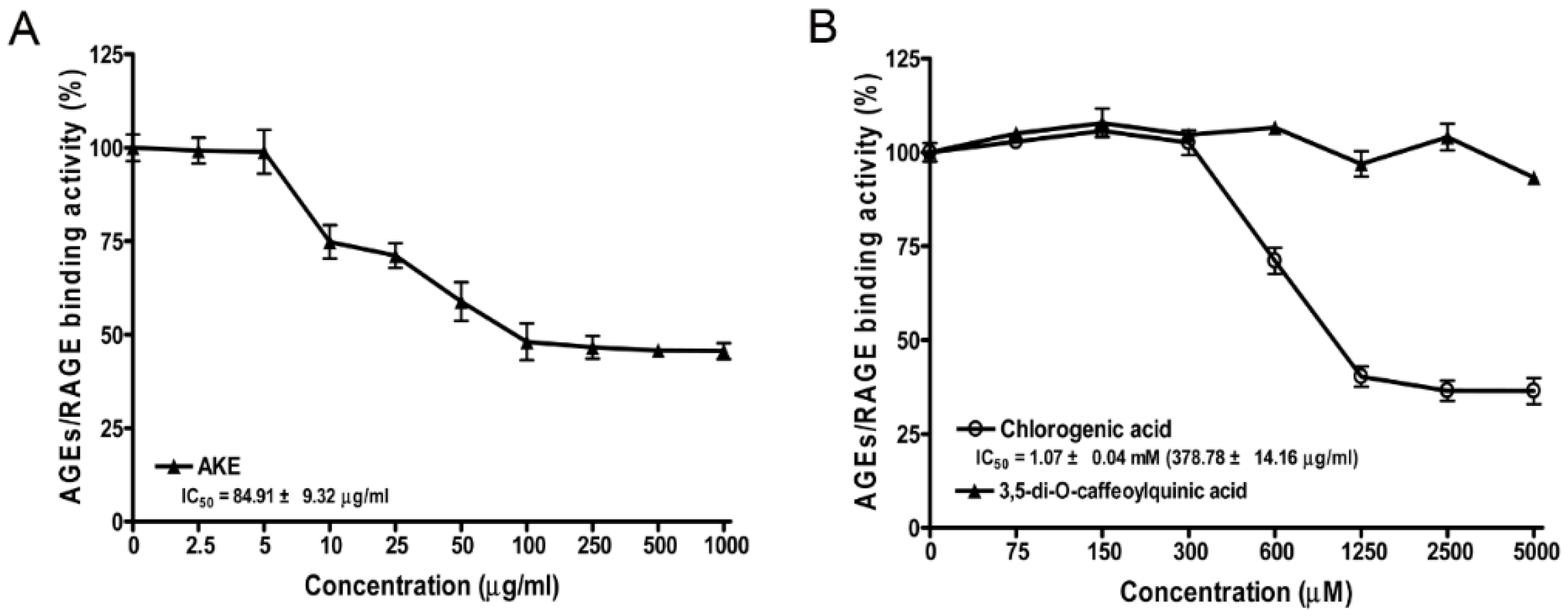
3.3. Inhibitory Effects of AKE, 3,5-di-O-Caffeoylquinic Acid and Chlorogenic Acid on AGE/RAGE Binding Activity in Vitro
3.4. Body Weight and Blood Glucose
3.5. Apoptosis of Retinal Microvascular Cells
3.6. AGE Staining of the Retinal Microvascular Cells
3.7. Expression of iNOS mRNA and iNOS Protein
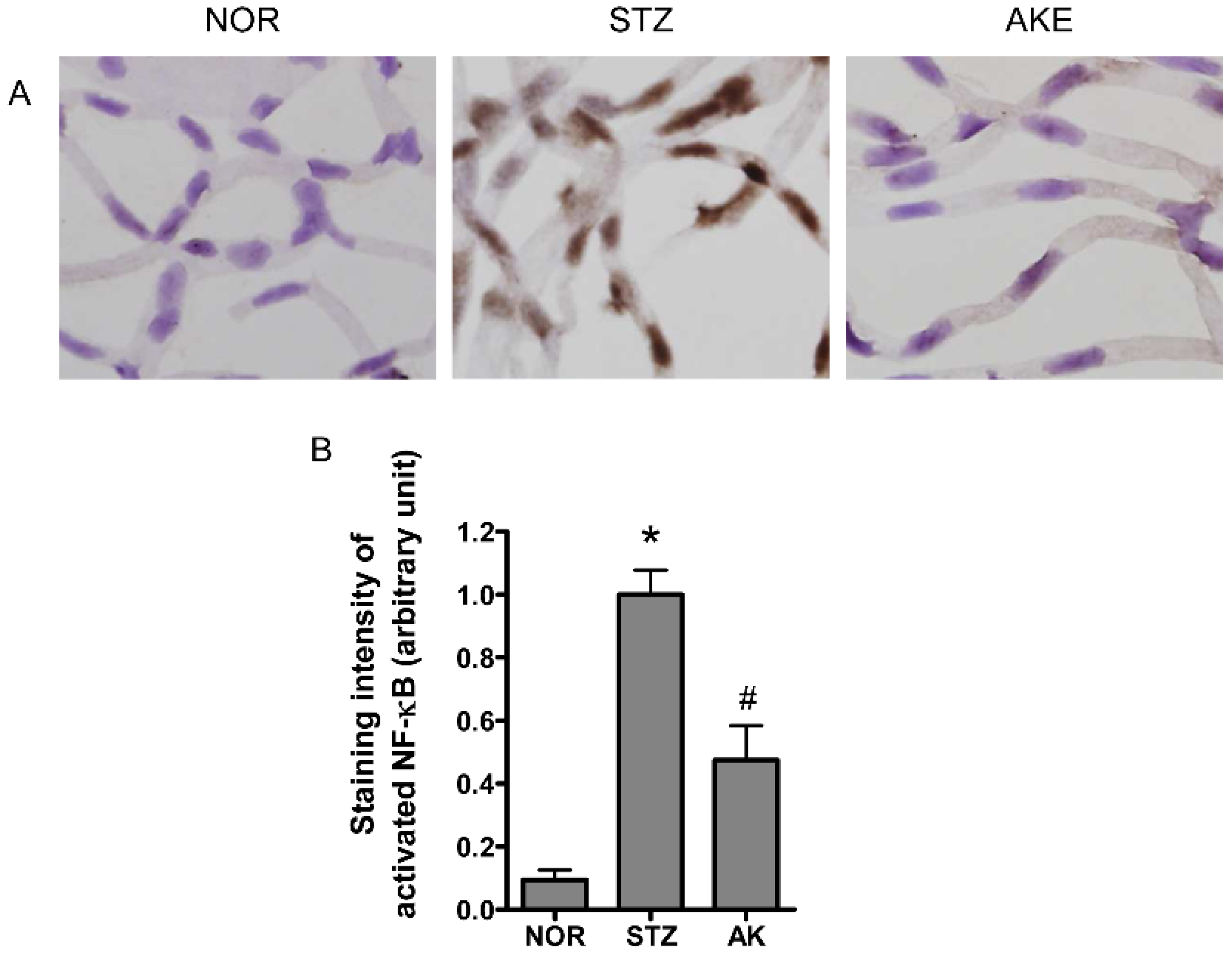
3.8. Activation of NF-κB in the Retinal Microvascular Cells
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mizutani, M.; Kern, T.S.; Lorenzi, M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J. Clin. Investig. 1996, 97, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Yamagishi, S.; Okamoto, T.; Inagaki, Y.; Amano, S.; Takeuchi, M.; Makita, Z. Serum levels of glucose-derived advanced glycation end products are associated with the severity of diabetic retinopathy in type 2 diabetic patients without renal dysfunction. Int. J. Clin. Pharmacol. Res. 2002, 22, 13–17. [Google Scholar] [PubMed]
- Miura, J.; Yamagishi, S.; Uchigata, Y.; Takeuchi, M.; Yamamoto, H.; Makita, Z.; Iwamoto, Y. Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with type 1 diabetes. J. Diabetes Complic. 2003, 17, 16–21. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Luo, D.; Huang, Y.; Zhu, J.; Wang, X.; Hu, H.; Patrick, C.P. Exogenous advanced glycosylation end products induce diabetes-like vascular dysfunction in normal rats: A factor in diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Roehlecke, C.; Witt, M.; Fehrenbach, H.; Hofer, A.; Miyata, T.; Weigert, C.; Funk, R.H.; Schleicher, E.D. Induction of apoptosis by glyoxal in human embryonic lung epithelial cell line L132. Am. J. Respir. Cell. Mol. Biol. 2000, 23, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Kaji, Y.; Amano, S.; Usui, T.; Oshika, T.; Yamashiro, K.; Ishida, S.; Suzuki, K.; Tanaka, S.; Adamis, A.P.; Nagai, R.; et al. Expression and function of receptors for advanced glycation end products in bovine corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 521–528. [Google Scholar] [CrossRef]
- Yamagishi, S.; Inagaki, Y.; Amano, S.; Okamoto, T.; Takeuchi, M.; Makita, Z. Pigment epithelium-derived factor protects cultured retinal pericytes from advanced glycation end product-induced injury through its antioxidative properties. Biochem. Biophys. Res. Commun. 2002, 296, 877–882. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Yun, H.Y.; Dawson, V.L.; Dawson, T.M. Nitric oxide in health and disease of the nervous system. Mol. Psychiatr. 1997, 2, 300–310. [Google Scholar] [CrossRef]
- Nathan, C.; Xie, Q.W. Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Brune, B.; von Knethen, A.; Sandau, K.B. Nitric oxide and its role in apoptosis. Eur. J. Pharmacol. 1998, 351, 261–272. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Desmet, S.; Meersschaert, A.; Dralands, L.; Missotten, L.; Geboes, K. Expression of the inducible isoform of nitric oxide synthase in the retinas of human subjects with diabetes mellitus. Am. J. Ophthalmol. 2001, 132, 551–556. [Google Scholar] [CrossRef]
- Carmo, A.; Cunha-Vaz, J.G.; Carvalho, A.P.; Lopes, M.C. Nitric oxide synthase activity in retinas from non-insulin-dependent diabetic Goto-Kakizaki rats: Correlation with blood-retinal barrier permeability. Nitric Oxide 2000, 4, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Smith, M.A.; Miller, C.M.; Kern, T.S. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J. Neurochem. 2002, 80, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.A.; Guberski, D.L.; Hutson, B.; Grant, M.B. Time course of NADH oxidase, inducible nitric oxide synthase and peroxynitrite in diabetic retinopathy in the BBZ/WOR rat. Nitric Oxide 2002, 6, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Du, Y.; Miller, C.; Gubitosi-Klug, R.A.; Ball, S.; Berkowitz, B.A.; Kern, T.S. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 2007, 50, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Chakrabarti, S. Cellular signaling and potential new treatment targets in diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 31867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, X.; Teng, X.; Snead, C.; Catravas, J.D. Molecular cloning and analysis of the rat inducible nitric oxide synthase gene promoter in aortic smooth muscle cells. Biochem. Pharmacol. 1998, 55, 1873–1880. [Google Scholar] [CrossRef]
- Lowenstein, C.J.; Alley, E.W.; Raval, P.; Snowman, A.M.; Snyder, S.H.; Russell, S.W.; Murphy, W.J. Macrophage nitric oxide synthase gene: Two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc. Natl. Acad. Sci. USA 1993, 90, 9730–9734. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar] [PubMed]
- Beck, K.F.; Sterzel, R.B. Cloning and sequencing of the proximal promoter of the rat iNOS gene: Activation of NFkappaB is not sufficient for transcription of the iNOS gene in rat mesangial cells. FEBS Lett. 1996, 394, 263–267. [Google Scholar] [CrossRef]
- De Vera, M.E.; Shapiro, R.A.; Nussler, A.K.; Mudgett, J.S.; Simmons, R.L.; Morris, S.M., Jr.; Billiar, T.R.; Geller, D.A. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: Initial analysis of the human NOS2 promoter. Proc. Natl. Acad. Sci. USA 1996, 93, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, K.S. Genetic diversity of Chrysanthemum zawadskii Herb. and the related groups in Korea using RAPDs. J. Korean Soc. Hort. Sci. 2000, 41, 230–236. [Google Scholar]
- Ahn, D.K. Illustrated Book of Korean Medicinal Herbs; Kyo-Hak Publishing: Seoul, Korea, 1998. [Google Scholar]
- KO, J.Y.; Lee, K.K. Effect of plant growth regulators on growth and flowering of potted Lychnis cognata, Aster koraiensis, and Campanula takesimana. RDA J. Agric. Sci. 1996, 38, 627–632. [Google Scholar]
- Sohn, E.; Kim, J.; Kim, C.S.; Kim, Y.S.; Jang, D.S.; Kim, J.S. Extract of the aerial parts of Aster koraiensis reduced development of diabetic nephropathy via anti-apoptosis of podocytes in streptozotocin-induced diabetic rats. Biochem. Biophys. Res. Commun. 2010, 391, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, Y.M.; Lee, B.W.; Kim, J.H.; Kim, J.S. Chemical constituents from the aerial parts of Aster koraiensis with protein glycation and aldose reductase inhibitory activities. J. Nat. Prod. 2012, 75, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, S.; Yamamoto, Y.; Tamei, H.; Matsuki, H.; Obata, K.; Hui, L.; Miura, J.; Osawa, M.; Uchigata, Y.; Iwamoto, Y.; Watanabe, T.; Yonekura, H.; Yamamoto, H. Development of an ELISA for esRAGE and its application to type 1 diabetic patients. Diabetes Res. Clin. Pract. 2006, 73, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Soulis, T.; Thallas, V.; Panagiotopoulos, S.; Long, D.M.; Vasan, S.; Wagle, D.; Jerums, G.; Cooper, M.E. Renoprotective effects of a novel inhibitor of advanced glycation. Diabetologia 2001, 44, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Vasan, S.; Zhang, X.; Kapurniotu, A.; Bernhagen, J.; Teichberg, S.; Basgen, J.; Wagle, D.; Shih, D.; Terlecky, I.; Bucala, R.; et al. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature 1996, 382, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.J.; Kim, C.S.; Kim, Y.S.; Jung, D.H.; Jang, D.S.; Lee, Y.M.; Kim, J.S. Effects of magnolol (5,5′-diallyl-2,2′-dihydroxybiphenyl) on diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. Life Sci. 2007, 80, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Knerlich, F.; Schilling, L.; Gorlach, C.; Wahl, M.; Ehrenreich, H.; Siren, A.L. Temporal profile of expression and cellular localization of inducible nitric oxide synthase, interleukin-1beta and interleukin converting enzyme after cryogenic lesion of the rat parietal cortex. Brain Res. Mol. Brain Res. 1999, 68, 73–87. [Google Scholar] [CrossRef]
- Kim, J.; Choi, C.; Chae, C. Pathogenesis of postweaning multisystemic wasting syndrome reproduced by co-infection with Korean isolates of porcine circovirus 2 and porcine parvovirus. J. Comp. Pathol. 2003, 128, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Takeuchi, M.; Matsui, T.; Nakamura, K.; Imaizumi, T.; Inoue, H. Angiotensin II augments advanced glycation end product-induced pericyte apoptosis through RAGE overexpression. FEBS Lett. 2005, 579, 4265–4270. [Google Scholar] [CrossRef] [PubMed]
- Romeo, G.; Liu, W.H.; Asnaghi, V.; Kern, T.S.; Lorenzi, M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 2002, 51, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Koppolu, P.; Chakrabarti, S.; Chen, S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic. Res. 2003, 37, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Yatoh, S.; Mizutani, M.; Yokoo, T.; Kozawa, T.; Sone, H.; Toyoshima, H.; Suzuki, S.; Shimano, H.; Kawakami, Y.; Okuda, Y.; Yamada, N. Antioxidants and an inhibitor of advanced glycation ameliorate death of retinal microvascular cells in diabetic retinopathy. Diabetes Metab. Res. Rev. 2006, 22, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.P. Pericytes and the pathogenesis of diabetic retinopathy. Horm. Metab. Res. 2005, 37, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Clements, R.S., Jr.; Robison, W.G., Jr.; Cohen, M.P. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J. Diabetes Complic. 1998, 12, 28–33. [Google Scholar] [CrossRef]
- Moore, T.C.; Moore, J.E.; Kaji, Y.; Frizzell, N.; Usui, T.; Poulaki, V.; Campbell, I.L.; Stitt, A.W.; Gardiner, T.A.; Archer, D.B.; Adamis, A.P. The role of advanced glycation end products in retinal microvascular leukostasis. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4457–4464. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Bhaduri, T.; McMullen, C.B.; Gardiner, T.A.; Archer, D.B. Advanced glycation end products induce blood-retinal barrier dysfunction in normoglycemic rats. Mol. Cell Biol. Res. Commun. 2000, 3, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Nakamura, K.; Matsui, T. Advanced glycation end products (AGEs) and their receptor (RAGE) system in diabetic retinopathy. Curr. Drug Discov. Technol. 2006, 3, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.D.; Schmidt, A.M.; Anderson, G.M.; Zhang, J.; Brett, J.; Zou, Y.S.; Pinsky, D.; Stern, D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem. 1994, 269, 9889–9897. [Google Scholar] [PubMed]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Becquet, F.; Courtois, Y.; Goureau, O. Nitric oxide in the eye: Multifaceted roles and diverse outcomes. Surv. Ophthalmol. 1997, 42, 71–82. [Google Scholar] [CrossRef]
- Inomata, M.; Hayashi, M.; Shumiya, S.; Kawashima, S.; Ito, Y. Involvement of inducible nitric oxide synthase in cataract formation in Shumiya cataract rat (SCR). Curr. Eye Res. 2001, 23, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nabekura, T.; Takeda, M.; Nakao, M.; Terao, M.; Hori, R.; Tomohiro, M. Nitric oxide participates in cataract development in selenite-treated rats. Curr. Eye Res. 2001, 22, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Chiou, G.C. Review: Effects of nitric oxide on eye diseases and their treatment. J. Ocul. Pharmacol. Ther. 2001, 17, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.J.; Park, K.M.; Lee, S.; Lim, H.J.; Go, S.H.; Eom, S.M.; Park, H.Y. Preconditioning with low concentration NO attenuates subsequent NO-induced apoptosis in vascular smooth muscle cells via HO-1-dependent mitochondrial death pathway. Toxicol. Appl. Pharmacol. 2006, 217, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.T.; Cherng, Y.G.; Chang, C.C.; Hwang, Y.P.; Chen, J.T.; Chen, R.M. Pretreatment with low nitric oxide protects osteoblasts from high nitric oxide-induced apoptotic insults through regulation of c-Jun N-terminal kinase/c-Jun-mediated Bcl-2 gene expression and protein translocation. J. Orthop. Res. 2007, 25, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Brynczka, C.; Merrick, B.A. Nerve growth factor potentiates p53 DNA binding but inhibits nitric oxide-induced apoptosis in neuronal PC12 cells. Neurochem. Res. 2007, 32, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.L.; Guh, J.H.; Chang, Y.L.; Kuo, S.C.; Lee, F.Y.; Teng, C.M. YC-1 prevents sodium nitroprusside-mediated apoptosis in vascular smooth muscle cells. Cardiovasc. Res. 2004, 61, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.C.; Chiou, S.H.; Lee, F.L.; Chou, C.K.; Chen, S.J.; Peng, C.H.; Kuo, Y.H.; Chen, C.F.; Ho, L.L.; Hsu, W.M. Possible involvement of nitric oxide in the progression of diabetic retinopathy. Ophthalmologica 2003, 217, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Engerman, R.L. Pathogenesis of diabetic retinopathy. Diabetes 1989, 38, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Amano, S.; Inagaki, Y.; Okamoto, T.; Koga, K.; Sasaki, N.; Yamamoto, H.; Takeuchi, M.; Makita, Z. Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem. Biophys. Res. Commun. 2002, 290, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Denis, U.; Lecomte, M.; Paget, C.; Ruggiero, D.; Wiernsperger, N.; Lagarde, M. Advanced glycation end-products induce apoptosis of bovine retinal pericytes in culture: Involvement of diacylglycerol/ceramide production and oxidative stress induction. Free Radic. Biol. Med. 2002, 33, 236–247. [Google Scholar] [CrossRef]
- Lecleire-Collet, A.; Tessier, L.H.; Massin, P.; Forster, V.; Brasseur, G.; Sahel, J.A.; Picaud, S. Advanced glycation end products can induce glial reaction and neuronal degeneration in retinal explants. Br. J. Ophthalmol. 2005, 89, 1631–1633. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Engerman, R.L. Pharmacological inhibition of diabetic retinopathy: Aminoguanidine and aspirin. Diabetes 2001, 50, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, E.O.; Rhee, Y.H.; Ahn, K.S.; Li, G.X.; Jiang, C.; Lu, J.; Kim, S.H. An oriental herbal cocktail, ka-mi-kae-kyuk-tang, exerts anti-cancer activities by targeting angiogenesis, apoptosis and metastasis. Carcinogenesis 2006, 27, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.S.; Jang, Y.J.; Hwang, M.K.; Kang, N.J.; Lee, K.W.; Lee, H.J. Attenuation of oxidative neuronal cell death by coffee phenolic phytochemicals. Mutat. Res. 2009, 661, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Chuang, H.C.; Wu, C.H.; Yen, G.C. Cytoprotective effects of phenolic acids on methylglyoxal-induced apoptosis in Neuro-2A cells. Mol. Nutr. Food Res. 2008, 52, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Shimazawa, M.; Mishima, S.; Hara, H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sci. 2007, 80, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Yurek-George, A.; Argirov, O.K. Kinetics and mechanism of the reaction of aminoguanidine with the alpha-oxoaldehydes glyoxal, methylglyoxal, and 3-deoxyglucosone under physiological conditions. Biochem. Pharmacol. 2000, 60, 55–65. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, I.H.; Kim, C.S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch. Pharm. Res. 2011, 34, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhao, T.; Yang, W.-W.; Wang, G.-H.; Yu, H.; Zhao, H.-X.; Yang, C.; Sun, L.-X. Comparative pharmacokinetics of chlorogenic acid after oral administration in rats. J. Pharm. Anal. 2011, 1, 270–274. [Google Scholar] [CrossRef]
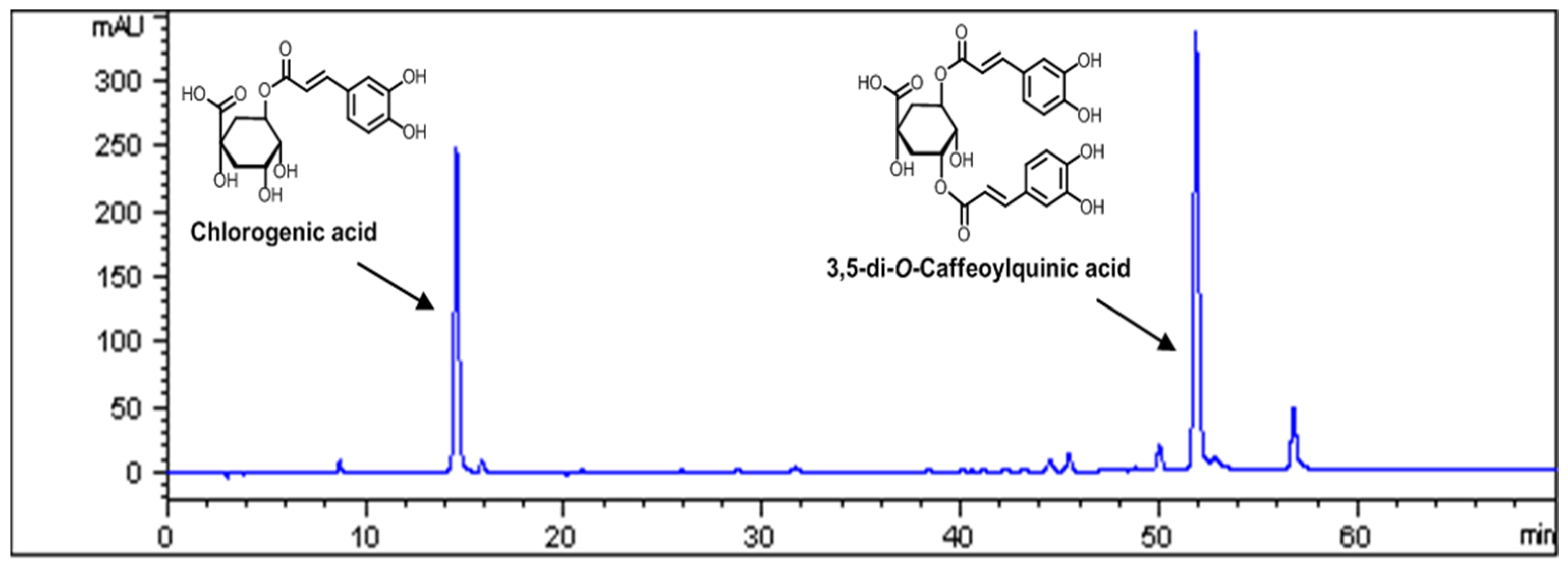



| Compound | Content | |
|---|---|---|
| mg/g | % | |
| Chlorogenic acid | 12.35 ± 0.22 | 1.24 ± 0.02 |
| 3,5-di-O-caffeoylquinic acid | 22.54 ± 0.51 | 2.25 ± 0.05 |
| Compound | Inhibitory Effect (IC50 Value) a | |
|---|---|---|
| μM | μg/mL | |
| Chlorogenic acid | 148.32 ± 3.14 | 52.55 ± 1.11 |
| 3,5-di-O-caffeoylquinic acid | 6.59 ± 0.04 | 3.40 ± 0.02 |
| Aminoguanidine b | 962 ± 29.52 | 71.19 ± 2.19 |
| NOR | STZ | AKE | |
|---|---|---|---|
| Body weight (g) | 494.46 ± 8.73 | 229.25 ± 10.66 * | 233.51 ± 11.69 * |
| Blood glucose (mg/dL) | 124.93 ± 10.8 | 409.78 ± 68.10 * | 383.82 ± 131.71 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jo, K.; Lee, I.-S.; Kim, C.-S.; Kim, J.S. The Extract of Aster Koraiensis Prevents Retinal Pericyte Apoptosis in Diabetic Rats and Its Active Compound, Chlorogenic Acid Inhibits AGE Formation and AGE/RAGE Interaction. Nutrients 2016, 8, 585. https://doi.org/10.3390/nu8090585
Kim J, Jo K, Lee I-S, Kim C-S, Kim JS. The Extract of Aster Koraiensis Prevents Retinal Pericyte Apoptosis in Diabetic Rats and Its Active Compound, Chlorogenic Acid Inhibits AGE Formation and AGE/RAGE Interaction. Nutrients. 2016; 8(9):585. https://doi.org/10.3390/nu8090585
Chicago/Turabian StyleKim, Junghyun, Kyuhyung Jo, Ik-Soo Lee, Chan-Sik Kim, and Jin Sook Kim. 2016. "The Extract of Aster Koraiensis Prevents Retinal Pericyte Apoptosis in Diabetic Rats and Its Active Compound, Chlorogenic Acid Inhibits AGE Formation and AGE/RAGE Interaction" Nutrients 8, no. 9: 585. https://doi.org/10.3390/nu8090585
APA StyleKim, J., Jo, K., Lee, I.-S., Kim, C.-S., & Kim, J. S. (2016). The Extract of Aster Koraiensis Prevents Retinal Pericyte Apoptosis in Diabetic Rats and Its Active Compound, Chlorogenic Acid Inhibits AGE Formation and AGE/RAGE Interaction. Nutrients, 8(9), 585. https://doi.org/10.3390/nu8090585





