Vitamin D in Fibromyalgia: A Causative or Confounding Biological Interplay?
Abstract
:1. Introduction
2. Fibromyalgia
2.1. Definition, Prevalence, and Diagnosis
2.2. Who Should Be Screened for Fibromyalgia?
2.3. How Do We “Understand” Pain in Fibromyalgia?
3. Fibromyalgia and Vitamin D
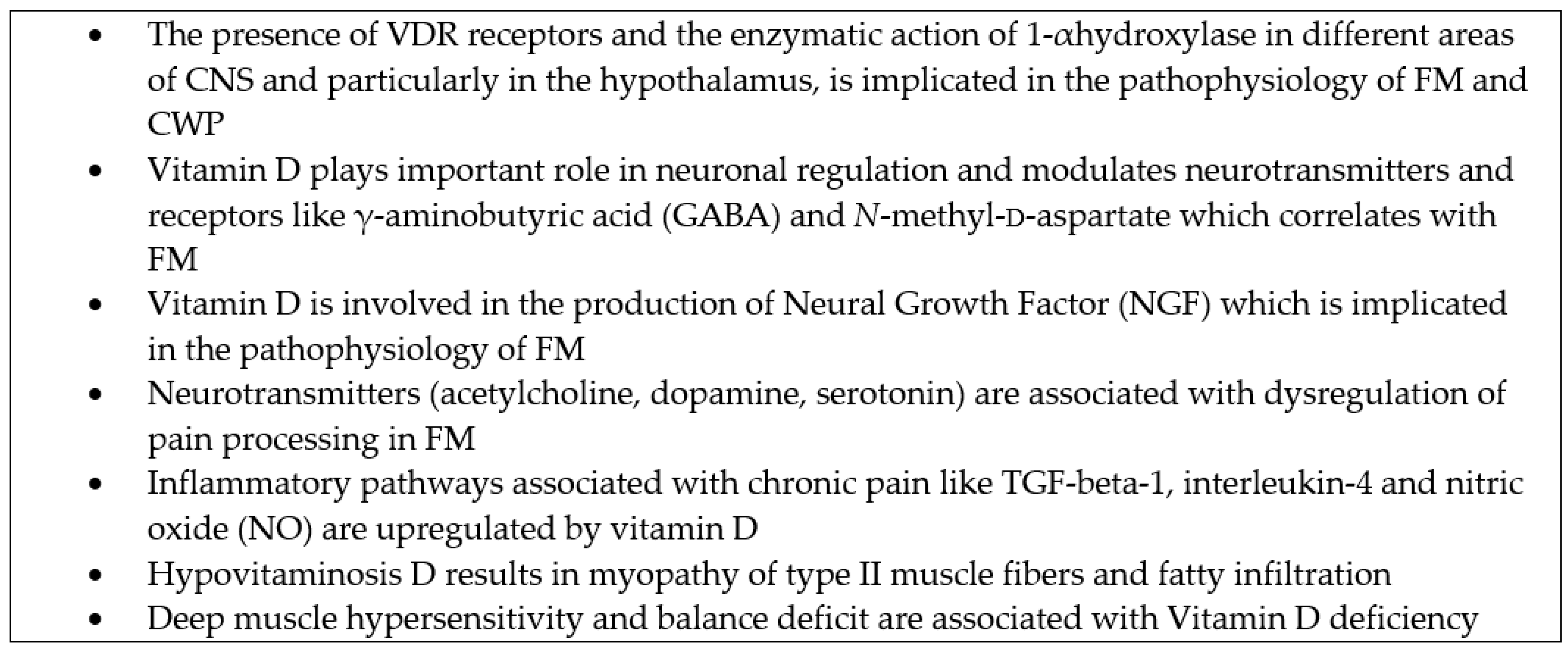
3.1. Pathophysiology
3.2. Data from Observational Studies and Meta-Analyses
3.3. Supplementation Studies
4. Future Perspectives of Vitamin D Clinical Trials in Fibromyalgia
5. Conclusions
Conflicts of Interest
References
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Branco, J.C.; Bannwarth, B.; Failde, I.; Abello Carbonell, J.; Blotman, F.; Spaeth, M.; Saraiva, F.; Nacci, F.; Thomas, E.; Caubère, J.P.; et al. Prevalence of fibromyalgia: A survey in five European countries. Semin. Arthritis Rheum. 2010, 39, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, B.; Blotman, F.; Lay, K.R.L.; Caubère, J.P.; André, E.; Taïeb, C. Fibromyalgia syndrome in the general population of France: A prevalence study. Jt. Bone Spine 2009, 76, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.L.; Burckhardt, C.; Crofford, L. Management of fibromyalgia syndrome. JAMA 2004, 292, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Picotto, G.; Liaudat, A.C.; Bohl, L.; Tolosa de Talamoni, N. Molecular aspects of vitamin D anticancer activity. Cancer Investig. 2012, 30, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- White, K.P.; Thompson, J. Fibromyalgia syndrome in an Amish community: A controlled study to determine disease and symptom prevalence. J. Rheumatol. 2003, 30, 1835–1840. [Google Scholar] [PubMed]
- Jacobsen, S.; Prescott, E.; Kjoller, M.; Bülow, P.M.; Danneskiold-samsøe, B.; Kamper-jørgensen, F. Fibromyalgia in the adult Danish population: I. A prevalence study. Scand. J. Rheumatol. 1993, 22, 233–237. [Google Scholar]
- Topbas, M.; Cakirbay, H.; Gulec, H.; Akgol, E.; Ak, I.; Can, G. The prevalence of fibromyalgia in women aged 20–64 in Turkey. Scand. J. Rheum. 2005, 34, 140–144. [Google Scholar] [PubMed]
- Makela, M.; Heliovaara, M. Prevalence of primary fibromyalgia in the Finish population. Br. Med. J. 1991, 303, 216–219. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Boulos, P. Inaccuracy in the diagnosis of fibromyalgia syndrome: analysis of referrals. Rheumatology (Oxf.) 2003, 42, 263–267. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Walitt, B.T.; Rasker, J.J.; Katz, R.S.; Häuser, W. The Use of Polysymptomatic Distress Categories in the Evaluation of Fibromyalgia (FM) and FM Severity. J. Rheumatol. 2015, 42, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Brahler, E.; Hinz, A.; Hauser, W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: Results from a survey of the general population. Arthritis Care Res. 2013, 65, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Russell, A.S. A questionnaire using the modified 2010 American College of Rheumatology Criteria for fibromyalgia: Specificity and sensitivity in clinical practice. J. Rheumatol. 2013, 40, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Russell, I.J.; Larson, A.A. Neurophysiopathogenesis of fibromyalgia syndrome: A unified hypothesis. Rheum Dis. Clin. N. Am. 2009, 35, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.A.; Clauw, D.J. Understanding fibromyalgia: Lessons from the broader pain research community. J. Pain 2009, 10, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Z.; Wu, X.; Mao, A.; Chang, F.; Deng, X.; Gao, H.; Ouyang, C.; Dery, K.J.; Le, K.; et al. Discovery of potential new gene variants and inflammatory cytokine associations with fibromyalgia syndrome by whole exome sequencing. PLoS ONE 2013, 8, e65033. [Google Scholar]
- Rodriguez-Revenga, L.; Madrigal, I.; Blanch-Rubió, J.; Elurbe, D.M.; Docampo, E.; Collado, A.; Vidal, J.; Carbonell, J.; Estivill, X.; Mila, M. Screening for the presence of FMR1 premutation alleles in women with fibromyalgia. Gene 2013, 512, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; Arnold, L.M.; McCarberg, B.H. The Science of Fibromyalgia. Mayo Clin. Proc. 2011, 86, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Ablin, K.; Clauw, D.J. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: Evolution of a clinical construct. Rheum. Dis. Clin. N. Am. 2009, 35, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Ferreira, E.A.; Matsutani, L.A.; Pereira, C.A.; Assumpção, A. Quantifying pain threshold and quality of life of fibromyalgia patients. Clin. Rheumatol. 2005, 24, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Gracely, R.H.; Petzke, F.; Wolf, J.M.; Clauw, D.J. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002, 46, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- McDermid, A.J.; Rollman, G.B.; McCain, G.A. Generalized hypervigilance in fibromyalgia: Evidence of perceptual amplification. Pain 1996, 66, 133–144. [Google Scholar] [CrossRef]
- Russell, I.J.; Vaeroy, H.; Javors, M.; Nyberg, F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992, 35, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, J.N.; Whalen, G.; Cunningham, J.; Clauw, D.J. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet. Disord. 2004, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Clauw, D.J.; Scott, D.J.; McLean, S.A.; Gracely, R.H.; Zubieta, J.K. Decreased central mu-opioid receptor availability in fibromyalgia. J. Neurosci. 2007, 27, 10000–10006. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M. The pathophysiology, diagnosis, and treatment of fibromyalgia. Psychiatr. Clin. N. Am. 2010, 33, 375–408. [Google Scholar] [CrossRef] [PubMed]
- May, A. Chronic pain may change the structure of the brain. Pain 2008, 137, 7–15. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, J.A.; Yezierski, R.P. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 2001, 90, 1–6. [Google Scholar] [CrossRef]
- Bosma, R.L.; Mojarad, E.A.; Leung, L.; Pukall, C.; Staud, R.; Stroman, P.W. FMRI of spinal and supra-spinal correlates of temporal pain summation in fibromyalgia patients. Hum. Brain Map. 2016, in press. [Google Scholar]
- Carrillo-de-la-Peña, M.T.; Vallet, M.; Pérez, M.I.; Gómez-Perretta, C. Intensity dependence of auditory-evoked cortical potentials in fibromyalgia patients: A test of the generalized hypervigilance hypothesis. J. Pain 2006, 7, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Atzeni, F.; Boccassini, L.; Cassisi, G.; Sarzi-Puttini, P. Physiopathology of pain in rheumatology. Reumatismology 2014, 66, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Tuohimaa, P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Shipton, E.A.; Shipton, E.E. Vitamin D and Pain: Vitamin D and Its Role in the Aetiology and Maintenance of Chronic Pain States and Associated Comorbidities. Pain Res. Treat. 2015, 2015, 904967. [Google Scholar] [CrossRef] [PubMed]
- Jirikowski, G.F.; Kauntzer, U.W.; Dief, A.E.E.; Caldwell, J.D. Distribution of vitamin D binding protein expressing neurons in the rat hypothalamus. Histochem. Cell Biol. 2009, 131, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Plotnikoff, G.A.; Quigley, J.M. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin. Proc. 2003, 78, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D receptor and 1-alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Nyagan, A.G.; Meyer, L.; Schaeffer, V.; Kibaly, C.; Patte-Mensah, C. Evidence for a key role of steroids in the modulation of pain. Psychoneuroendocrinology 2009, 34, S169–S177. [Google Scholar] [CrossRef] [PubMed]
- Russell, I.J.; Holman, A.J.; Swick, T.J.; Alvarez-Horine, S.; Wang, Y.G.; Guinta, D.; Sodium Oxybate 06-008 FM Study Group. Sodium oxybate reduces pain, fatigue, and sleep disturbance and improves functionality in fibromyalgia: Results from a 14-week, randomized, double-blind, placebo-controlled study. Pain 2011, 152, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Neveu, I.; Naveilhan, P.; Jehan, F.; Baudet, C.; Wion, D.; De Luca, H.F.; Brachet, P. 1,25-dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res. Mol. Brain Res. 1994, 24, 70–76. [Google Scholar] [CrossRef]
- Sanchez, B.; Relova, J.L.; Gallego, R.; Ben-Batalla, I.; Perez-Fernandez, R. 1,25-Dihydroxyvitamin D3 administration to 6-hydroxydopamine-lesioned rats increases glial cell line-derived neurotrophic factor and partially restores tyrosine hydroxylase expression in substantia nigra and striatum. J. Neurosci. Res. 2009, 87, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Alberti, A.; Candeliere, A.; Floridi, A.; Capocchi, G.; Calabresi, P. Glial cell line-derived neurotrophic factor and somatostatin levels in cerebrospinal fluid of patients affected by chronic migraine and fibromyalgia. Cephalalgia 2006, 26, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; MacKie, P.J.; Kareken, D.A.; Hutchins, G.D.; Chumin, E.J.; Christian, B.T.; Yoder, K.K. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 2015. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Leung, L.; Cahill, C.M. TNF-𝛼 and neuropathic pain—A review. J. Neuroinflamm. 2010. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Fernandez, B.O.; Hamilton, A.; Lang, N.N.; Gallagher, J.M.; Newby, D.E.; Feelisch, M.; Weller, R.B. UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase. J. Investig. Dermatol. 2014, 134, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Opländer, C.; Volkmar, C.M.; Paunel-Görgülü, A.; van Faassen, E.E.; Heiss, C.; Kelm, M.; Halmer, D.; Mürtz, M.; Pallua, N.; Suschek, C.V. Whole body UVA irradiation lowers systemic blood pressure by release of nitric oxide from intracutaneous photolabile nitric oxide derivates. Circ. Res. 2009, 105, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Boland, R. Role of Vitamin D in skeletal muscle function. Endocr. Rev. 1986, 7, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Von Känel, R.; Müller-Hartmannsgruber, V.; Kokinogenis, G.; Egloff, N. Vitamin D and central hypersensitivity in patients with chronic pain. Pain Med. 2014, 15, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Olama, S.M.; Senna, M.K.; Elarman, M.M.; Elhawary, G. Serum vitamin D level and bone mineral density in premenopausal Egyptian women with fibromyalgia. Rheumatol. Int. 2013, 33, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Mateos, F.; Valero, C.; Olmos, J.M.; Casanueva, B.; Castillo, J.; Martínez, J.; Hernández, J.L.; González Macías, J. Bone mass and vitamin D levels in women with a diagnosis of fibromyalgia. Osteoporos. Int. 2014, 25, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Tague, S.E.; Clarke, G.L.; Winter, M.K.; McCarson, K.E.; Wright, D.E.; Smith, P.G. Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyperinnervation. J. Neurosci. 2011, 31, 13728–13738. [Google Scholar] [CrossRef] [PubMed]
- Jesus, C.A.; Feder, D.; Peres, M.F. The role of vitamin D in pathophysiology and treatment of fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 355. [Google Scholar] [CrossRef] [PubMed]
- Karvelas, D.; Vasudevan, S.V. Fibromyalgia syndrome. Pain Manag. 2011, 1, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Huisman, A.M.; White, K.P.; Algra, A.; Harth, M.; Vieth, R.; Jacobs, J.W.; Bijlsma, J.W.; Bell, D.A. Vitamin D levels in women with systemic lupus erythematosus and fibromyalgia. J. Rheumatol. 2001, 28, 2535–2539. [Google Scholar] [PubMed]
- Bhatty, S.A.; Shaikh, N.A.; Irfan, M.; Kashif, S.M.; Vaswani, A.S.; Sumbhai, A.; Gunpat, J. Vitamin D deficiency in fibromyalgia. JPMA 2010, 60, 949–951. [Google Scholar]
- McCabe, P.S.; Pye, S.R.; Beth, J.M.; Lee, D.M.; Tajar, A.; Bartfai, G.; Boonen, S.; Bouillon, R.; Casanueva, F.; Finn, J.D.; et al. Low vitamin D and the risk of developing chronic widespread pain: Results from the European male ageing study. BMC Musculoskelet. Disord. 2016, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Tandeter, H.; Grynbaum, M.; Zuili, I.; Shany, S.; Shvartzman, P. Serum 25-OH vitamin D levels in patients with fibromyalgia. Israel Med. Assoc J. 2009, 11, 339–342. [Google Scholar]
- Armstrong, D.J.; Meenagh, G.K.; Bickle, I.; Lee, A.S.; Curran, E.S.; Finch, M.B. Vitamin D deficiency is assiciated with anxiety and depression in fibromyalgia. Clin. Rheumatol. 2007, 26, 551–554. [Google Scholar] [CrossRef] [PubMed]
- De Rezende Pena, C.; Grillo, L.P.; Das Chagas Medeiros, M.M. Evaluation of 25-hydroxyvitamin D serum levels in patients with fibromyalgia. J. Clin. Rheumatol. 2010, 16, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Anagnostis, P.; Paschou, S.A.; Kandaraki, E.; Goulis, D.G. Vitamin D status during pregnancy: Time for a more unified approach beyond borders? Eur. J. Clin. Nutr. 2015, 69, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Karras, S.N.; Athyros, V.G.; Annweiler, C.; Karagiannis, A. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes. Lancet Diabetes Endocrinol. 2014, 2, 362–363. [Google Scholar] [CrossRef]
- Hsiao, M.Y.; Hung, C.Y.; Chang, K.V.; Han, D.S.; Wang, T.G. Is serum hypovitaminosis D associated with chronic widespread pain including fibromyalgia? A meta-analysis of observational studies. Pain Phys. 2015, 18, E877–E887. [Google Scholar]
- Badsha, H.; Daher, M.; Ooi Kong, K. Myalgias or non-specific muscle pain in Arab or Indo-Pakistani patients may indicate vitamin D deficiency. Clin. Rheumatol. 2009, 28, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Harari, M.; Dramsdahl, E.; Shany, S.; Baumfeld, Y.; Ingber, A.; Novack, V.; Sukenik, S. Increased vitamin D serum levels correlate with clinical improvement of rheumatic diseases after Dead Sea climatotherapy. Israel Med. Assoc. J. 2011, 13, 212–215. [Google Scholar]
- Matthana, M.H. The relation between vitamin D deficiency and fibromyalgia syndrome in women. Saudi Med. J. 2011, 32, 925–929. [Google Scholar] [PubMed]
- Abokrysha, N.T. Vitamin D deficiency in women with fibromyalgia in Saudi Arabia. Pain Med. 2012, 13, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.E.; Arnspiger, S.A. Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. J. Clin. Rheumatol. 2008, 14, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Wepner, F.; Scheuer, R.; Schuetz-Wieser, B.; Machacek, P.; Pieler-Bruha, E.; Cross, H.S.; Hahne, J.; Friedrich, M. Effects of vitamin D on patients with fibromyalgia syndrome: A randomized placebo-controlled trial. Pain 2014, 155, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hypponen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Gandini, S.; Mullie, P. A systematic review: Influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J. Clin. Endocrinol. Metab. 2012, 97, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-de-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Anagnostis, P.; Naughton, D.; Annweiler, C.; Petroczi, A.; Goulis, D.G. Vitamin D during pregnancy: Why observational studies suggest deficiency and interventional studies show no improvement in clinical outcomes? A narrative review. J. Endocrinol. Invest. 2015, 38, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R.; Chan, P.C.; MacFarlane, G.D. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am. J. Clin. Nutr. 2001, 73, 288–294. [Google Scholar] [PubMed]
- Karras, S.N.; Anagnostis, P.; Annweiler, C.; Naughton, D.P.; Petroczi, A.; Bili, E.; Harizopoulou, V.; Tarlatzis, B.C.; Persinaki, A.; Papadopoulou, F.; et al. Maternal vitamin D status during pregnancy: The Mediterranean reality. Eur. J. Clin. Nutr. 2014, 68, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, I.M.; Middelkoop, B.J.; Boeke, A.J.; Lips, P. Prevalence of vitamin D deficiency among Turkish, Moroccan, Indian and sub-Sahara African populations in Europe and their countries of origin: An overview. Osteoporos. Int. 2011, 22, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
| Older Criteria | Widespread Pain, Both Halves of the Body, ≥3 Consecutive Months ≥11 of the Tender Points (see Text) |
|---|---|
| Current Criteria (as referred in ACR official website) | Pain and symptoms over the past week, based on the total number of: painful areas out of 19 parts of the body PLUS level of severity of these symptoms: a. Fatigue; b. Waking unrefreshed; c. Cognitive (memory or thought) problems PLUS number of other general physical symptoms. Symptoms lasting at least three months at a similar level. No other health problem that would explain the pain and other symptoms |
| Study and Year of Publication | Population | Methodology | Outcome | Comment |
|---|---|---|---|---|
| Badsha et al., 2009 [67] | FM Patients (n = 139) or muscle pain | N = 50 i.m. 600.000 IU Vit D3 (<15 ng/dL), single dose | Follow up 1–2 months | Serum 25(OH)D after supplementation not reported |
| N = 20 p.o. 50.000 IU Vit D3/week for 8 weeks (<15 ng/dL) | 90% of patients reported clinical improvement | |||
| 95% female (age 40 ± 11.5) | ||||
| N = p.o. 1 mg alphacalcidiol (16–20 ng/dL) | ||||
| Middle East | ||||
| Harari et al., 2011 [68] | Patients (n = 60) age 62.8 ± 11.6 | Daily sun exposure, balneotherapy | Group 1: 25(OH)D: 88.8 ± 23.8 nmol/L (35.5 ± 9.5 ng/mL) | Data on vitamin D supplementation prior to inclusion to the study not reported |
| Females 47, males 13 | ||||
| Group 1 (n = 33) FM 25(OH)D: 75.5 ± 28.1 nmol/L (30.2 ± 11.2 ng/mL) | Group 2: 25(OH)D: 84.7 ± 22,4 nmol/L (33.9 ± 8.81 ng/mL) | |||
| Group 2 R.A.(n = 16), 25(OH)D: 63.6 ± 27.8 nmol/L (25.4 ± 11.1 ng/mL) | ||||
| Group 3: 25(OH)D :97.3 ± 23 nmol/L (38.9 ± 9.21 ng/mL) | ||||
| Group 3 OA n = 11, 25(OH)D: 71.8 ± 18.5 nmol/L (28.7 ± 7.4 ng/mL) | VAS levels improved significantly from 4.88 ± 1.63 to 7.26 ± 1.46 | |||
| Matthana et al., 2011 [69] | FM female patients (n = 100) n = 61 Vit D deficient Saudi Arabia | Patients with vit. D deficiency treated with vit.D2 50.000 IU/week until their blood level of 25(OH)D exceeded 50 ng/mL | 42 reported significant clinical improvement (FIQR) (25(OH)D ≥30 ng/mL) | No controls included |
| Abokrysha et al., 2012 [70] | FM patients (n = 30) age 34.56 ± 8.1 | Patients received either i.m 600.000 IU Vit D3 | Patients demonstrated significant clinical improvement (ACR criteria) | No controls included concentrations of 25(OH)D were not available after supplementation |
| Vit D 4.76 ± 1.46 ng/mL Saudi Arabia | Single dose or p.o. 50.000 IU Vit D3/week (8 weeks) | |||
| Warner et al., 2008 [71] | FM female patients (n = 50) (Diffuse pain) Vit D ≤ 20 ng/mL | n = 25 placebo (age 56.7 ± 11.3) n = 25 vit.D2 (50.000 IU/week) for 3 months (age 58 ± 7.3) | Vit D in the active treatment group higher than placebo 31.2 ± 6.1 ng/mL vs. 19.3 ± 6.5 ng/mL No improvement in FPS and pain on VAS | Data on vitamin D supplementation prior to inclusion to the study not reported |
| Wepner et al., 2014 [72] | FM female patients(n = 30) Randomized in treatment group (TG) and control group (CG) 25(OH)D < 32 ng/mL | Randomized placebo controlled trial 20 weeks oral vit.D3 (First evaluation) Re-evaluation after 24 weeks without supplementation | Marked reduction in pain in TG (VAS) Optimization of 25(OH)D in FM had a positive effect on the perception on SFHS 36 | Data on vitamin D supplementation prior to inclusion to the study not reported |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karras, S.; Rapti, E.; Matsoukas, S.; Kotsa, K. Vitamin D in Fibromyalgia: A Causative or Confounding Biological Interplay? Nutrients 2016, 8, 343. https://doi.org/10.3390/nu8060343
Karras S, Rapti E, Matsoukas S, Kotsa K. Vitamin D in Fibromyalgia: A Causative or Confounding Biological Interplay? Nutrients. 2016; 8(6):343. https://doi.org/10.3390/nu8060343
Chicago/Turabian StyleKarras, Spyridon, Eleni Rapti, Stauros Matsoukas, and Kalliopi Kotsa. 2016. "Vitamin D in Fibromyalgia: A Causative or Confounding Biological Interplay?" Nutrients 8, no. 6: 343. https://doi.org/10.3390/nu8060343
APA StyleKarras, S., Rapti, E., Matsoukas, S., & Kotsa, K. (2016). Vitamin D in Fibromyalgia: A Causative or Confounding Biological Interplay? Nutrients, 8(6), 343. https://doi.org/10.3390/nu8060343






