Maternal Low Quality Protein Diet Alters Plasma Amino Acid Concentrations of Weaning Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals and Diets
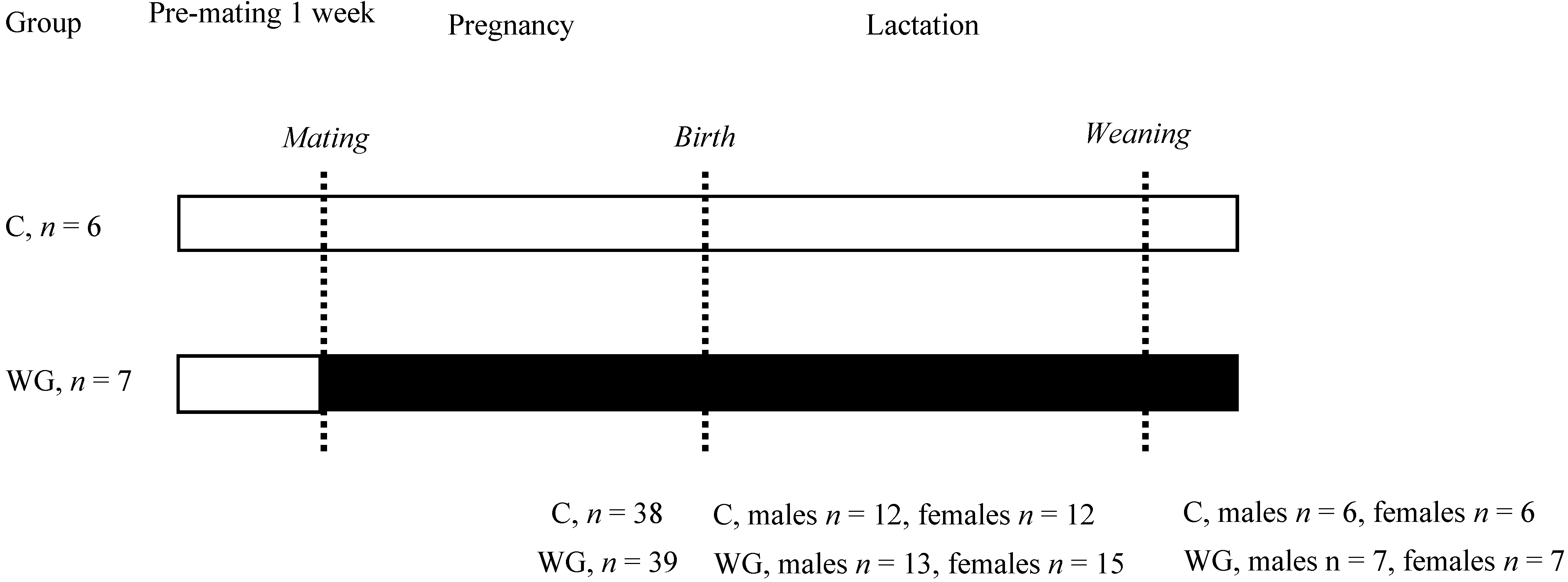
2.2. Estimation of Milk Yield
2.3. Plasma Amino Acid Concentration
2.4. Statistical Analysis
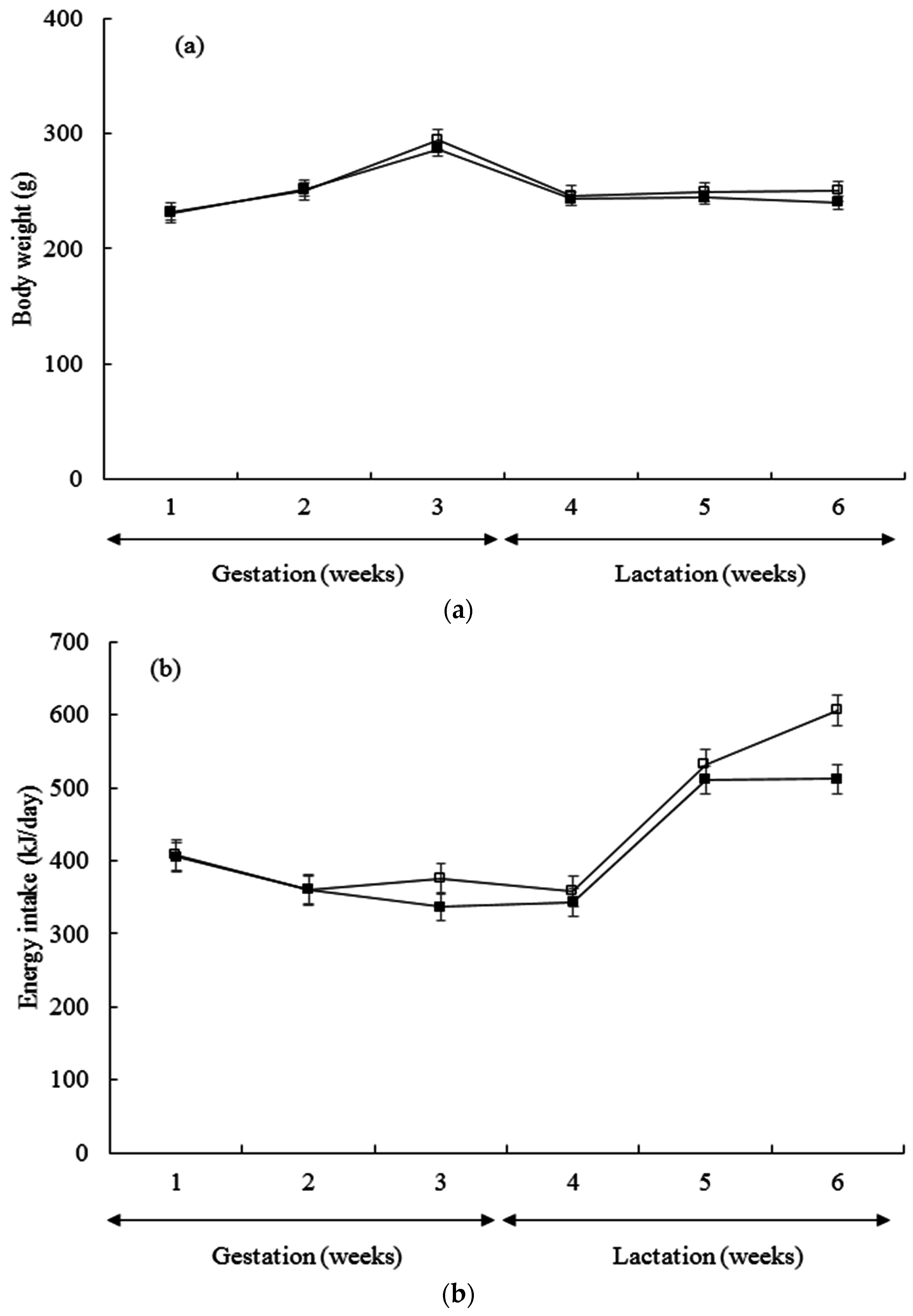
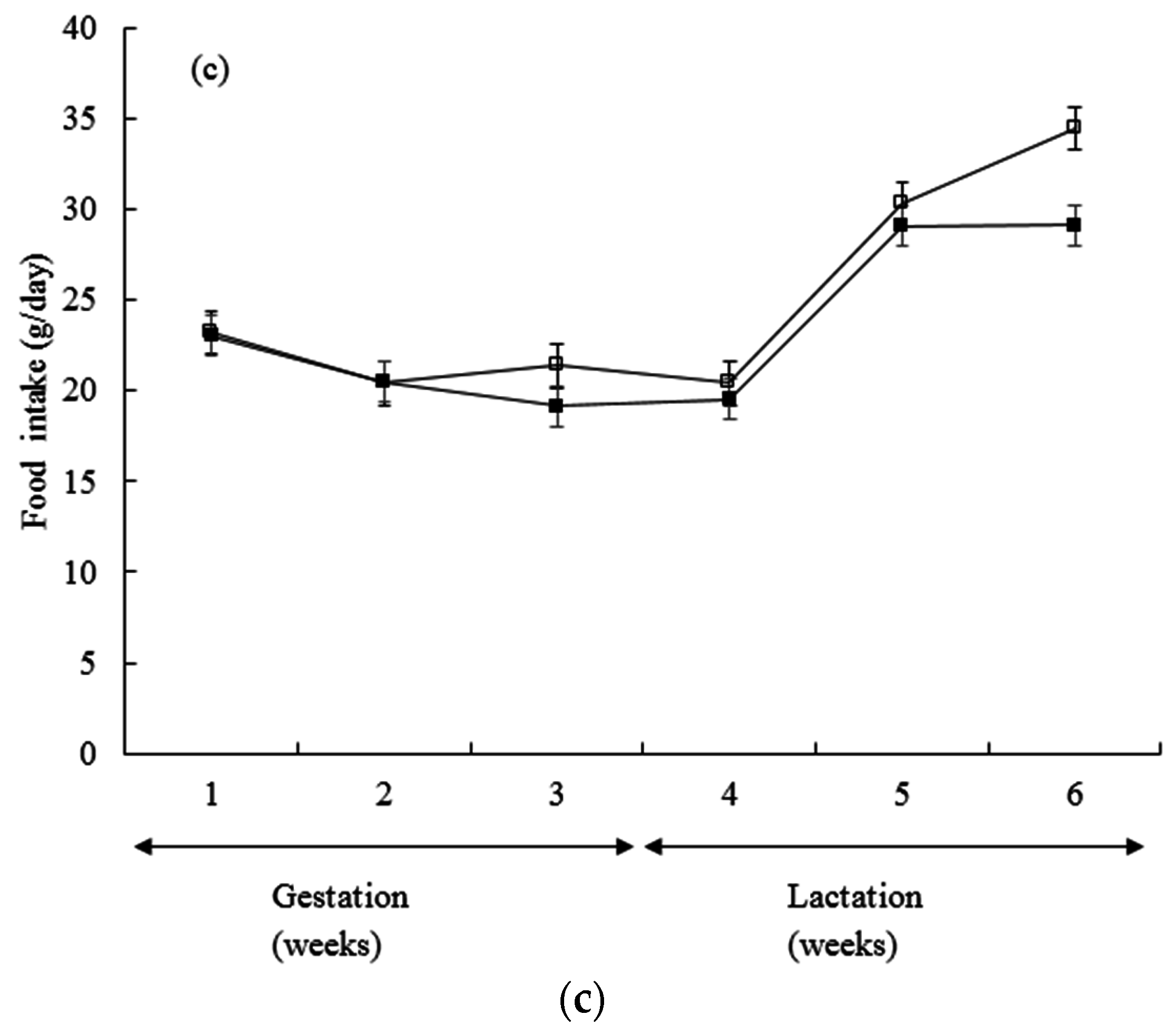
3. Results
| Males | Females | |||
|---|---|---|---|---|
| C | WG | C | WG | |
| Birth weight (g) | 5.96 ± 0.14 | 6.15 ± 0.14 | 5.53 ± 0.14 | 5.58 ± 0.13 |
| Body weight at 1st week (g) * | 11.41 ± 0.82 | 11.45 ± 0.79 | 10.71 ± 0.82 | 10.82 ± 0.73 |
| Body weight at 2nd week (g) * | 22.61 ± 0.82 | 22.55 ± 0.79 | 21.95 ± 0.82 | 22.03 ± 0.73 |
| Body weight at 3rd week(g) * | 35.83 ± 0.82 | 34.40 ± 0.79 | 34.80 ± 0.82 | 33.56 ± 0.73 |
| Estimation of milk yield (g/day) | 5.17 ± 0.39 | 4.95 ± 0.39 | 5.12 ± 0.39 | 5.63 ± 0.39 |
| Organ (% Body Weight) | At birth (Both Genders) | End of Lactation (Males) | End of Lactation (Females) | |||
|---|---|---|---|---|---|---|
| C | WG | C | WG | C | WG | |
| Liver | 3.47 ± 0.20 | 3.77 ± 0.19 | 3.61 ± 0.21 | 3.56 ± 0.19 | 3.81 ± 0.26 | 3.72 ± 0.24 |
| Brain | 2.92 ± 0.15 | 3.23 ± 0.14 | 3.08 ± 0.14 | 2.77 ± 0.13 | 3.29 ± 0.14 | 3.28 ± 0.13 |
| Heart | 0.54 ± 0.03 | 0.52 ± 0.02 | 0.40 ± 0.05 | 0.36 ± 0.04 | 0.43 ± 0.05 | 0.35 ± 0.05 |
| Left kidney | 0.48 ± 0.28 | 0.49 ± 0.03 | 0.63 ± 0.03 | 0.66 ± 0.02 | 0.64 ± 0.03 | 0.67 ± 0.02 |
| Right kidney | 0.49 ± 0.28 | 0.51 ± 0.03 | 0.65 ± 0.03 | 0.69 ± 0.02 | 0.66 ± 0.03 | 0.70 ± 0.03 |
| Amino Acid (µmol/L) | Maternal | Offspring | Offspring /Maternal | |||
|---|---|---|---|---|---|---|
| Essential amino acids | C | WG | C | WG | C | WG |
| Lysine *,§ | 25.67 ± 4.57 | 34.70 ± 4.23 | 177.90 ± 41.96 | 38.11 ± 38.85 | 8.65 ± 1.34 | 1.25 ± 1.25 |
| Phenylalanine | 859.12 ± 54.98 | 759.60 ± 50.90 | 688.99 ± 59.07 | 638.89 ± 54.69 | 0.90 ± 0.13 | 0.88 ± 0.12 |
| Leucine | 307.73 ± 23.02 | 259.49 ± 21.31 | 267.55 ± 25.51 | 261.76 ± 23.62 | 0.91 ± 0.07 | 1.02 ± 0.06 |
| Isoleucine | 237.55 ± 18.73 | 203.53 ± 17.34 | 243.11 ± 19.19 | 224.45 ± 17.76 | 1.04 ± 0.07 | 1.12 ± 0.06 |
| Methionine *,‡ | 90.15 ± 6.60 | 74.20 ± 6.11 | 493.59 ± 117.86 | 183.88 ± 109.12 | 7.62 ± 1.54 | 2.51 ± 1.42 |
| Valine § | 337.05 ± 23.43 | 287.98 ± 21.69 | 130.89 ± 57.56 | 257.86 ± 53.29 | 0.23 ± 0.15 | 0.89 ± 0.14 |
| Histidine | 1700.26 ± 151.67 | 1499.39 ± 140.42 | 1347.24 ± 167.45 | 1483.52 ± 155.03 | 0.82 ± 0.14 | 1.06 ± 0.13 |
| Threonine | 414.30 ± 38.45 | 363.86 ± 35.60 | 335.35 ± 31.12 | 398.02 ± 28.81 | 0.89 ± 0.09 | 1.12 ± 0.08 |
| Non-essential amino acids | ||||||
| Proline | 292.34 ± 19.67 | 258.47 ± 18.21 | 423.57 ± 65.52 | 447.99 ± 60.66 | 1.59 ± 0.18 | 1.70 ± 0.16 |
| Hydroxyproline ‡ | 38.84 ± 3.00 | 34.20 ± 2.78 | 70.83 ± 10.74 | 43.94 ± 9.94 | 2.27 ± 0.31 | 1.27 ± 0.29 |
| Glycyl-proline **,‡ | 129.90 ± 16.41 | 121.75 ± 15.19 | 96.65 ± 29.32 | 227.54 ± 27.15 | 0.67 ± 0.39 | 2.09 ± 0.36 |
| Serine †,§ | 465.83 ± 31.84 | 368.11 ± 29.48 | 352.42 ± 40.34 | 458.58 ± 37.34 | 0.81 ± 0.09 | 1.25 ± 0.08 |
| Glycine | 658.13 ± 74.92 | 603.96 ± 69.36 | 1068.50 ± 90.78 | 1144.75 ± 84.05 | 1.70 ± 0.20 | 1.98 ± 0.19 |
| Aspartic acid **,§ | 495.58 ± 48.37 | 452.08 ± 44.78 | 136.09 ± 56.83 | 419.29 ± 51.88 | 0.28 ± 0.13 | 0.90 ± 0.12 |
| Asparagine | 138.77 ± 8.84 | 115.44 ± 8.18 | 136.21 ± 6.83 | 131.00 ± 6.32 | 1.02 ± 0.05 | 1.16 ± 0.05 |
| Cystathionine | 56.85 ± 31.22 | 87.07 ± 28.90 | 76.37 ± 26.77 | 137.37 ± 21.86 | 9.66 ± 3.88 | 4.70 ± 3.17 |
| Ornithine *,‡ | 594.09 ± 195.16 | 438.89 ± 180.69 | 416.47 ± 235.44 | 1104.42 ± 217.97 | 0.79 ± 1.82 | 6.35 ± 1.68 |
| Tyrosine | 833.19 ± 83.97 | 767.22 ± 77.74 | 716.87 ± 124.31 | 909.47 ± 115.08 | 0.94 ± 0.09 | 1.18 ± 0.08 |
| Cystine | 71.34 ± 15.37 | 59.99 ± 14.23 | 75.20 ± 12.11 | 68.70 ± 11.21 | 1.21 ± 0.44 | 1.62 ± 0.41 |
| Alanine | 956.86 ± 93.76 | 891.79 ± 86.80 | 704.67 ± 109.46 | 850.89 ± 101.34 | 0.81 ± 0.09 | 0.98 ± 0.08 |
| Sarcosine | 11.95 ± 0.72 | 12.52 ± 0.61 | 20.06 ± 1.42 | 20.04 ± 1.31 | 1.75 ± 0.18 | 1.63 ± 0.15 |
| Glutamic acid | 706.61 ± 46.32 | 646.88 ± 42.88 | 724.82 ± 66.08 | 748.66 ± 61.18 | 1.06 ± 0.05 | 1.15 ± 0.05 |
| Glutamine * | 244.59 ± 109.55 | 283.26 ± 92.59 | 332.48 ± 83.39 | 73.72 ± 77.21 | 30.50 ± 12.85 | 9.80 ± 10.86 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Freeman, H.J.; Kim, Y.S.; Sleisenger, M.H. Protein digestion and absorption in man. Normal mechanisms and protein-energy malnutrition. Am. J. Med. 1979, 67, 1030–1036. [Google Scholar] [CrossRef]
- Young, V.R.; Marchini, J.S.; Cortiella, J. Assessment of protein nutritional status. J. Nutr. 1990, 120, 1496–1502. [Google Scholar] [PubMed]
- Mitsuhashi, S. Current topics in the biotechnological production of essential amino acids, functional amino acids, and dipeptides. Curr. Opin. Biotechnol. 2014, 26, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Bayham, B.E.; Greenway, F.L.; Johnson, W.D.; Dhurandhar, N.V. A randomized trial to manipulate the quality instead of quantity of dietary proteins to influence the markers of satiety. J. Diabetes Complicat. 2014, 28, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G. Advantages and limitations of the protein digestibility-corrected amino acid score (PDCAAS) as a method for evaluating protein quality in human diets. Br. J. Nutr. 2012, 108, S333–S336. [Google Scholar] [CrossRef] [PubMed]
- Pellet, P.L. World essential amino acid supply with special reference to South-East Asia. Food Nutr. Bull. 1996, 17, 204–234. [Google Scholar]
- FAO/WHO-United Nations University. Energy and protein requirements. In World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 1985; Volume 724, pp. 1–206. [Google Scholar]
- Gilani, G.S.; Wu Xiao, C.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Hales, C.N.; Fall, C.H.; Osmond, C.; Phipps, K.; Clark, P.M. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia 1993, 36, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 2002, 13, 364–368. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Forsén, T.; Tuomilehto, J.; Jaddoe, V.W.; Osmond, C.; Barker, D.J. Effects of size at birth and childhood growth on the insulin resistance syndrome in elderly individuals. Diabetologia 2002, 45, 342–348. [Google Scholar] [CrossRef] [PubMed]
- McMullen, S.; Mostyn, A. Animal models for the study of the developmental origins of health and disease. Proc. Nutr. Soc. 2009, 68, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S.C.; Nwagwu, M. Impaired growth and increased glucocorticoid-sensitive enzyme activities in tissues of rat fetuses exposed to maternal low protein diets. Life Sci. 1998, 63, 605–615. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, X.; Zhang, Q.; Yu, M.; Xu, J.; Wang, Z. Maternal protein restriction induces early-onset glucose intolerance and alters hepatic genes expression in the peroxisome proliferator-activated receptor pathway in offspring. J. Diabetes Investig. 2015, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Li, A.; Li, L.; Kitlinska, J.B.; Zukowska, Z. Maternal low-protein diet up-regulates the neuropeptide Y system in visceral fat and leads to abdominal obesity and glucose intolerance in a sex- and time-specific manner. FASEB J. 2012, 26, 3528–3536. [Google Scholar] [CrossRef] [PubMed]
- Elmes, M.J.; Gardner, D.S.; Langley-Evans, S.C. Fetal exposure to a maternal low-protein diet is associated with altered left ventricular pressure response to ischaemia-reperfusion injury. Br. J. Nutr. 2007, 98, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Gao, G.; Song, H.; Cai, D.; Yang, X.; Zhao, R. Low-protein diet fed to crossbred sows during pregnancy and lactation enhances myostatin gene expression through epigenetic regulation in skeletal muscle of weaning piglets. Eur. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Alfenas, R.C.G.; Bressan, J.; Paiva, A.C. Effects of protein quality on appetite and energy metabolism in normal weight subjects. Arq. Bras. Endocrinol. Metabol. 2010, 54, 45–51. [Google Scholar] [CrossRef]
- Holmer-Jensen, J.; Mortensen, L.S.; Astrup, A.; de Vrese, M.; Holst, J.J.; Thomsen, C.; Hermansen, K. Acute differential effects of dietary protein quality on postprandial lipemia in obese non-diabetic subjects. Nutr. Res. 2013, 33, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.S.; Hartvigsen, M.L.; Brader, L.J.; Astrup, A.; Schrezenmeir, J.; Holst, J.J.; Thomsen, C.; Hermansen, K. Differential effects of protein quality on postprandial lipemia in response to a fat-rich meal in type 2 diabetes: Comparison of whey, casein, gluten, and cod protein. Am. J. Clin. Nutr. 2009, 90, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Milton, J.E.; Packard, D.P.; Shuler, L.A.; Short, R.A. Dietary amino acids and blood pressure: A cohort study of patients with cardiovascular disease. Am. J. Kidney Dis. 2012, 59, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, C.; Mølgaard, C.; Vaag, A.; Barkholt, V.; Michaelsen, K.F. High intakes of milk, but not meat, increase s-insulin and insulin resistance in 8-year-old boys. Eur. J. Clin. Nutr. 2005, 59, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.A.; LeFaivre, M.H.; Milner, J.A. Effects of dietary protein quantity and quality on the growth of dogs and rats. J. Nutr. 1982, 112, 1843–1853. [Google Scholar] [PubMed]
- Mercer, L.P.; Watson, D.F.; Ramlet, J.S. Control of food intake in the rat by dietary protein concentration. J. Nutr. 1981, 111, 1117–1123. [Google Scholar] [PubMed]
- Park, M.S.; Liepa, G.U. Effects of dietary protein and amino acids on the metabolism of cholesterol-carrying lipoproteins in rats. J. Nutr. 1982, 112, 1892–1898. [Google Scholar] [PubMed]
- Bozzini, C.E.; Champin, G.M.; Alippi, R.M.; Bozzini, C. Biomechanical properties of the mandible, as assessed by bending test, in rats fed a low-quality protein. Arch. Oral Biol. 2013, 58, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Alippi, R.M.; Picasso, E.; Huygens, P.; Bozzini, C.E.; Bozzini, C. Growth-dependent effects of dietary protein concentration and quality on the biomechanical properties of the diaphyseal rat femur. Endocrinol. Nutr. 2012, 59, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Sampson, D.A.; Jansen, G.R. Measurement of milk yield in the lactating rat from pup weight and weight gain. J. Pediatr. Gastroenterol. Nutr. 1984, 3, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; Morgan, C.J.; Turner, J.A. Application of the Phenomenex EZ:faasttrade mark amino acid analysis kit for rapid gas-chromatographic determination of concentrations of plasma tryptophan and its brain uptake competitors. Amino Acids 2008, 34, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Mattson, D.L.; Meister, C.J.; Marcelle, M.L. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 2005, 45, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Yagi, I.; Saeki, T.; Kotaru, M.; Iwami, K. Quantity as well as quality of dietary protein affects serine dehydratase gene expression in rat liver. J. Nutr. Sci. Vitaminol. 2003, 49, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Harper, A.E. Amino acid balance and food intake: Effect of different dietary amino acid patterns on the plasma amino acid pattern of rats. J. Nutr. 1970, 100, 429–437. [Google Scholar] [PubMed]
- Menaker, L.; Navia, J.M. Appetite regulation in the rat under various physiological conditions: The role of dietary protein and calories. J. Nutr. 1973, 103, 347–352. [Google Scholar] [PubMed]
- Tews, J.K.; Kim, Y.W.; Harper, A.E. Induction of threonine imbalance by dispensable amino acids: Relationships between tissue amino acids and diet in rats. J. Nutr. 1980, 110, 394–408. [Google Scholar] [PubMed]
- Jansen, G.R.; Schibly, M.B.; Masor, M.; Sampson, D.A.; Longenecker, J.B. Free amino acid levels during lactation in rats: Effects of protein quality and protein quantity. J. Nutr. 1986, 116, 376–387. [Google Scholar] [PubMed]
- Kwong, E.; Barnes, R.H. Comparative contributions of dietary protein quality and quantity to growth during gestation, lactation and postweaning in the rat. J. Nutr. 1977, 107, 420–425. [Google Scholar] [PubMed]
- Haq, A.U.; Bundrant, H.M.; Mercer, L.P. Food intake is inversely correlated with central nervous system histamine receptor (H1) concentrations in male Sprague-Dawley rats fed normal, low protein, low energy or poor quality protein diets. J. Nutr. 1996, 126, 3083–3089. [Google Scholar] [PubMed]
- Wood-Bradley, R.J.; Barrand, S.; Giot, A.; Armitage, J.A. Understanding the role of maternal diet on kidney development; an opportunity to improve cardiovascular and renal health for future generations. Nutrients 2015, 7, 1881–1905. [Google Scholar] [CrossRef] [PubMed]
- Zohdi, V.; Lim, K.; Pearson, J.T.; Black, M.J. Developmental programming of cardiovascular disease following intrauterine growth restriction: Findings utilising a rat model of maternal protein restriction. Nutrients 2014, 7, 119–152. [Google Scholar] [CrossRef] [PubMed]
- Jansen, G.R.; Hunsaker, H. Effect of dietary protein and energy on protein synthesis during lactation in rats. J. Nutr. 1986, 116, 957–968. [Google Scholar] [PubMed]
- Jansen, G.R.; Grayson, C.; Hunsaker, H. Wheat gluten during pregnancy and lactation: Effects on mammary gland development and pup viability. Am. J. Clin. Nutr. 1987, 46, 250–257. [Google Scholar] [PubMed]
- Jansen, G.R.; Binard, R.; Longenecker, J.B. Protein quality and quantity influence free amino acid levels in the brain and serum of rats during lactation. J. Nutr. 1991, 121, 1187–1194. [Google Scholar] [PubMed]
- Labuschagne, C.F.; van den Broek, N.J.; Mackay, G.M.; Vousden, K.H.; Maddocks, O.D. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 2014, 7, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Pooya, S.; Blaise, S.; Garcia, M.M.; Giudicelli, J.; Alberto, J.M.; Guéant-Rodriguez, R.M.; Jeannesson, E.; Gueguen, N.; Bressenot, A.; Nicolas, B.; et al. Methyl donor deficiency impairs fatty acid oxidation through PGC-1α hypomethylation and decreased ER-α, ERR-α, and HNF-4α in the rat liver. J. Hepatol. 2012, 57, 344–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konycheva, G.; Dziadek, M.A.; Ferguson, L.R.; Krägeloh, C.U.; Coolen, M.W.; Davison, M.; Breier, B.H. Dietary methyl donor deficiency during pregnancy in rats shapes learning and anxiety in offspring. Nutr. Res. 2011, 31, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Johnson, G.A.; Wu, G. Amino acids and conceptus development during the peri-implantation period of pregnancy. Adv. Exp. Med. Biol. 2015, 843, 23–52. [Google Scholar] [PubMed]
- Yokogoshi, H.; Hayase, K.; Yoshida, A. The quality and quantity of dietary protein affect brain protein synthesis in rats. J. Nutr. 1992, 122, 2210–2217. [Google Scholar] [PubMed]
- Ishida, A.; Kyoya, T.; Nakashima, K.; Katsumata, M. Muscle protein metabolism during compensatory growth with changing dietary lysine levels from deficient to sufficient in growing rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2011, 57, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.S.; Kwon, D.H.; Bong, J.J.; Jeong, J.Y.; Nam, Y.S.; Lee, M.S.; Liu, X.; Baik, M. Severe dietary lysine restriction affects growth and body composition and hepatic gene expression for nitrogen metabolism in growing rats. J. Anim. Physiol. Anim. Nutr. (Berl.) 2014, 98, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Aissa, A.F.; Gomes, T.D.; Almeida, M.R.; Hernandes, L.C.; Darin, J.D.; Bianchi, M.L.; Antunes, L.M. Methionine concentration in the diet has a tissue-specific effect on chromosomal stability in female mice. Food Chem. Toxicol. 2013, 62, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Morales, D.; Adamian, L.; Shi, D.; Liang, J. Lysine carboxylation: Unveiling a spontaneous post-translational modification. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 48–57. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Nie, J.; Sun, W.J.; Zheng, Z.Q.; Yang, X.J. Lysine acetylation: Enzymes, bromodomains and links to different diseases. Essays Biochem. 2012, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xie, C.; Zhang, Y.; Fan, Z.; Yin, Y.; Blachier, F. Glutamate-glutamine cycle and exchange in the placenta-fetus unit during late pregnancy. Amino Acids 2015, 47, 45–53. [Google Scholar]
- Sales, F.A.; Pacheco, D.; Blair, H.T.; Kenyon, P.R.; Nicholas, G.; Senna Salerno, M.; McCoard, S.A. Identification of amino acids associated with skeletal muscle growth in late gestation and at weaning in lambs of well-nourished sheep. J. Anim. Sci. 2014, 92, 5041–5052. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Aragão, R.; Guzmán-Quevedo, O.; Pérez-García, G.; Manhães-de-Castro, R.; Bolaños-Jiménez, F. Maternal protein restriction impairs the transcriptional metabolic flexibility of skeletal muscle in adult rat offspring. Br. J. Nutr. 2014, 112, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Cabeço, L.C.; Budri, P.E.; Baroni, M.; Castan, E.P.; Carani, F.R.; de Souza, P.A.; Boer, P.A.; Matheus, S.M.; Dal-Pai-Silva, M. Maternal protein restriction induce skeletal muscle changes without altering the MRFs MyoD and myogenin expression in offspring. J. Mol. Histol. 2012, 43, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Palou, A.; Arola, L.; Alemany, M. Plasma amino acid concentrations in pregnant rats and in 21-day foetuses. Biochem. J. 1977, 166, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, C.; Barcelo, A.C.; Alippi, R.M.; Leal, T.L.; Bozzini, C.E. The concentration of dietary casein required for normal mandibular growth in the rat. J. Dent. Res. 1989, 68, 840–842. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabasakal Cetin, A.; Dasgin, H.; Gülec, A.; Onbasilar, İ.; Akyol, A. Maternal Low Quality Protein Diet Alters Plasma Amino Acid Concentrations of Weaning Rats. Nutrients 2015, 7, 9847-9859. https://doi.org/10.3390/nu7125508
Kabasakal Cetin A, Dasgin H, Gülec A, Onbasilar İ, Akyol A. Maternal Low Quality Protein Diet Alters Plasma Amino Acid Concentrations of Weaning Rats. Nutrients. 2015; 7(12):9847-9859. https://doi.org/10.3390/nu7125508
Chicago/Turabian StyleKabasakal Cetin, Arzu, Halil Dasgin, Atila Gülec, İlyas Onbasilar, and Asli Akyol. 2015. "Maternal Low Quality Protein Diet Alters Plasma Amino Acid Concentrations of Weaning Rats" Nutrients 7, no. 12: 9847-9859. https://doi.org/10.3390/nu7125508
APA StyleKabasakal Cetin, A., Dasgin, H., Gülec, A., Onbasilar, İ., & Akyol, A. (2015). Maternal Low Quality Protein Diet Alters Plasma Amino Acid Concentrations of Weaning Rats. Nutrients, 7(12), 9847-9859. https://doi.org/10.3390/nu7125508





