Ameliorating Effect of Akebia quinata Fruit Extracts on Skin Aging Induced by Advanced Glycation End Products
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Antibodies
2.2. Plant Preparation and Extraction
2.3. In Vitro Glycation of Bovine Serum Albumin (BSA)
2.4. Determination of AGEs Formation
2.5. Determination of Nε-CML
2.6. Cell Line and Cell Culture
2.7. DPPH Scavenging Activity Assay
2.8. ABTS+ Scavenging Capacity Assay
2.9. Determination of Ferric Reducing Capacity
2.10. Intracellular ROS Measurement
2.11. Senescence-Associated Galactosidase (SA-β-Gal) Activity
2.12. Human Skin Explants
2.13. Antiglycation Activity in Human Skin Explants
2.14. Image Analysis of Cow’s Feet Wrinkle
2.15. Statistical Analysis
3. Results and Discussion
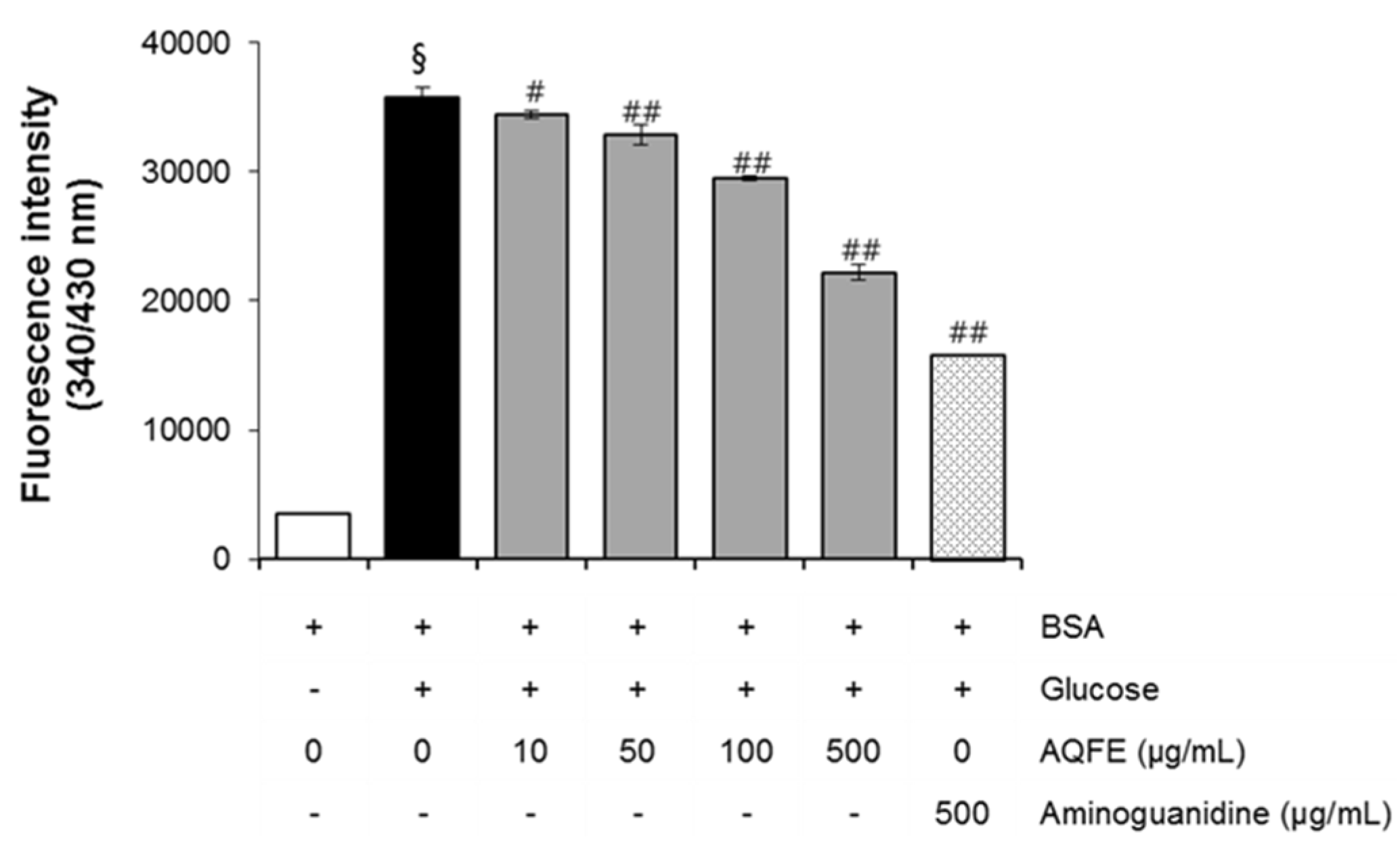
3.1. The Effect of AQFE on AGEs Formation
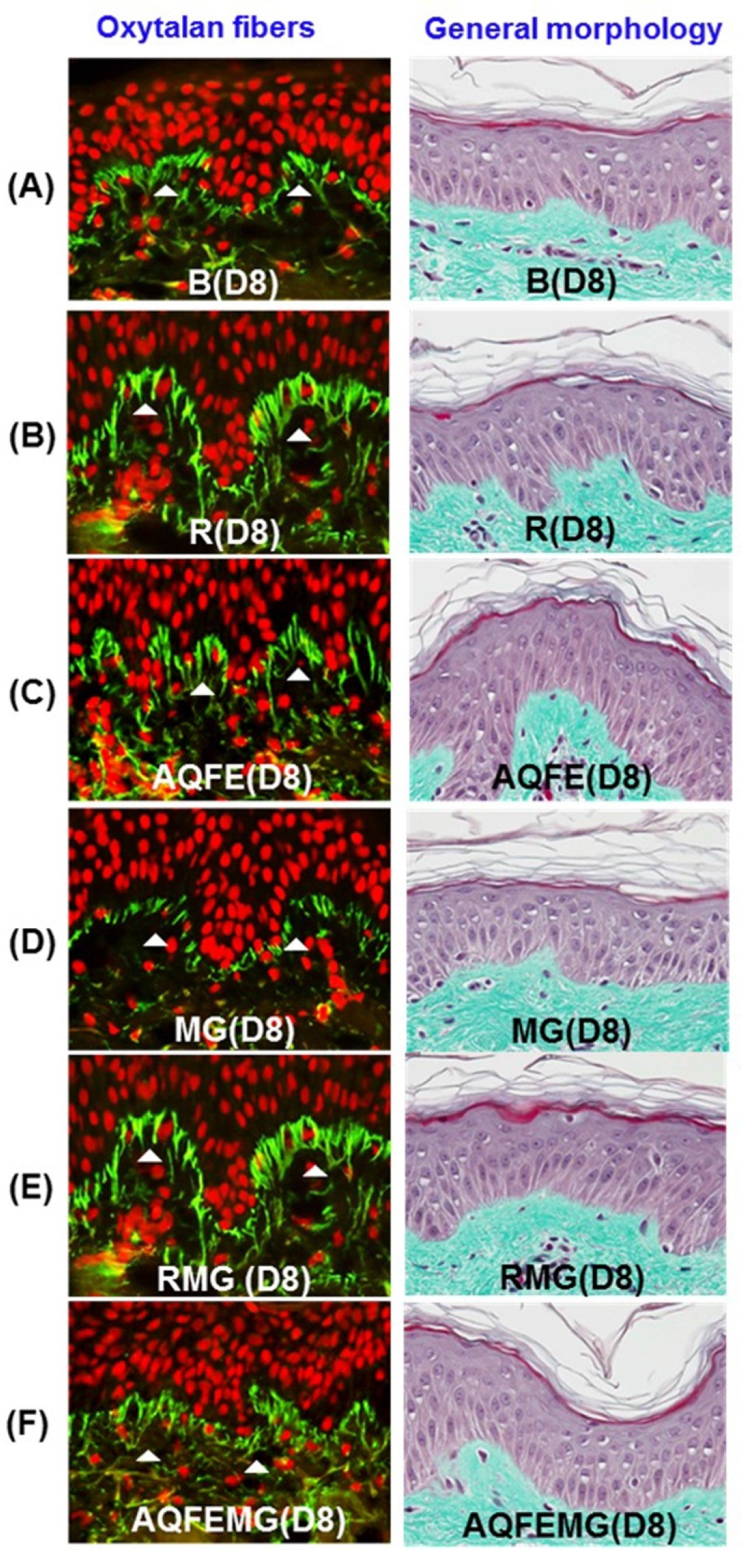
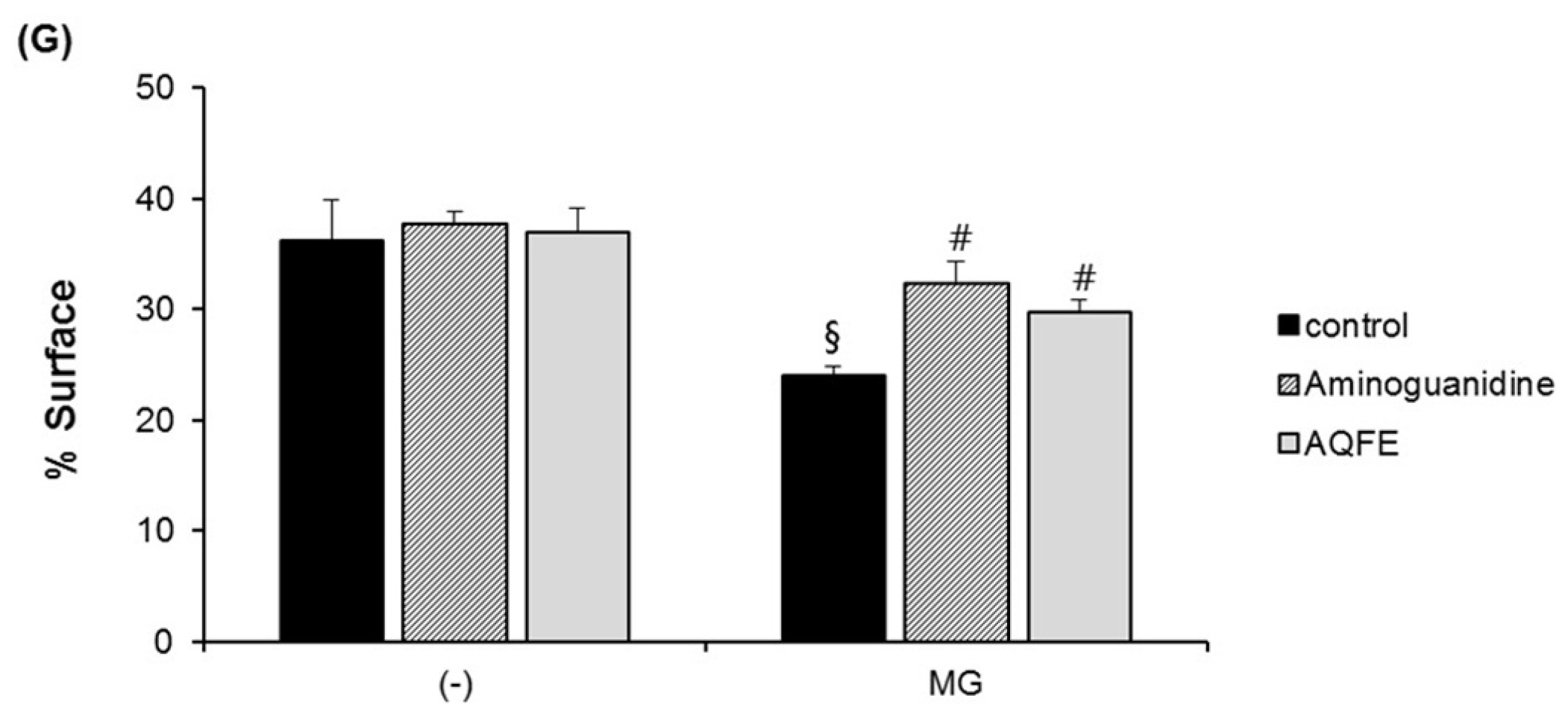
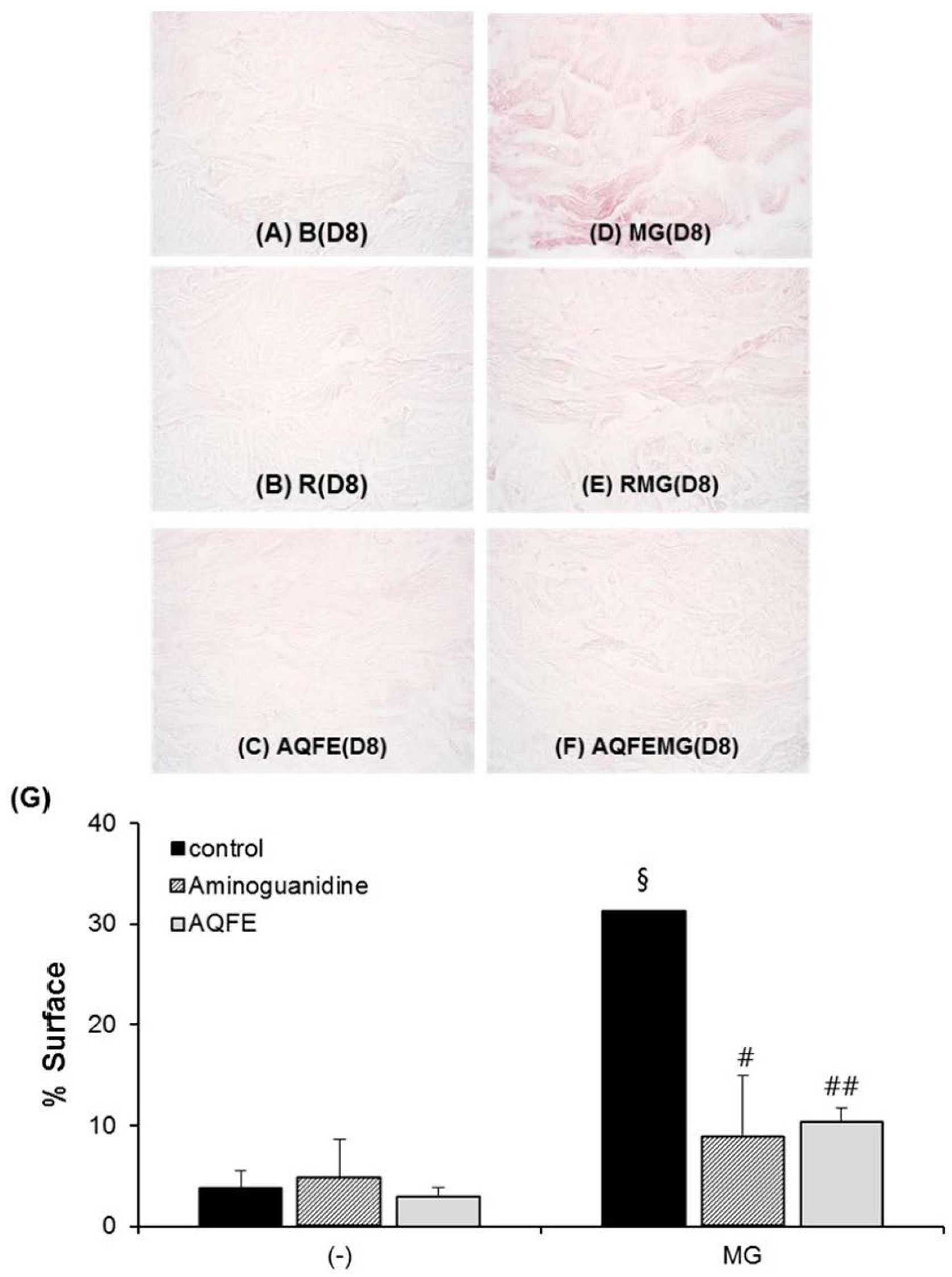
3.2. Topical Antiglycation Activity of AQFE
3.3. Antioxidant Capacities of AQFE
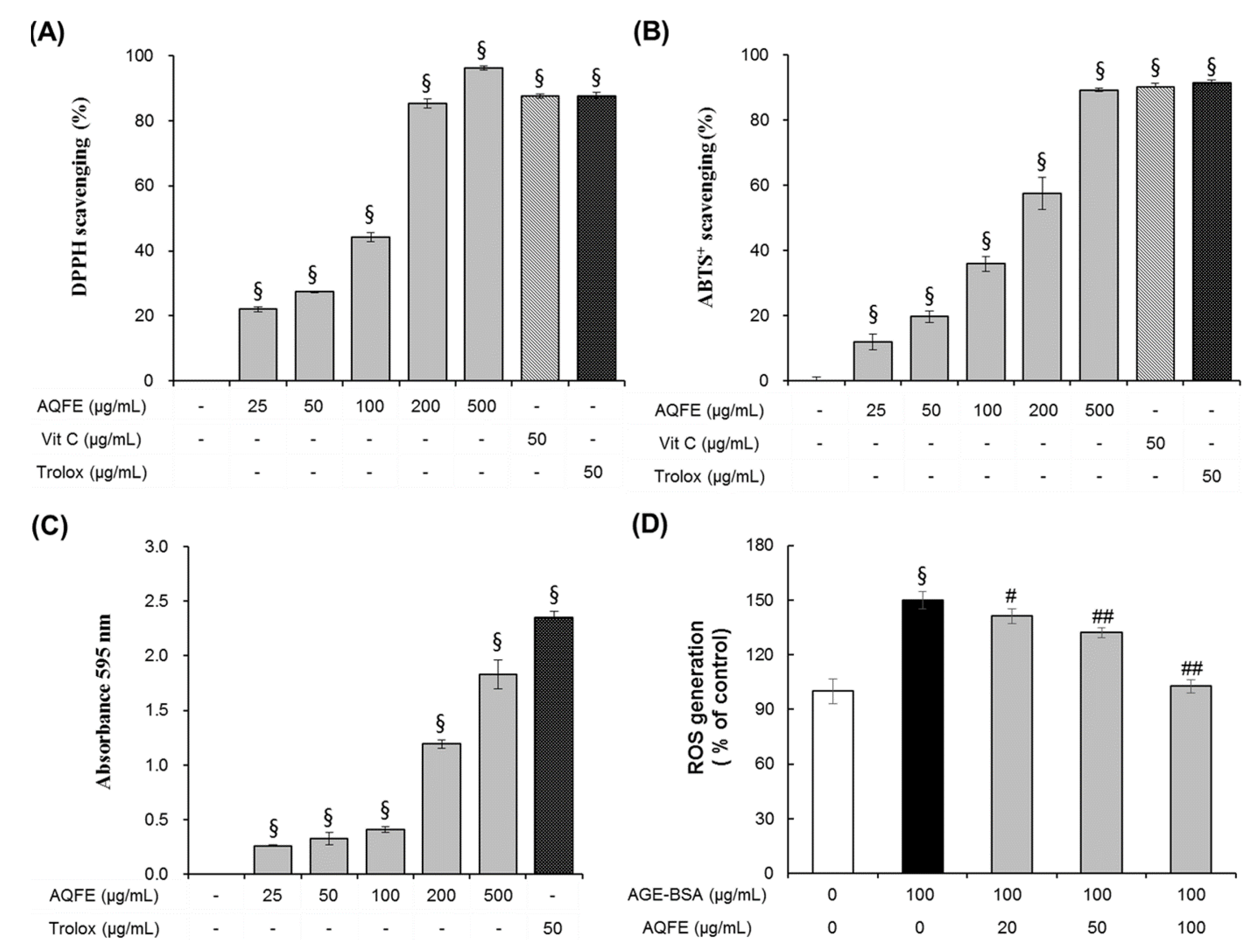
3.4. The Effect of AQFE on Cellular Senescence
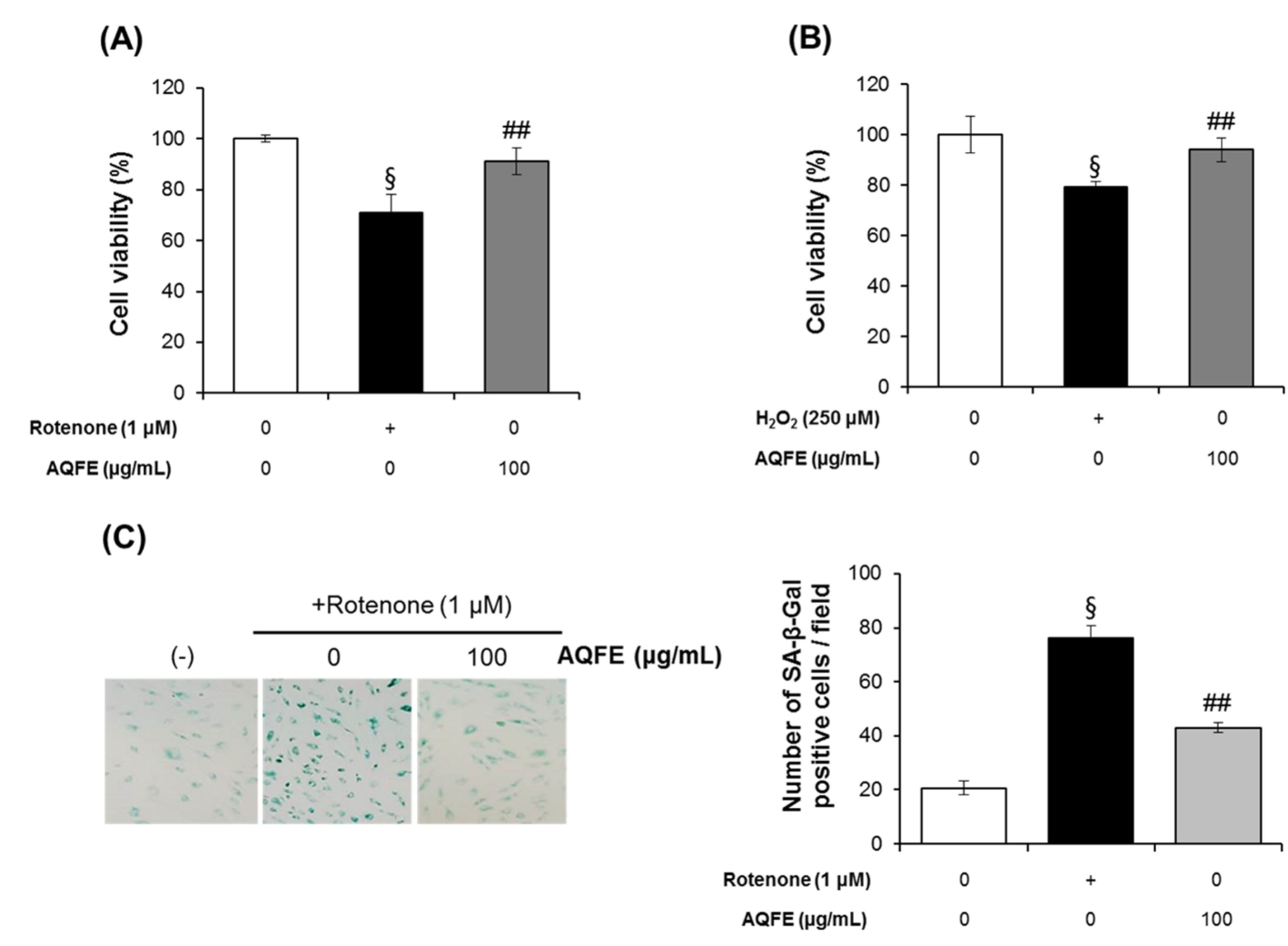
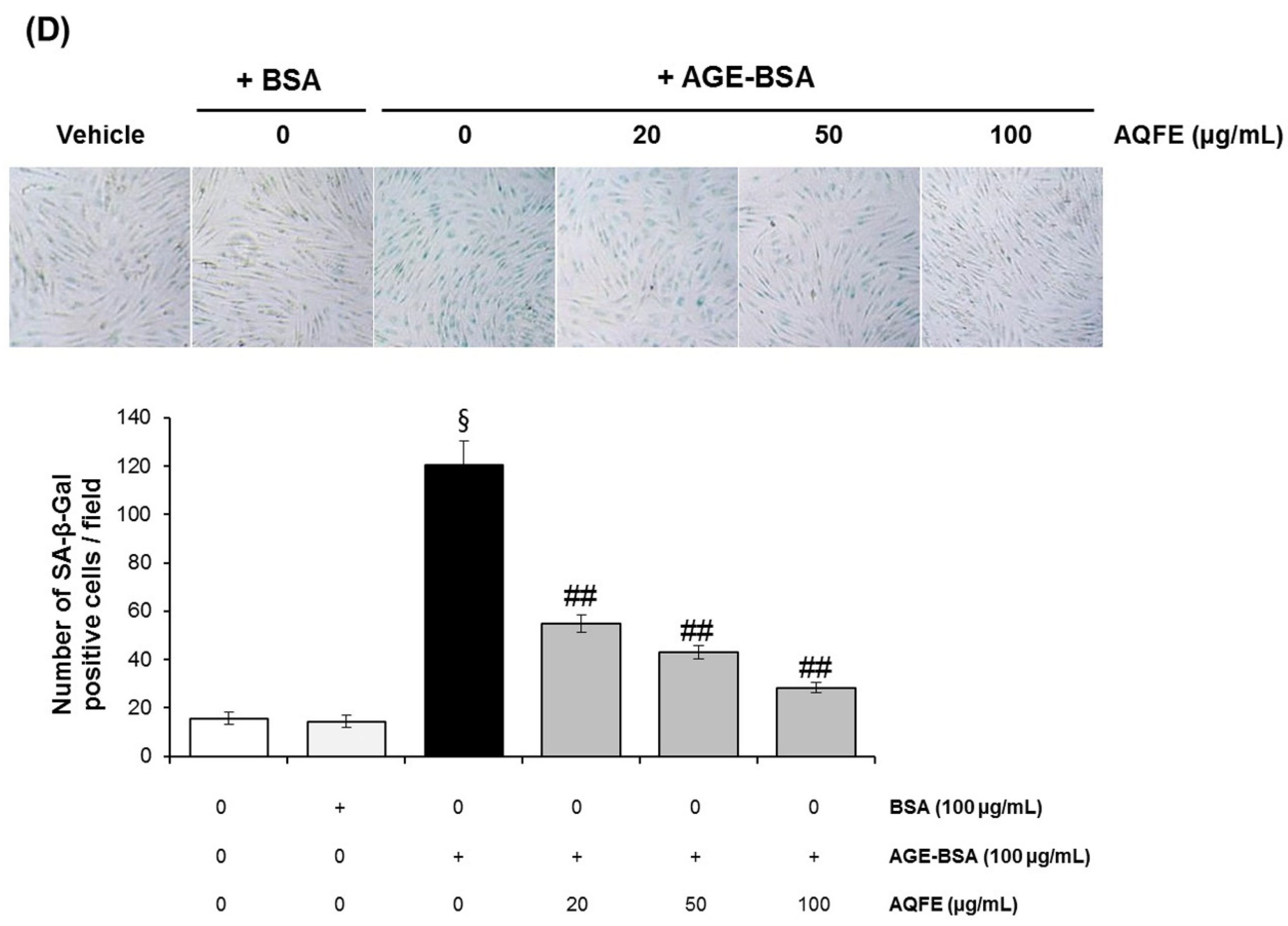
3.5. Anti-Skin Aging Properties of AQFE in Vivo
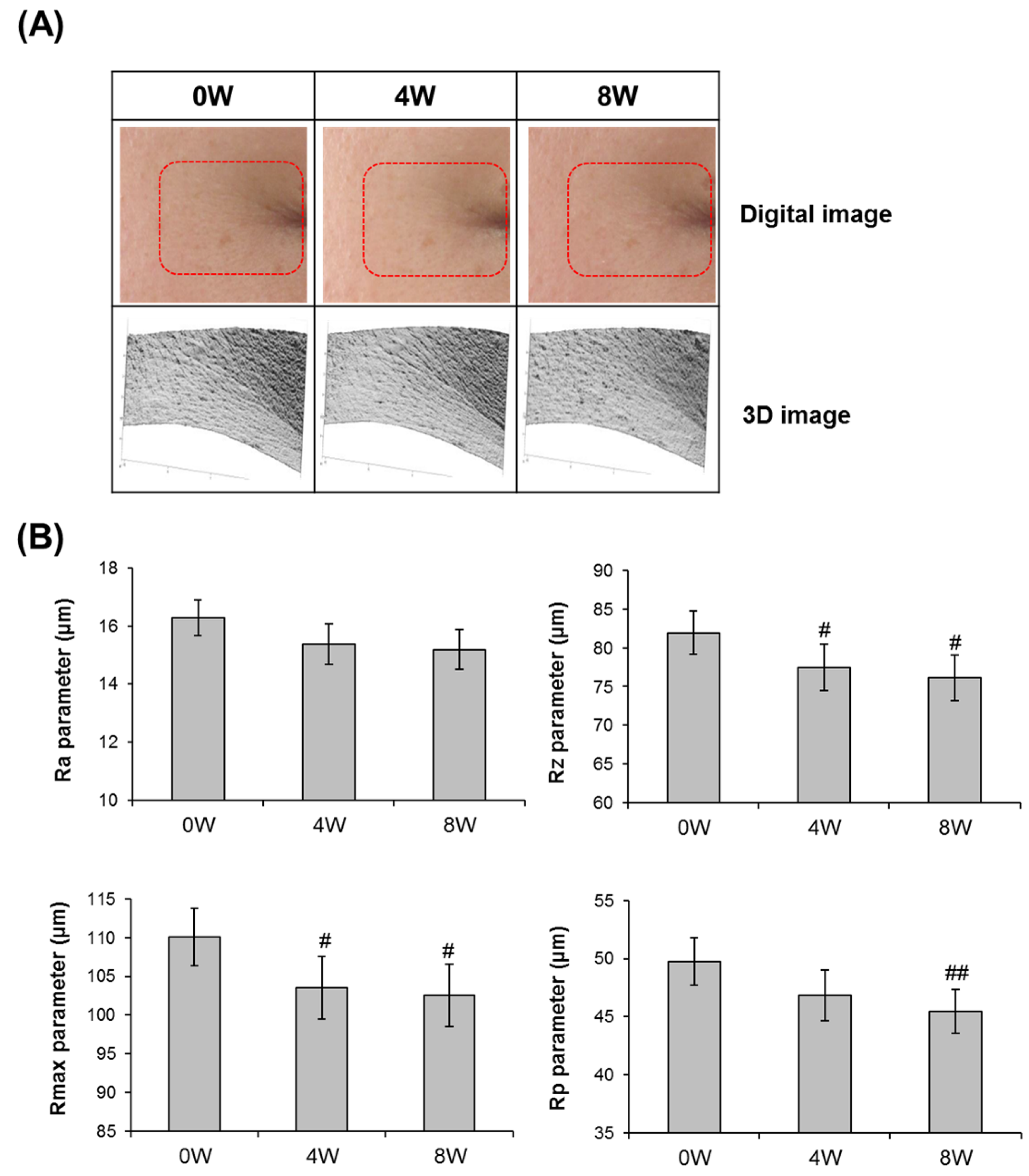
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verzijl, N.; DeGroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.; Lafeber, F.P.; Baynes, J.W.; TeKoppele, J.M. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.A.; Patrick, J.S.; Thorpe, S.R.; Baynes, J.W. Oxidation of glycated proteins: Age-dependent accumulation of N epsilon-(carboxymethyl) lysine in lens proteins. Biochemistry 1989, 28, 9464–9468. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Monnier, V.M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J. Biol. Chem. 1989, 264, 21597–21602. [Google Scholar] [PubMed]
- Jeanmaire, C.; Danoux, L.; Pauly, G. Glycation during human dermal intrinsic and actinic ageing: An in vivo and in vitro model study. Br. J. Dermatol. 2001, 145, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H. Reaction of glycation and human skin: The effects on the skin and its components, reconstructed skin as a model. Pathol. Biol. 2010, 58, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, Z.; Alikhani, M.; Boyd, C.M.; Nagao, K.; Trackman, P.C.; Graves, D.T. Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J. Biol. Chem. 2005, 280, 12087–12095. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.; Maclellan, C.M.; Raptis, M.; Vora, S.; Trackman, P.C.; Graves, D.T. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. Am. J. Physiol. Cell Physiol. 2007, 292, C850–C856. [Google Scholar] [CrossRef] [PubMed]
- Ravelojaona, V.; Robert, A.M.; Robert, L. Expression of senescence associated beta galactosidase (SA-beta-Gal) by human skin fibroblasts, effect of advanced glycation end-products and fucose or rhamnose-rich polysaccharides. Arch. Gerontol. Geriatr. 2009, 48, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Ravelojaona, V.; Péterszegi, G.; Molinari, J.; Gesztesi, J.L.; Robert, L. Demonstration of the cytotoxic effect of Advanced Glycation Endproducts (AGE-s). J. Soc. Biol. 2007, 201, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Okano, Y.; Masaki, H.; Sakurai, H. Dysfunction of dermal fibroblasts induced by advanced glycation end-products (AGEs) and the contribution of a nonspecific interaction with cell membrane and AGEs. J. Dermatol. Sci. 2002, 29, 171–180. [Google Scholar] [CrossRef]
- Molinari, J.; Ruszova, E.; Velebny, V.; Robert, L. Effect of advanced glycation end products on gene expression profiles of human dermal fibroblasts. Biogerontology 2008, 9, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Bakala, H.; Monnier, V.M.; Asselineau, D. Collagen glycation triggers the formation of aged skin in vitro. Eur. J. Dermatol. 2007, 17, 12–20. [Google Scholar] [PubMed]
- Barlovic, D.P.; Soro-Paavonen, A.; Jandeleit-Dahm, K.A. RAGE biology, atherosclerosis and diabetes. Clin. Sci. 2011, 121, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Reihsner, R.; Melling, M.; Pfeiler, W.; Menzel, E.J. Alterations of biochemical and two-dimensional biomechanical properties of human skin in diabetes mellitus as compared to effects of in vitro non-enzymatic glycation. Clin. Biomech. 2000, 15, 379–386. [Google Scholar] [CrossRef]
- Corstjens, H.; Dicanio, D.; Muizzuddin, N.; Neven, A.; Sparacio, R.; Declercq, L.; Maes, D. Glycation associated skin autofluorescence and skin elasticity are related to chronological age and body mass index of healthy subjects. Exp. Gerontol. 2008, 43, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zheng, Z.; Cheng, K.W.; Shan, F.; Ren, G.; Chen, F.; Wang, M. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008, 106, 475–481. [Google Scholar] [CrossRef]
- Kiho, T.; Usui, S.; Hirano, K.; Aizawa, K.; Inakuma, T. Tomato paste fraction inhibiting the formation of advanced glycation end-products. Biosci. Biotechnol. Biochem. 2004, 68, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Hsieh, C.L.; Wang, H.Y.; Chen, H.Y. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, J.; Zeng, X.; Huangfu, J.; Jiang, Y.; Wang, M.; Chen, F. Astaxanthin is responsible for antiglycoxidative properties of microalga Chlorella zofingiensis. Food Chem. 2011, 126, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, J.; Zeng, X.; Huangfu, J.; Jiang, Y.; Wang, M.; Chen, F. Protective actions of microalgae against endogenous and exogenous advanced glycation endproducts (AGEs) in human retinal pigment epithelial cells. Food Funct. 2011, 2, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Eisenbrand, G. Akebia quinata (Thunb.) Decne. In Chinese Drugs of Plant Origin; Springer: Heidelberg, Germany, 1992; pp. 59–67. [Google Scholar]
- Choi, W.H.; Seo, B.I. The effect of chekamuiyiin-tang on biochemical and histological changes of rats fed high diet. J. Korean Med. 2000, 21, 31–39. [Google Scholar]
- Rim, A.R.; Kim, S.J.; Jeom, K.I.; Park, E.; Park, H.R.; Lee, S.C. Antioxidant activity of extracts from Akebia quinata Decne. J. Food Sci. Nutr. 2006, 11, 84–87. [Google Scholar] [CrossRef]
- Chen, Y.F.; Roan, H.Y.; Lii, C.K.; Huang, Y.C.; Wang, T.S. Relationship between antioxidant and antiglycation ability of saponins, polyphenols, and polysaccharides in Chinese herbal medicines used to treat diabetes. J. Med. Plants Res. 2011, 5, 2322–2331. [Google Scholar]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 2013, 6, 561–569. [Google Scholar] [CrossRef]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, M.; de Craen, A.J.; de Meijer, P.H.; Beekman, M.; Blauw, G.J.; Slagboom, P.E.; Westendorp, R.G. Evidence of genetic enrichment for exceptional survival using a family approach: The Leiden Longevity Study. Eur. J. Hum. Genet. 2006, 14, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Roudkenar, M.H.; Halabian, R.; Ghasemipour, Z.; Roushandeh, A.M.; Rouhbakhsh, M.; Nekogoftar, M.; Kuwahara, Y.; Fukumoto, M.; Shokrgozar, M.A. Neutrophil gelatinase-associated lipocalin acts as a protective factor against H2O2 toxicity. Arch. Med. Res. 2008, 39, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.S.; Sung, H.Y.; Lim, H.M.; Kwon, K.S.; Park, S.S. PI3K-ERK1/2 activation contributes to extracellular H2O2 generation in amyloid β toxicity. Neurosci. Lett. 2012, 526, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Y.; Wang, R.; Xia, C.; Zhang, R.; Lian, K.; Luan, R.; Sun, L.; Yang, L.; Lau, W.B.; et al. Advanced glycation end products accelerate ischemia/reperfusion injury through receptor of advanced end product/nitrative thioredoxin inactivation in cardiac microvascular endothelial cells. Antioxid. Redox Signal. 2011, 15, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Byun, S.; Kim, S.; Kim, M.; Park, D.; Lee, J. Isomenthone protects human dermal fibroblasts from TNF-α-induced death possibly by preventing activation of JNK and p38 MAPK. Food Chem. Toxicol. 2012, 50, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Murrant, C.L.; Reid, M.B. Detection of reactive oxygen and reactive nitrogen species in skeletal muscle. Microsc. Res. Tech. 2001, 55, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, E.L.; Empsen, C.; Geerts, A.; van Grunsven, L.A. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J. Hepatol. 2010, 52, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Tsuruga, E.; Nakashima, K.; Sawa, Y.; Ishikawa, H. Fibulin-4 and -5, but not Fibulin-2, are Associated with Tropoelastin Deposition in Elastin-Producing Cell Culture. Acta Histochem. Cytochem. 2010, 43, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Yagi, M.; Nomoto, K.; Shimode, A.; Ogura, M.; Yonei, Y. Inhibition of advanced glycation end product formation by herbal teas and its relation to anti-skin aging. Antiaging Med. 2012, 9, 135–148. [Google Scholar]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structual and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.R.; Thornalley, P.J. Mechanism of the degradation of non-enzymatically glycated proteins under physiological conditions. Eur. J. Biochem. 1992, 210, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Ikeda, K.; Higashi, T.; Sano, H.; Jinnouchi, Y.; Araki, T.; Horiuchi, S. Hydroxyl radical mediates Nᵋ-(Carboxymethyl) lysine formation from Amadori product. Biochem. Biophys. Res. Commun. 1997, 234, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Huang, S.M.; Yen, G.C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011, 2, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Elosta, A.; Ghous, T.; Ahmed, N. Natural products as antiglycation agents: Possible therapeutic potential for diabetic complications. Curr. Diabetes Rev. 2012, 8, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.V.; Bottoms, M.A.; Mitchinson, M.J. Oxidative alterations in the experimental glycation model of diabetes mellitus are due to protein-glucose adduct oxidation. Some fundamental differences in proposed mechanisms of glucose oxidation and oxidant production. Biochem. J. 1993, 291 Pt 2, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, R.; Haenen, G.R.M.M.; van den Berg, H.; Bast, A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Benzie, I.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Onder, T.T.; Young, J.J.; McFaline, J.L.; Pang, B.; Dedon, P.C.; Weinberg, R.A. Continuous elimination of oxidized nucleotides is necessary to prevent rapid onset of cellular senescence. Proc. Natl. Acad. Sci. USA 2009, 106, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, M.; Naaldijk, Y.; Stolzing, A.F. Regulating aging in adult stem cells with microRNA. Z. Gerontol. Geriatr. 2013, 46, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar] [PubMed]
- Ramakrishnan, S.; Sulochana, K.N.; Punitham, R. Two new functions of inositol in the eye lens: Antioxidation and antiglycation and possible mechanisms. Indian J. Biochem. Biophys. 1999, 36, 129–133. [Google Scholar] [PubMed]
- Gülçin, I.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. The antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(beta-d-glucopyranosyl)-hederagenin. Phytother. Res. 2006, 20, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.; Arnold, F.; Peno-Mazzarino, L.; Bouzoud, D.; Luu, M.T.; Lati, E.; Mercier, M. Glycation induction and antiglycation activity of skin care ingredients on living human skin explants. Int. J. Cosmet. Sci. 2011, 33, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeong, I.H.; Kim, C.S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch. Pharm. Res. 2011, 34, 495–500. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Son, D.; Kim, M.; Lee, S.; Roh, K.-B.; Ryu, D.; Lee, J.; Jung, E.; Park, D. Ameliorating Effect of Akebia quinata Fruit Extracts on Skin Aging Induced by Advanced Glycation End Products. Nutrients 2015, 7, 9337-9352. https://doi.org/10.3390/nu7115478
Shin S, Son D, Kim M, Lee S, Roh K-B, Ryu D, Lee J, Jung E, Park D. Ameliorating Effect of Akebia quinata Fruit Extracts on Skin Aging Induced by Advanced Glycation End Products. Nutrients. 2015; 7(11):9337-9352. https://doi.org/10.3390/nu7115478
Chicago/Turabian StyleShin, Seoungwoo, Dahee Son, Minkyung Kim, Seungjun Lee, Kyung-Baeg Roh, Dehun Ryu, Jongsung Lee, Eunsun Jung, and Deokhoon Park. 2015. "Ameliorating Effect of Akebia quinata Fruit Extracts on Skin Aging Induced by Advanced Glycation End Products" Nutrients 7, no. 11: 9337-9352. https://doi.org/10.3390/nu7115478
APA StyleShin, S., Son, D., Kim, M., Lee, S., Roh, K.-B., Ryu, D., Lee, J., Jung, E., & Park, D. (2015). Ameliorating Effect of Akebia quinata Fruit Extracts on Skin Aging Induced by Advanced Glycation End Products. Nutrients, 7(11), 9337-9352. https://doi.org/10.3390/nu7115478





