Association of Dietary Vitamin A and β-Carotene Intake with the Risk of Lung Cancer: A Meta-Analysis of 19 Publications
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
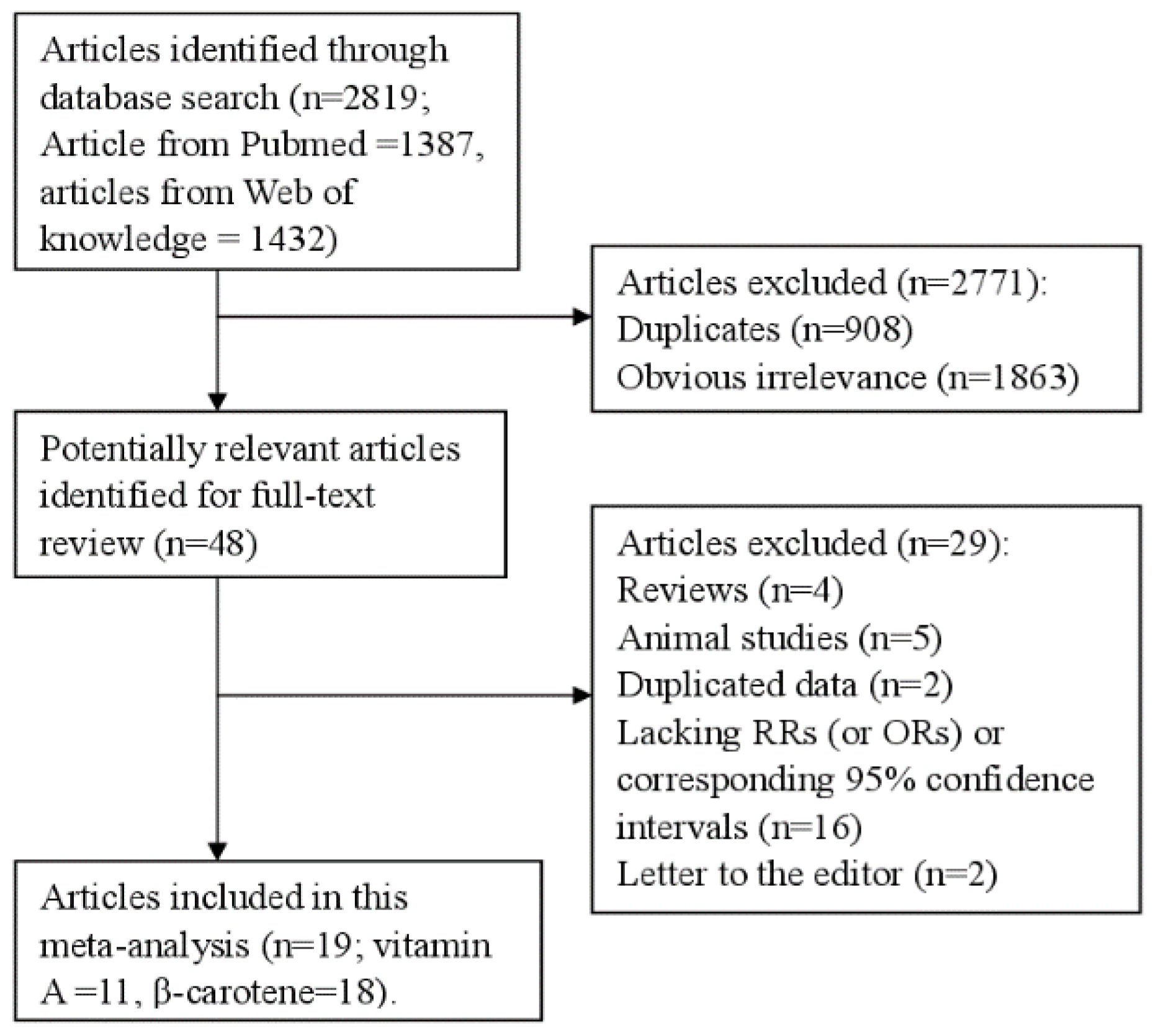
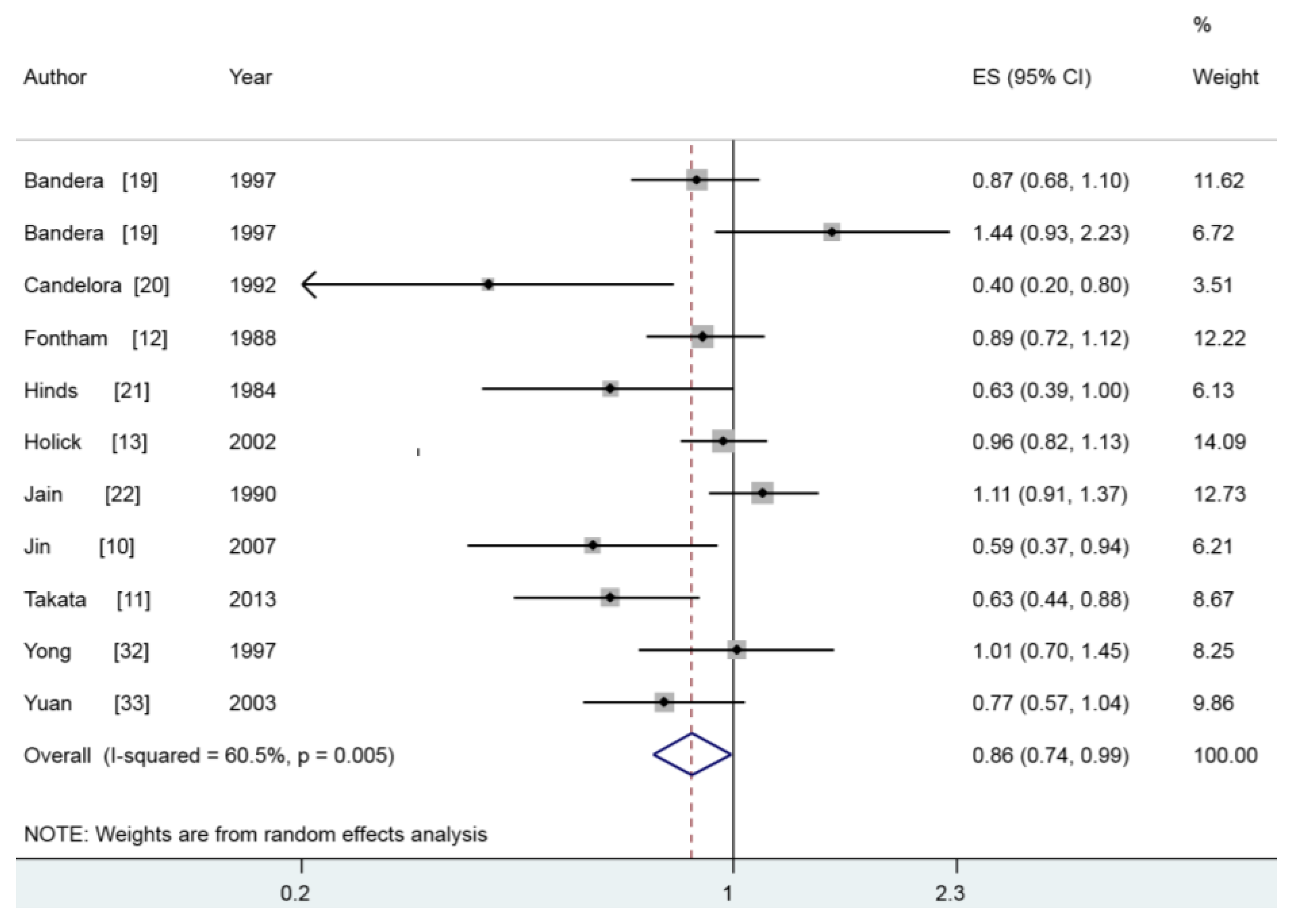
3.2. Vitamin A and Lung Cancer
| Study, Year | Country | Study Design | Participants (Cases) | Age (Years) | Categories | RR (95% CI) for Each Category | Adjustment for Covariates |
|---|---|---|---|---|---|---|---|
| Bandera et al., 1997 [19] | United States | Prospective | 48,000 (525) | 40–80 | Vitamin A Males Tertiles 1 Tertiles 2 Tertiles 3 Females Tertiles 1 Tertiles 2 Tertiles 3 | Vitamin A Males 1 0.82 (0.64–1.05) 0.87(0.68–1.10) Females 1 1.19(0.75–1.88) 1.44(0.93–2.23) | Adjusted for age, education, cigarettes/day, years smoking, and total energy intake (except calories) based on Cox Proportional Hazards Model. |
| Candelora et al., 1992 [20] | United States | Case-control | 387 (124) | Case: 71.9 Control: 69.8 | Vitamin A Quartile 1 Quartile 2 Quartile 3 Quartile 4 β-carotene Quartile 1 Quartile 2 Quartile 3 Quartile 4 | Vitamin A 1 0.60(0.30–1.20) 0.60 (0.30–1.20) 0.40 (0.20–0.80) β-carotene 1 0.50 (0.30–1.00) 0.50 (0.30–0.90) 0.40 (0.20–0.80) | Adjusted for age, education (≤8 and >8 grades), and total calories. |
| Fontham et al., 1988 [12] | United States | Case-control | 2527 (1253) | <40–≥70 | Vitamin A Low Moderate High β-carotene Low Moderate High | Vitamin A 1 0.85 (0.68–1.06) 0.89 (0.72–1.12) β-carotene 1 0.96 (0.76–1.20) 0.88 (0.70–1.11) | Adjusted in logistic regression model for age, race, sex, and pack years of cigarette use. |
| Hinds et al., 1984 [21] | United States | Case-control | 991 (364) | ≥30 | Vitamin A (IU) 0–51,799 51,800–78,099 78,100–115,199 115,200 + | Vitamin A 1 0.88 (0.49–1.26) 1.06 (0.58–1.54) 0.63 (0.39–1.00) | Adjustment by multiple logistic regression for age, ethnicity, cholesterol intake, occupational status, vitamin A intake, pack-years of cigarette smoking, and sex where appropriate. |
| Holick et al., 2002 [13] | Finland | Prospective | 27,084 (1644) | 50–69 | Vitamin A (μg/day) <717 717–1044 1045–1481 1482–2138 >2138 β-carotene (μg/day) <977 977–1440 1441–2029 2030–3015 >3015 | Vitamin A 1 0.97(0.83–1.14) 1.02(0.87–1.20) 1.03(0.88–1.21) 0.96(0.82–1.13) β-carotene 1 0.92(0.79–1.06) 0.90(0.78–1.04) 0.79(0.68–0.92) 0.92 (0.79–1.07) | Adjusted for age, years smoked cigarettes per day, intervention (α-tocopherol and β-carotene supplement), supplement use (β-carotene and vitamin A), energy intake, cholesterol, and fat. |
| Jain et al., 1990 [22] | Canada | Case-control | 1611 (839) | 20–75 | Vitamin A Highest vs. Lowest β-carotene Highest vs. Lowest | Vitamin A 1.11 (0.91–1.37) β-carotene 1.00 (0.79–1.27) | Adjusted for cumulative cigarette smoking |
| Jin et al., 2007 [10] | China | Case-control | 903 (301) | ≤80 | Vitamin A (RE/day) ≤947 947–1742 1742–3630 ≥3630 β-carotene (μg/day) ≤3734 3735–7440 7440–15,363 ≥15,363 | Vitamin A 1 0.78 (0.51–1.18) 0.62 (0.41–0.95) 0.59 (0.37–0.94) β-carotene 1 0.74 (0.49–1.12) 0.69 (0.45–1.06) 0.52 (0.32–0.83) | Adjusted for pack-years of cigarette smoking, occupational exposure, passive smoking exposure from mother and friends, medical insurance status and education levels. |
| Le Marchand et al., 1989 [23] | United States | Case-control | 1197 (332) | 30–85 | β-carotene Males Quartile 1 Quartile 2 Quartile 3 Quartile 4 Females Quartile 1 Quartile 2 Quartile 3 Quartile 4 | β-carotene Males 1 1.25 (0.76–1.74) 0.81 (0.52–1.10) 0.63 (0.36–1.11) Females 1 0.81 (0.51–1.11) 0.62 (0.31–0.93) 0.39 (0.16–1.00) | Adjusted for age, ethnicity, smoking status, pack-years of cigarette smoking, cholesterol intake (for males only), and intakes of other nutrients in the table. |
| Neuhouser et al., 2003 [24] | United States | Prospective | 14,120 (742) | Case: 60.4 Control: 57.6 | β-carotene (μg/day) ≤1156 1157–1714 1715–2331 2332–3428 ≥3429 | β-carotene 1 0.90 (0.63–1.28) 0.92 (0.65–1.30) 1.03 (0.73–1.45) 0.95 (0.67–1.36) | Adjusted for sex, age, smoking status, total pack-years of smoking, asbestos exposure, race/ethnicity, and enrollment center. |
| Ocke et al., 1997 [25] | Netherlands | Prospective | 561 (54) | Case: 59.3 Control: 59.5 | β-carotene (mg) <1.07 1.07–1.31 >1.31 | β-carotene 1 0.61 (0.21–1.04) 0.74 (0.41–1.35) | Adjusted for age, pack-years of cigarettes, and energy intake, |
| Rohan et al., 2002 [26] | Canada | Prospective | 5516 (155) | 40–59 | β-carotene Quartile 1 Quartile 2 Quartile 3 Quartile 4 | β-carotene 1 1.78 (1.04–3.05) 1.83 (1.03–3.24) 1.40 (0.76–2.59) | Adjusted for age, study allocation, study center, cigarette smoking, vitamin C intake, folate intake, dietary fiber intake, and energy intake. |
| Speizer et al., 1999 [27] | United States | Prospective | 121,700 (593) | 30–55 | β-carotene Q5 vs.Q1 | β-carotene 0.80 (0.60–1.11) | Age, total energy intake, smoking (past and current amount in 1980; 1±4, 5±14, 15±24, 25±34, 35±44, 45+) and age of starting to smoke. |
| Stefani et al., 1999 [28] | Uruguay | Case-control | 981 (541) | 30–89 | β-carotene (μg/day) <1938 1939–3330 3331–5862 ≥5863 | β-carotene 1 0.83 (0.56–1.22) 0.61 (0.42–0.89) 0.42 (0.28–0.63) | Adjusted for age, residence, urban/rural status, education, family history of a lung cancer in 1st-degree relative, body mass index, tobacco smoking (pack-yr), and total energy and total fat intakes, IQR, interquartile range. |
| Steinmetz et al., 1993 [29] | United States | Prospective | 41,837 (179) | 55–69 | β-carotene Quartile 1 Quartile 2 Quartile 3 Quartile 4 | β-carotene 1 0.76 (0.47–1.25) 0.67 (0.39–1.14) 0.81 (0.48–1.38) | Adjusted by inclusion of continuous variables for age, energy intake, and pack-years of smoking in multivariate logistic regression models. |
| Takata et al., 2013 [11] | China | Prospective | 61,491 (359) | 40–74 | Vitamin A (μg/day) 359.4 549.8 729.2 1046.1 β-carotene (μg/day) 1449.8 2045.8 3346.9 5025.5 | Vitamin A 1 0.86 (0.65–1.13) 0.85 (0.64–1.14) 0.63 (0.44–0.88) β-carotene 1 0.83 (0.63–1.09) 0.82 (0.62–1.10) 0.64 (0.46–0.88) | Adjusted for age, years of smoking, the number of cigarettes smoked per day, current smoking status, total caloric intake, education, BMI category, ever consumption of tea, history of chronic bronchitis, and family history of lung cancer among first-degree relatives. |
| Voorrips et al., 2000 [30] | Netherlands | Prospective | 58,279 (939) | 55–69 | β-carotene Quartile 1 Quartile 2 Quartile 3 Quartile 4 Quartile 5 | β-carotene 1 0.83 (0.60–1.14) 1.00 (0.72–1.39) 1.14 (0.80–1.62) 1.11 (0.76–1.60) | Adjusted for current smoking, years of smoking cigarettes, number of cigarettes per day, highest educational level, family history of lung cancer, and age. |
| Wright et al., 2003 [31] | United States | Case-control | 1211 (587) | 35–84 | β-carotene (μg/day) <823.58 823.58–1145.95 1145.96–1526.06 1526.07–2323.54 >2323.54 | β-carotene 1 0.71 (0.49–1.00) 0.60 (0.41–0.87) 0.71 (0.48–1.10) 0.58 (0.39–0.86) | Adjusted for age, total calorie intake, pack-years of smoking, and education. |
| Yong et al., 1997 [32] | United States | Prospective | 1068 (248) | 25–74 | Vitamin A (IU) 1 2 3 4 β-carotene (IU) 1 2 3 4 | Vitamin A 1 0.98 (0.68–1.39) 0.98 (0.69–1.40) 1.01 (0.70–1.45) β-carotene 1 0.66 (0.46–0.94) 0.78 (0.56–1.10) 0.74 (0.52–1.06) | Adjusted for sex race, educational attainment, nonrecreabonal activity level, body mass index, family history, smoking status/pack-years of smoking, total calorie intake, and alcohol intake. |
| Yuan et al., 2003 [33] | China | Prospective | 63,257 (482) | 45–74 | Vitamin A Quartile 1 Quartile 2 Quartile 3 Quartile 4 Quartile 5 β-carotene Quartile 1 Quartile 2 Quartile 3 Quartile 4 Quartile 5 | Vitamin A 1 0.71 (0.54–0.92) 0.75 (0.58–0.99) 0.99 (0.76–1.28) 0.77 (0.57–1.04) β-carotene 1 0.77 (0.59–1.00) 0.81 (0.62–1.06) 0.98 (0.75–1.28) 0.85 (0.63–1.14) | Adjusted for age at baseline, sex, dialect group, year of interview, level of education, and BMI, number of cigarettes smoked per day, number of years of smoking, and number of years since quitting smoking for former smokers. |
| Subgroups | No. | No. | Risk Estimate (95% CI) | Heterogeneity Test | |
|---|---|---|---|---|---|
| (Cases) | Studies | I2 (%) | p-value | ||
| Vitamin A | 6139 | 11 | 0.855 (0.739–0.989) | 60.5 | 0.005 |
| Study design | |||||
| Prospective | 3258 | 6 | 0.869 (0.758–0.980) | 52.9 | 0.060 |
| Case-control | 2881 | 5 | 0.754 (0.560–1.016) | 72.7 | 0.005 |
| Geographic locations | |||||
| America | 3353 | 7 | 0.915 (0.751–1.114) | 61.4 | 0.016 |
| Asia | 1142 | 3 | 0.682 (0.556–0.837) | 0.0 | 0.549 |
| Sex | |||||
| Males | 1981 | 4 | 0.697 (0.553–0.879) | 38.5 | 0.181 |
| Females | 644 | 4 | 0.811 (0.415–1.584) | 73.8 | 0.010 |
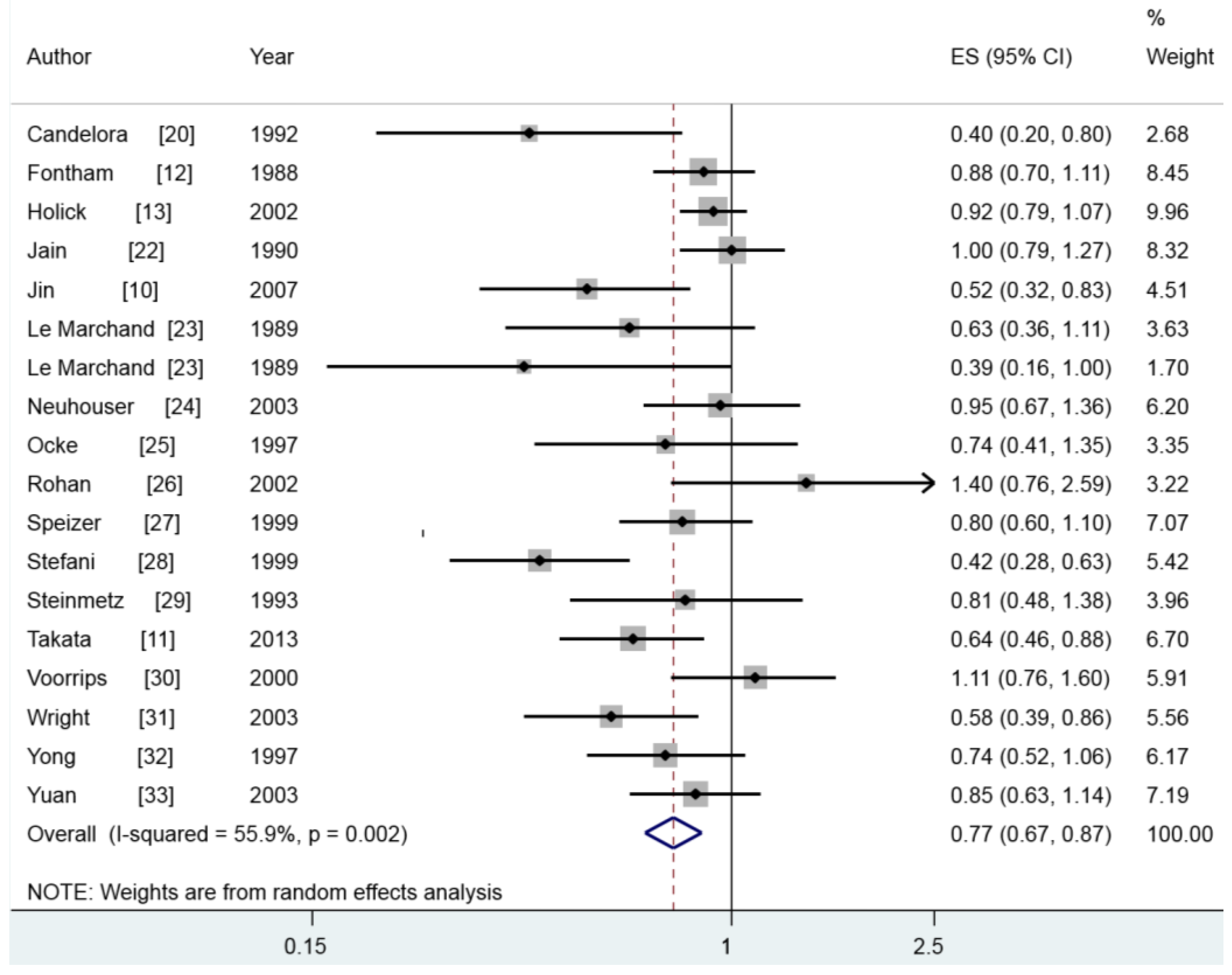
| β-Carotene | 9372 | 18 | 0.768 (0.675–0.874) | 55.9 | 0.002 |
| Study design | |||||
| Prospective | 5395 | 10 | 0.867 (0.782–0.962) | 6.5 | 0.382 |
| Case-control | 3977 | 8 | 0.616 (0.469–0.809) | 71.6 | 0.001 |
| Geographic locations | |||||
| America | 5593 | 12 | 0.742 (0.618–0.890) | 59.9 | 0.004 |
| Europe | 2637 | 3 | 0.933 (0.814–1.070) | 0.0 | 0.484 |
| Asia | 1142 | 3 | 0.685 (0.523–0.896) | 41.8 | 0.180 |
| Sex | |||||
| Males | 2494 | 4 | 0.786 (0.612–1.010) | 45.0 | 0.142 |
| Females | 2027 | 7 | 0.730 (0.549–0.972) | 49.0 | 0.067 |
| Histological type | |||||
| Squamous cell carcinoma | 1039 | 6 | 0.693 (0.480–0.982) | 71.2 | 0.004 |
| Small cell carcinoma | 228 | 3 | 0.654 (0.416–1.027) | 0.0 | 0.863 |
| Adenocarcinoma | 609 | 6 | 0.695 (0.452–1.069) | 68.1 | 0.008 |
3.3. β-Carotene and Lung Cancer
3.4. Meta-Regression
3.5. Sensitivity Analysis and Small-Study Effect
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, C.; Tao, H.; Cheng, Y.; Han, L.; Li, X.; Hu, Y. Statin use and risk of lung cancer: A meta-analysis of observational studies and randomized controlled trials. PLoS ONE 2013, 8, e77950. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shen, L.; Zheng, D. Association between vitamin C intake and lung cancer: A dose-response meta-analysis. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, J.; Hong, X.; Chai, Z.; Li, Q. Dietary vitamin E intake could reduce the risk of lung cancer: Evidence from a meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 6631–6637. [Google Scholar] [PubMed]
- D’Archivio, M.; Santangelo, C.; Scazzocchio, B.; Vari, R.; Filesi, C.; Masella, R.; Giovannini, C. Modulatory effects of polyphenols on apoptosis induction: Relevance for cancer prevention. Int. J. Mol. Sci. 2008, 9, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Khoshyomn, S.; Nathan, D.; Manske, G.C.; Osler, T.M.; Penar, P.L. Synergistic effect of genistein and BCNU on growth inhibition and cytotoxicity of glioblastoma cells. J. Neurooncol. 2002, 57, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.A.; Eldridge, A.L.; Peters, J.C. Dietary carotenoids and lung cancer: A review of recent research. Nutr. Rev. 1999, 57, 133–145. [Google Scholar] [CrossRef] [PubMed]
- WCRF/AICR. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. WCRF/AICR: Washington, DC, USA, 2007; Available online: http://www.dietandcancerreport.org/cancer_resource_center/downloads/summary/spanish.pdf (accessed on 30 October 2015).
- Jin, Y.R.; Lee, M.S.; Lee, J.H.; Hsu, H.K.; Lu, J.Y.; Chao, S.S.; Chen, K.T.; Liou, S.H.; Ger, L.P. Intake of vitamin A-rich foods and lung cancer risk in Taiwan: With special reference to garland chrysanthemum and sweet potato leaf consumption. Asia Pac. J. Clin. Nutr. 2007, 16, 477–488. [Google Scholar] [PubMed]
- Takata, Y.; Xiang, Y.B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.T.; Zheng, W.; Shu, X.O. Intakes of fruits, vegetables, and related vitamins and lung cancer risk: Results from the Shanghai Men’s Health Study (2002–2009). Nutr. Cancer 2013, 65, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Fontham, E.T.; Pickle, L.W.; Haenszel, W.; Correa, P.; Lin, Y.P.; Falk, R.T. Dietary vitamins A and C and lung cancer risk in Louisiana. Cancer 1988, 62, 2267–2273. [Google Scholar] [CrossRef]
- Holick, C.N.; Michaud, D.S.; Stolzenberg-Solomon, R.; Mayne, S.T.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Dietary carotenoids, serum β-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, β-carotene cohort study. Am. J. Epidemiol. 2002, 156, 536–547. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Controlling the risk of spurious findings from meta-regression. Stat. Med. 2004, 23, 1663–1682. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Tobias, A. Assessing the in fluence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999, 47, 15–17. [Google Scholar]
- Bandera, E.V.; Freudenheim, J.L.; Marshall, J.R.; Zielezny, M.; Priore, R.L.; Brasure, J.; Baptiste, M.; Graham, S. Diet and alcohol consumption and lung cancer risk in the New York State Cohort (United States). Cancer Causes Control 1997, 8, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Candelora, E.C.; Stockwell, H.G.; Armstrong, A.W.; Pinkham, P.A. Dietary intake and risk of lung cancer in women who never smoked. Nutr. Cancer 1992, 17, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Hinds, M.W.; Kolonel, L.N.; Hankin, J.H.; Lee, J. Dietary vitamin A, carotene, vitamin C and risk of lung cancer in Hawaii. Am. J. Epidemiol. 1984, 119, 227–237. [Google Scholar] [PubMed]
- Jain, M.; Burch, J.D.; Howe, G.R.; Risch, H.A.; Miller, A.B. Dietary factors and risk of lung cancer: Results from a case-control study, Toronto, 1981–1985. Int. J. Cancer 1990, 45, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Le Marchand, L.; Yoshizawa, C.N.; Kolonel, L.N.; Hankin, J.H.; Goodman, M.T. Vegetable consumption and lung cancer risk: A population-based case-control study in Hawaii. J. Natl. Cancer Inst. 1989, 81, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Patterson, R.E.; Thornquist, M.D.; Omenn, G.S.; King, I.B.; Goodman, G.E. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the β-carotene and retinol efficacy trial (CARET). Cancer Epidemiol. Biomark. Prev. 2003, 12, 350–358. [Google Scholar]
- Ocke, M.C.; Bueno-de-Mesquita, H.B.; Feskens, E.J.; van Staveren, W.A.; Kromhout, D. Repeated measurements of vegetables, fruits, β-carotene, and vitamins C and E in relation to lung cancer. The Zutphen Study. Am. J. Epidemiol. 1997, 145, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Rohan, T.E.; Jain, M.; Howe, G.R.; Miller, A.B. A cohort study of dietary carotenoids and lung cancer risk in women (Canada). Cancer Causes Control 2002, 13, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Speizer, F.E.; Colditz, G.A.; Hunter, D.J.; Rosner, B.; Hennekens, C. Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA). Cancer Causes Control 1999, 10, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Stefani, E.D.; Boffetta, P.; Deneo-Pellegrini, H.; Mendilaharsu, M.; Carzoglio, J.C.; Ronco, A.; Olivera, L. Dietary antioxidants and lung cancer risk: A case-control study in Uruguay. Nutr. Cancer 1999, 34, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.A.; Potter, J.D.; Folsom, A.R. Vegetables, fruit, and lung cancer in the Iowa Women’s Health Study. Cancer Res. 1993, 53, 536–543. [Google Scholar] [PubMed]
- Voorrips, L.E.; Goldbohm, R.A.; Brants, H.A.; van Poppel, G.A.; Sturmans, F.; Hermus, R.J.; van den Brandt, P.A. A prospective cohort study on antioxidant and folate intake and male lung cancer risk. Cancer Epidemiol. Biomark. Prev. 2000, 9, 357–365. [Google Scholar]
- Wright, M.E.; Mayne, S.T.; Swanson, C.A.; Sinha, R.; Alavanja, M.C. Dietary carotenoids, vegetables, and lung cancer risk in women: The Missouri women’s health study (United States). Cancer Causes Control 2003, 14, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Yong, L.C.; Brown, C.C.; Schatzkin, A.; Dresser, C.M.; Slesinski, M.J.; Cox, C.S.; Taylor, P.R. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1997, 146, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.M.; Stram, D.O.; Arakawa, K.; Lee, H.P.; Yu, M.C. Dietary cryptoxanthin and reduced risk of lung cancer: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2003, 12, 890–898. [Google Scholar]
- The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Epstein, K.R. The role of carotenoids on the risk of lung cancer. Semin. Oncol. 2003, 30, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Roberts, A.B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983, 43, 3034–3040. [Google Scholar] [PubMed]
- Clinton, S.K.; Emenhiser, C.; Schwartz, S.J.; Bostwick, D.G.; Williams, A.W.; Moore, B.J.; Erdman, J.W., Jr. Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol. Biomark. Prev. 1996, 5, 823–833. [Google Scholar]
- Xu, X.; Yu, E.; Liu, L.; Zhang, W.; Wei, X.; Gao, X.; Song, N.; Fu, C. Dietary intake of vitamins A, C, and E and the risk of colorectal adenoma: A meta-analysis of observational studies. Eur. J. Cancer Prev. 2013, 22, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, H.; Chen, J.; Shi, Y.; Cai, J.; Yang, J.; Wu, Y. Association between dietary antioxidant vitamins intake/blood level and risk of gastric cancer. Int. J. Cancer 2014, 135, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, T.; Meng, Q.; Zhai, S. Association of carotenoids with risk of gastric cancer: A meta-analysis. Clin. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ye, Y.; Shi, Y.; Li, P.; Xu, J.; Chen, K.; Xu, E.; Yang, J. Association between vitamin A, retinol intake and blood retinol level and gastric cancer risk: A meta-analysis. Clin. Nutr. 2015, 34, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qin, S.; Zhang, T.; Song, X.; Zhang, S. The effect of fruit and vegetable intake on the development of lung cancer: A meta-analysis of 32 publications and 20 414 cases. Eur. J. Clin. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Van’tVeer, P.; Jansen, M.C.; Klerk, M.; Kok, F.J. Fruits and vegetables in the prevention of cancer and cardiovascular disease. Public Health Nutr. 2000, 3, 103–107. [Google Scholar] [PubMed]
- Ziegler, R.G. Vegetables, fruits, and carotenoids and the risk of cancer. Am. J. Clin. Nutr. 1991, 53, 251S–259S. [Google Scholar] [PubMed]
- Wettasinghe, M.; Bolling, B.; Plhak, L.; Xiao, H.; Parkin, K. Phase II enzyme-inducing and antioxidant activities of beetroot (Beta vulgaris L.) extracts from phenotypes of different pigmentation. J. Agric. Food Chem. 2002, 50, 6704–6709. [Google Scholar] [CrossRef] [PubMed]
- Munafo, M.R.; Flint, J. Meta-analysis of genetic association studies. Trends Genet. 2004, 20, 439–444. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, N.; Su, X.; Wang, Z.; Dai, B.; Kang, J. Association of Dietary Vitamin A and β-Carotene Intake with the Risk of Lung Cancer: A Meta-Analysis of 19 Publications. Nutrients 2015, 7, 9309-9324. https://doi.org/10.3390/nu7115463
Yu N, Su X, Wang Z, Dai B, Kang J. Association of Dietary Vitamin A and β-Carotene Intake with the Risk of Lung Cancer: A Meta-Analysis of 19 Publications. Nutrients. 2015; 7(11):9309-9324. https://doi.org/10.3390/nu7115463
Chicago/Turabian StyleYu, Na, Xinming Su, Zanfeng Wang, Bing Dai, and Jian Kang. 2015. "Association of Dietary Vitamin A and β-Carotene Intake with the Risk of Lung Cancer: A Meta-Analysis of 19 Publications" Nutrients 7, no. 11: 9309-9324. https://doi.org/10.3390/nu7115463
APA StyleYu, N., Su, X., Wang, Z., Dai, B., & Kang, J. (2015). Association of Dietary Vitamin A and β-Carotene Intake with the Risk of Lung Cancer: A Meta-Analysis of 19 Publications. Nutrients, 7(11), 9309-9324. https://doi.org/10.3390/nu7115463




