Effects of Black Adzuki Bean (Vigna angularis) Extract on Proliferation and Differentiation of 3T3-L1 Preadipocytes into Mature Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Reagents
2.2. Determination of Antioxidant Activity
2.3. Cell Culture and Differentiation
2.4. MTT Assay
2.5. Bromodeoxyuridine Incorporation Assays
2.6. Triglyceride Assay and Oil Red O Staining
2.7. Quantitative Real-Time Polymerase Chain Reaction Analysis
2.8. Statistical Analysis
3. Results
3.1. Antioxidant Activity of Bean Extracts
| IC50(mg/mL) | Ascorbic Acid | Trolox | BAB | RAB | BB |
|---|---|---|---|---|---|
| DPPH | 0.06 ± 0.01 d | 0.05 ± 0.02 d | 0.98 ± 0.01 c | 1.34 ± 0.02 b | 9.59 ± 0.14 a |
| ABTS | 0.10 ± 0.02 d | 0.19 ± 0.01 d | 2.00 ± 0.07 c | 2.53 ± 0.04 b | 7.35 ± 0.09 a |
3.2. Effects of Black Adzuki Bean on Proliferation
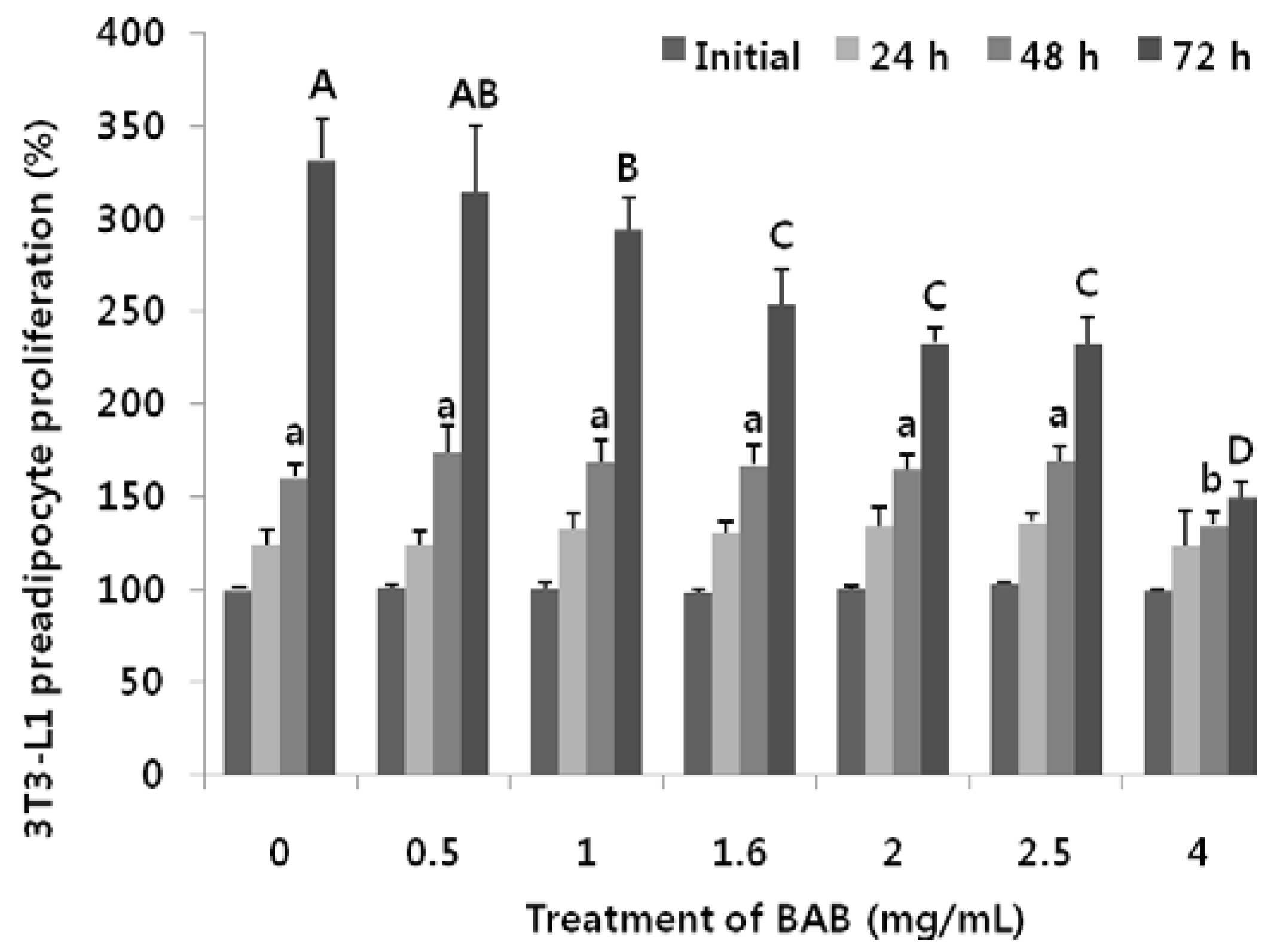
3.3. Antiadipogenic Function of Black Adzuki Bean in the Early Phase of Adipogenesis


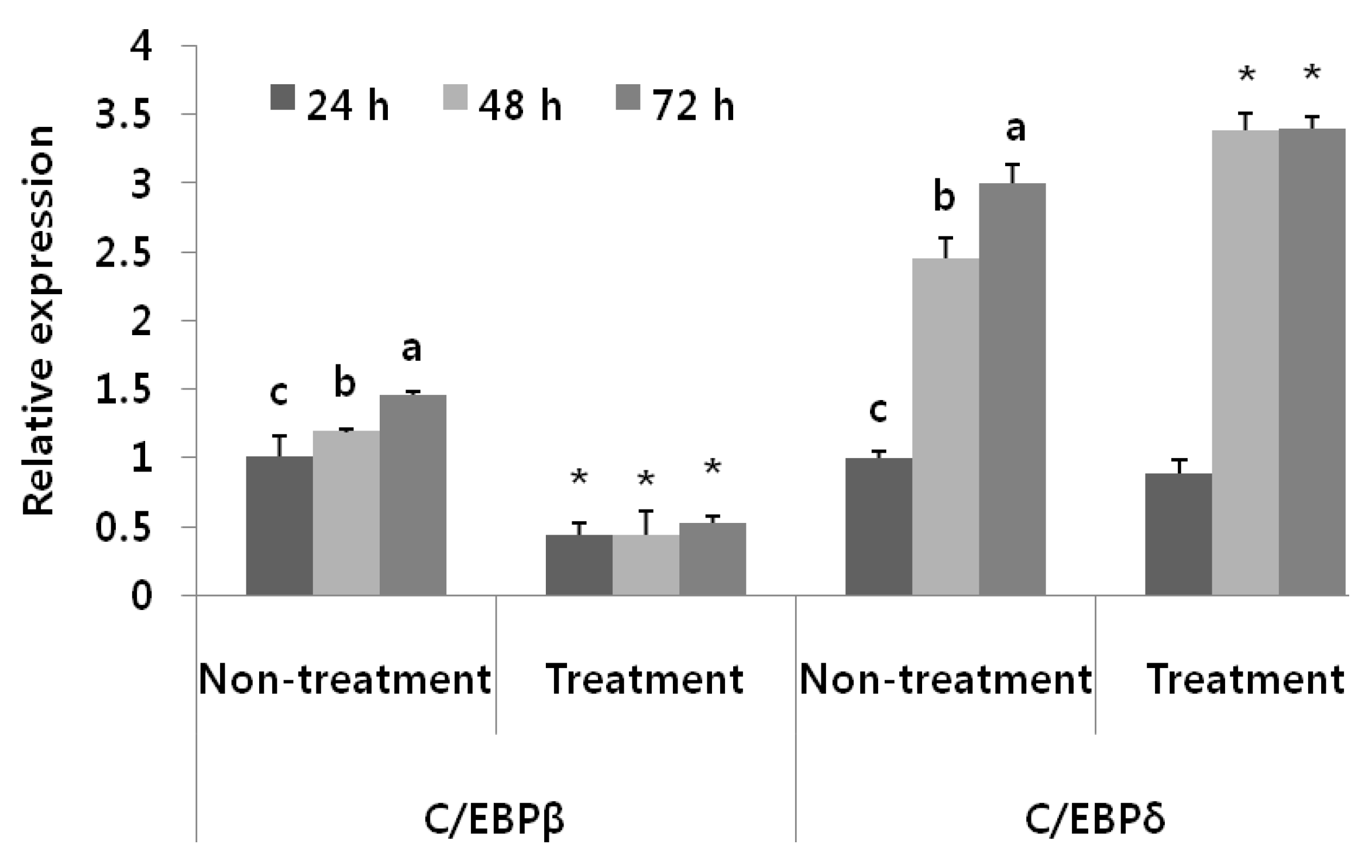
3.4. Adipogenic Levels and Lipid Metabolic Gene Profiles Were Highly Modulated Due to Black Adzuki Bean Extract Treatment

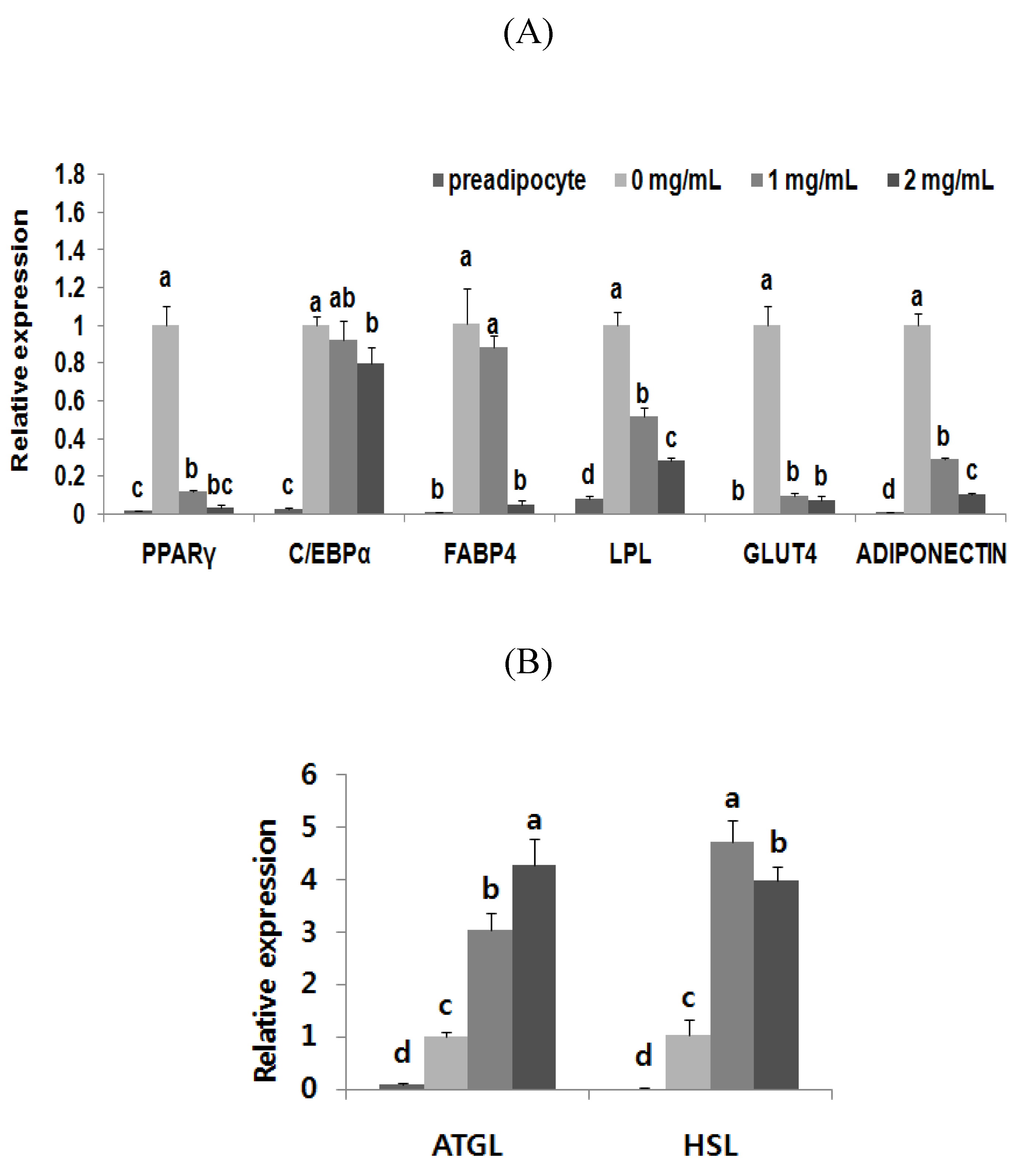
4. Discussion
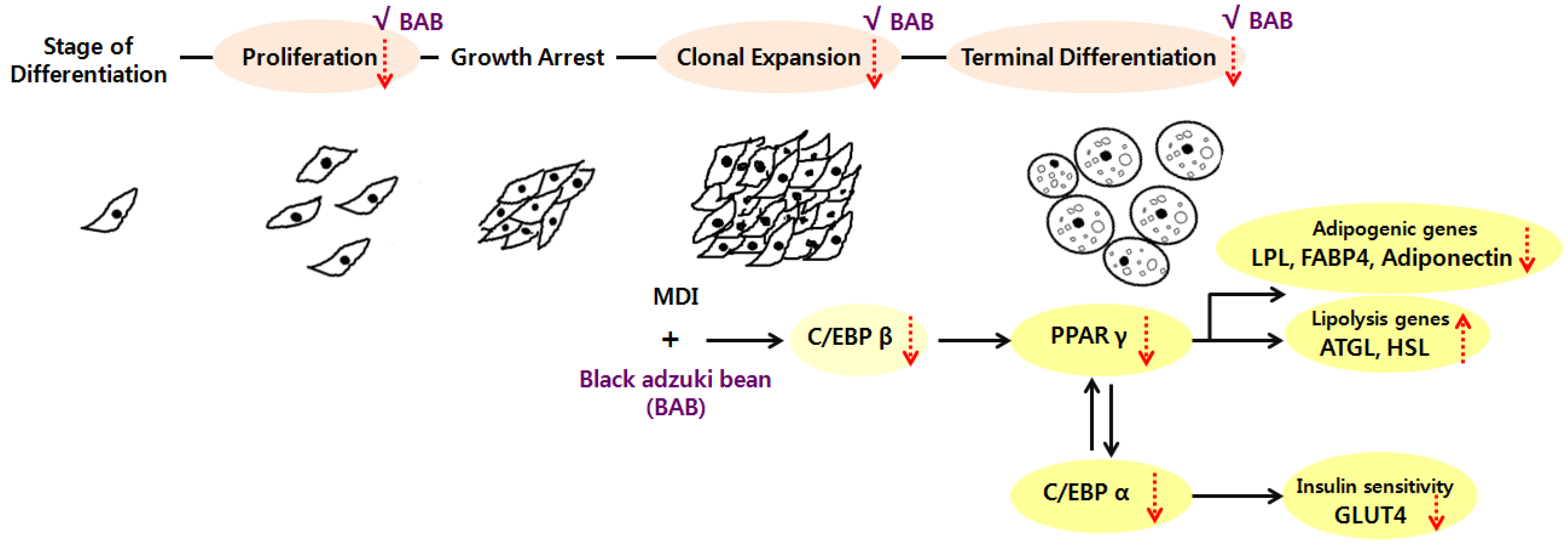
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Phytochemicals and regulation of the adipocyte life cycle. J. Nutr. Biochem. 2008, 19, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.; Afandi, B.; AbuHaltem, M.; Yassin, J.; Habib, H.; Ibrahim, W. Oxidative damage and inflammation in obese diabetic Emirati subjects supplemented with antioxidants and B-vitamins: A randomized placebo-controlled trail. Nutr. Metab. 2013, 10, 21–28. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Fu, M.; Bookout, A.L.; Kliewer, S.A.; Mangelsdorf, D.J. MicroRNA let-7 regulates 3T3-L1 adipogenesis. Mol. Endocrinol. 2009, 23, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Tanaka, M.; Takanashi, M.; Hussain, A.; Yuan, B.; Toyoda, H.; Kuroda, M. Anthocyanidins-enriched bilberry extracts inhibit 3T3-L1 adipocyte differentiation via the insulin pathway. Nutr. Metab. 2011, 8, 1–9. [Google Scholar] [CrossRef]
- Yu, T.; Ahn, H.M.; Shen, T.; Yoon, K.J.; Jang, H.J.; Lee, Y.J.; Yang, H.M.; Kim, J.H.; Kim, C.H.; Han, M.H.; et al. Anti-inflammatory activity of ethanol extract derived from Phaseolus angularis beans. J. Ethnopharmacol. 2011, 137, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Mukai, Y.; Yamate, J.; Kato, J.; Kurasaki, M.; Hatai, A.; Sagai, M. Effect of polyphenol-containing azuki bean (Vigna angularis) extract on blood pressure elevation and macrophage infiltration in the heart and kidney of spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Yuuka, M.; Shin, S. Polyphenol-containing azuki bean (Vigna angularis) seed coats attenuate vascular oxidative stress and inflammation in sponaneously hypertensive rats. J. Nutr. Biochem. 2011, 22, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.S.; Song, S.B.; Ko, J.Y.; Seo, M.C.; Lee, J.S.; Kang, J.R.; Oh, B.G.; Nam, M.H.; Jeong, H.S.; Lee, J.S. Antioxidant components and antioxidant activities of methanolic extract from adzuki beans (Vigna angularis var. nipponensis). Korean J. Food Sci. Technol. 2010, 42, 693–698. [Google Scholar]
- Shigenori, N.; Yusuke, S.; Chihiro, S.; Jun, K.; Hiroshi, K.; Kazunori, H.; Michiyuki, K. Suppression of serum cholesterol levels in mice by adzuki bean polyphenols. Food Sci. Technol. Res. 2008, 14, 217–220. [Google Scholar] [CrossRef]
- Han, K.H.; Iijuka, M.; Shimada, K.I.; Sekikawa, M.; Kuramochi, K.; Ohba, K.; Ruvini, L.; Chiji, H.; Fukushima, M. Adzuki resistant starch lowered serum cholesterol and hepatic 3-hydroxy-3-mehtylglutaryl-CoA mRNA levels and increased hepatic LDL-receptor and cholesterol 7α-hydroxylase mRNA levels in rats fed a cholesterol diet. Br. J. Nutr. 2005, 94, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Tomohiro, I.; Misato, K.; Fumihiko, H.; Yukio, F. Hypoglycemic effect of hot-water extract of adzuki (Vigna angularis) in spontaneously diabetic KK-Ay mice. Nutrition 2009, 25, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Fernández-Arroyo, S.; Pérez-Sanchez, A.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Menéndez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Titta, L.; Trinei, M.; Stendardo, M.; Berniakovich, I.; Petroni, K.; Tonelli, C.; Riso, P.; Porrini, M.; Minucci, S.; Pelicci, P.G.; et al. Blood orange juice inhibits fat accumulation in mice. Int. J. Obes. 2010, 34, 578–588. [Google Scholar] [CrossRef]
- Lila, M.A. From beans to berries and beyond: Teamwork between plant chemicals for protection of optimal human health. Ann. N Y Acad. Sci. 2007, 1114, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Soundararajan, K.; Ramaswamy, R.; Ramasamy, V.V.; Paul, C.; Mohammad, A.A. Effect of Caralluma fimbriata extract on 3T3-L1 preadipocyte cell division. Food Nutr. Sci. 2011, 2, 329–336. [Google Scholar]
- Ramirez-Zacarias, J.L.; Castro-Munozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil Red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, R.; Joshi, V.C. Hormonal regulation of stearoyl coenzyme a desaturase activity and lipogenesis during adipose conversion of 3T3-Ll cells. J. Biol. Chem. 1982, 257, 12224–12230. [Google Scholar] [PubMed]
- Choi, H.; Lee, H.; Kim, T.H.; Kim, H.J.; Lee, Y.J.; Lee, S.J.; Yu, J.H.; Kim, D.; Kim, K.S.; Park, S.W.; et al. G0/G1 switch gene 2 has a critical role in adipocyte differentiation. Cell Death. Differ. 2014, 21, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Francien, M.G.; Cynthia, M.S.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [PubMed]
- Kim, C.Y.; Le, T.T.; Chen, C.; Cheng, J.X.; Kim, K.H. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. J. Nutr. Biochem. 2011, 22, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, M.H.; Jeong, M.K.; Yeo, J.Y.; Cho, W.I.; Chang, P.S.; Chung, J.H.; Lee, J.H. Radical scavenging activity and anti-obesity effectsc in 3T3-L1 preadipocyte differentiation of Ssuk (Artemisia princeps Pamp.) extract. Food Sci. Biotechnol. 2010, 19, 535–540. [Google Scholar] [CrossRef]
- Shelly, H.; Corene, C.; Shi, S.; Xiuxiu, S.; Kequan, Z. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J. Agric. Food Chem. 2010, 25, 11250–11256. [Google Scholar]
- Liang, C.H.; Chan, L.P.; Chou, T.H.; Chiang, F.Y.; Yen, C.M.; Chen, P.J.; Ding, H.Y.; Lin, R.J. Brazilein from Caesalpinia sappan L. antioxidant inhibits adipocyte differentiation and induces apoptosis through caspase-3 activity and anthelmintic activities against Hymenolepis nana and Anisakis simplex. Evid. Based Complement. Altern. Med. 2013, 2013, 1–14. [Google Scholar]
- Chu, W.L.; Lim, Y.W.; Radhakrishnan, A.K.; Lim, P.E. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement. Altern. Med. 2010, 10, 53–61. [Google Scholar] [PubMed]
- Qusti, S.Y.; Abo-khatwa, A.N.; Mona, A.B.L. Screening of antioxidant activity and phenolic content of selected food items cited in the holy Quran. EJBS 2010, 2, 40–51. [Google Scholar]
- Barana, C.J.; Tomomi, H.; Han, K.H.; Hiroshi, I.; Tomoko, O.; Shinichi, S.; Michihiro, F.; Mitsuo, S.; Shimada, K.I. Utilization of adzuki bean extract as a natural antioxidant in cured and uncured cooked pork sausages. Meat Sci. 2011, 89, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef] [PubMed]
- Anne, W.H.; Joyce, B.H. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am. J. Physiol. Cell Physiol. 2001, 280, 807–813. [Google Scholar]
- Kitano-Okada, T.; Ito, A.; Koide, A.; Nakamura, Y.; Han, K.H.; Shimada, K.; Sasaki, K.; Ohba, K.; Sibayama, S.; Fukushima, M. Anti-obesity role of adzuki bean extract containing polyphenols: In vivo and in vitro effects. J. Sci. Food Agric. 2012, 92, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Macdouglad, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/enhancer-binding protein β is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acd. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef]
- Kuri-Harcuch, W.; Marsch-Moreno, M. DNA synthesis and cell division related to adipose differentiation of 3T3-L1 cells. J. Cell. Physiol. 1983, 114, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Schwarz, E.J.; Dimaculangan, D.D.; Lazar, M.A. Peroxisome proliferator-activated receptor (PPAR) gamma: Adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 1994, 135, 798–800. [Google Scholar] [PubMed]
- Warnke, I.; Goralczyk, R.; Fuhrer, E.; Schwager, J. Dietary constituents reduce lipid accumulation in murine C3H10 T1/2 adipocytes: A novel fluorescent method to quantify fat droplets. Nutr. Metab. 2011, 8, 1–13. [Google Scholar] [CrossRef]
- Ayala-Sumuano1, J.T.; Velez-Delvalle, C.; Beltra´n-Langarica, A.; Marsch-Moreno, M.; Cerbón-Solorzano1, J.; Kuri-Harcuch, W. Srebf1a is a key regulator of transcriptional control for adipogenesis. Sci. Rep. 2011, 1, 178–186. [Google Scholar] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.J.; Govers, R.; James, D.E. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 2002, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Weems, J.; Olson, A.L. Class II histone deacetylases limit GLUT4 gene expression during adipocyte differentiation. J. Biol. Chem. 2011, 286, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Sumuano, J.T.; Velez-delValle, C.; Beltrán-Langarica, A.; Marsch-Moreno, M.; Hernandez-Mosqueira, C.; Kuri-Harcuch, W. Glucocorticoid paradoxically recruits adipose progenitors and impairs lipid homeostasis and glucose transport in mature Adipocytes. Sci. Rep. 2013, 3, 2573. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Hamm, J.K.; Verhagen, L.A.; Peroni, O.; Katic, M.; Flier, J.S. Adipose triglyceridelipase function, regulation by insulin, and comparison with adiponutrin. Diabetes 2006, 55, 148–157. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Park, J.-E.; Song, S.-B.; Cha, Y.-S. Effects of Black Adzuki Bean (Vigna angularis) Extract on Proliferation and Differentiation of 3T3-L1 Preadipocytes into Mature Adipocytes. Nutrients 2015, 7, 277-292. https://doi.org/10.3390/nu7010277
Kim M, Park J-E, Song S-B, Cha Y-S. Effects of Black Adzuki Bean (Vigna angularis) Extract on Proliferation and Differentiation of 3T3-L1 Preadipocytes into Mature Adipocytes. Nutrients. 2015; 7(1):277-292. https://doi.org/10.3390/nu7010277
Chicago/Turabian StyleKim, Mina, Jeong-Eun Park, Seok-Bo Song, and Youn-Soo Cha. 2015. "Effects of Black Adzuki Bean (Vigna angularis) Extract on Proliferation and Differentiation of 3T3-L1 Preadipocytes into Mature Adipocytes" Nutrients 7, no. 1: 277-292. https://doi.org/10.3390/nu7010277
APA StyleKim, M., Park, J.-E., Song, S.-B., & Cha, Y.-S. (2015). Effects of Black Adzuki Bean (Vigna angularis) Extract on Proliferation and Differentiation of 3T3-L1 Preadipocytes into Mature Adipocytes. Nutrients, 7(1), 277-292. https://doi.org/10.3390/nu7010277




