1. Introduction
An interaction between exercise-induced responses and nutrient availability has long been recognized [
1]. It seems that altering the substrate supply during exercise can modify a training impulse, yet it has not been clearly determined to what extent. Skeletal muscle energy status exerts profound effects on resting metabolism and fuel use during exercise, exercise capacity, regulation of cell signaling and gene expression, as well as numerous processes involved in training adaptation. Some of the more recent studies on nutrition and exercise metabolism have attempted to examine scientific evidence for the hypothesis that endurance training undertaken with low carbohydrate availability promotes greater adaptive changes compared to high carbohydrate availability [
1,
2,
3].
Athletes in endurance sports, lasting one hour or more, are constantly searching for new nutrition strategies to enhance performance. Knowledge on energy metabolism has placed the focus on dietary carbohydrates in the past 3–4 decades, with most athletes experiencing carbohydrate loading for different periods of time before competition [
4,
5,
6]. High carbohydrate diets increase muscle and liver glycogen stores, improving endurance performance, yet at the same time, they increase the rate of carbohydrate utilization during exercise. Having this in mind, scientists and athletes have begun experimenting with dietary procedures that would decrease the rate of carbohydrate utilization, while increasing fat metabolism during prolonged physical work [
7,
8,
9]. It seems that such an alternative in exercise metabolism can be induced by a high fat, low carbohydrate diet. Very low carbohydrate ketogenic diets have been used for years in fighting obesity and different common and rare disease states [
10].
Research suggests that mild ketosis may offer therapeutic potential in diseases related to substrate insufficiency or insulin resistance, those resulting from free radical damage and hypoxia [
10,
11]. On the other hand, there are some data indicating that blood ketones are related to fatigue and perceived effort during exercise [
12]. Most of these studies have been conducted with untrained and/or obese subjects. Research with competitive athletes in different sport disciplines is scarce, and conflicting results have been presented. Several studies with competitive athletes have indicated that low carbohydrate ketogenic diets do not compromise aerobic endurance and explosive strength performance, while decreasing body weight and fat mass [
11,
13,
14]. Most studies with endurance athletes have indicated that prolonged ketosis results in an adaptation, after which free fatty acids become the major metabolic fuel, and carbohydrate utilization is markedly reduced during moderate, but exhausting exercise [
7,
9,
15,
16]. Thus, the justification for a low carbohydrate, high fat diet in endurance sports is to utilize a more concentrated fuel source to slow down the rate of carbohydrate use during exercise [
15,
16].
Having this in mind, new concepts of improving endurance performance have been created, with the hypothesis that a low carbohydrate high fat diet will increase the rate of free fatty acid (FFA) metabolism during exercise, while muscle glycogen will be preserved for later stages of an event, especially for the more intense parts [
9,
13,
17]. The major drawback in fat loading is the fact that per unit of time, more ATP can be generated from carbohydrate than from fat oxidation. When blood-borne FFA are oxidized, the maximum rate of ATP resynthesis is about 0.40 moL/min, while an aerobic or anaerobic breakdown of glycogen can generate from 1.0 to 2.0 mol of ATP/min [
18,
19]. During high intensity exercise, the rate of ATP breakdown is too high to be matched by the rate of ATP synthesis from FFA. This phenomenon limits the use of fat loading in sport disciplines that require high intensity efforts from the athletes. High intensity exercise also suppresses lipolysis, thereby reducing the availability of fatty acids to the muscles [
20]. An increased rate of glycolysis and lactate production during exercise also hinder the oxidation of fat by reducing the entry of long chain fatty acids into the mitochondria [
21].
A major metabolic adaptation to endurance training is an increased capacity for fat oxidation [
15]. Cross country cycling is a predominantly endurance sport event in which training sessions last from 1 to 4 h. The intensity of effort in this sport discipline varies from low to maximal; thus, both metabolic pathways improve significantly with training. The contribution of fat to the total energy expenditure increases after endurance training at both relative and absolute exercise intensity [
22]. Most importantly, the trained muscles of athletes have a greater mitochondrial and capillary density, which enables them to oxidize more fat compared to the untrained muscles of sedentary subjects [
17]. This sparing of glycogen effect allows endurance athletes to exercise longer before experiencing glycogen depletion and associated fatigue. Another important adaptive mechanism to endurance training includes increased activity of hormone-sensitive lipase (HSL) and decreased secretion of insulin, both at rest and during exercise [
9,
17].
Endurance trained individuals deliver more blood and oxygen to the working muscles due to a higher cardiac output and an increased arteriovenous oxygen difference. These athletes also produce less lactate at the same load due to a higher lactate threshold. Both of these adaptive changes facilitate fat oxidation. Theoretically, since endurance athletes can metabolize fat more efficiently, low carbohydrate, high fat diets should be preferred to carbohydrate loading as a nutritional strategy for increased performance. Numerous studies examining the benefits of these dietary procedures with athletes of different sport disciplines and different sports level have given conflicting results [
4,
13,
14,
16,
22,
23,
24]. A ketogenic diet is high in fat and low in carbohydrate and protein content. Although the “ketogenic diet” is deficient in one nutrient (carbohydrates), it provides an alternative fuel source for the brain and skeletal muscles, which includes ketones. These ketone bodies include β-hydroxybutyrate and acetoacetate. A ketogenic diet may be recommended in neurological diseases (epileptogenesis), during the disruption of GLUT-1 transporters and during the low activity of pyruvate dehydrogenase (E1).
Despite some therapeutic benefits, ketogenic diets create several physiological consequences of which the most significant for physical exercise includes ketosis. Other side effects of ketogenic diets for sport performance include dehydration, hypoglycemia and increased risk of kidney stones [
25,
26,
27]. Additionally, high fat, low carbohydrate ketogenic diets may induce metabolic disturbances, causing acidosis, weight loss, inadequate growth, hyperlipidemia, vitamin and trace elements deficiency (zinc, selenium and copper), hypoglycemia, hyperuricemia, anemia and leukopenia [
27].
Objective of the Research and Main Hypothesis
The main objective of this research was to determine the effects of a long-term, low carbohydrate, ketogenic diet, rich in polyunsaturated fatty acids, on aerobic performance in off-road cyclists. Additionally, the effects of this diet on body mass and body composition were evaluated, as well as those that occurred in the lipid and lipoprotein profiles, due to the dietary intervention. The effects of the high fat diet on resting and exercise concentrations of chosen hormones and metabolites were also determined. The main hypothesis stated that a long-term, low carbohydrate, ketogenic diet, applied in off-road cyclists, would decrease body mass and body fat content, while increasing FFA metabolism during continuous exercise with moderate intensity, with a concomitant decrease in insulin levels and glucose uptake. It was also hypothesized that the ketogenic diet would allow the subjects to maintain the level of aerobic power and capacity as evaluated by the value of maximal oxygen uptake (VO2max) and lactate threshold (LT). The study was approved by the Bioethical Committee for Research at the Academy of Physical Education in Katowice.
3. Results
A two-way repeated measures ANOVA revealed a statistically significant effect of the diet intervention program on β-hydroxybutyrate concentration evaluated at rest (β-HGB). Tukey’s HSD post hoc test revealed a statistically significant increase, from 0.04 mmoL/L (mixed), to 0.15 mmoL/L, after the ketogenic diet in β-HGB concentration (F = 18.45, η2 = 0.615, p = 0.001).
Table 3 presents the characteristics of body mass and body composition after the mixed and ketogenic diets, while,
Table 4 shows the lipid and lipoprotein profiles during the mixed and ketogenic diets at rest and during the exercise protocol.
Table 3.
Body mass and body composition in off-road cyclists after a mixed (Mix) and ketogenic (Ket) diet.
Table 3.
Body mass and body composition in off-road cyclists after a mixed (Mix) and ketogenic (Ket) diet.
| Variables | Mix | Ket | η2 | p |
|---|
| X | SD | X | SD |
|---|
| Body mass (kg) | 80.14 | 7.26 | 78.26 | 7.86 | 0.552 | 0.011 |
| BMI (kg/m2) | 24.87 | 3.09 | 23.89 | 3.10 | 0.471 | 0.012 |
| FAT (%) | 14.88 | 3.78 | 11.02 | 3.66 | 0.747 | 0.001 |
The diet intervention significantly differentiated T-Ch (
F = 13.26;
p = 0.0083), HDL-Ch (
F = 8.12;
p = 0.024) and LDL-Ch (
F = 20.22;
p = 0.0027). The exercise protocol caused statistically significant changes in the concentration of TG (
F = 5.05;
p = 0.0086) (HDL-Ch (
F = 16.47;
p = 0.0001)). A significant interaction in the concentration of TG was registered (diet × exercise) (
F = 3.28;
p = 0.041). Significant differences due to the diet intervention occurred during the 90 min of the exercise protocol in regard to T-Ch, (
p < 0.01) and HDL-Ch, (
p = 0.05), while during maximal effort, such differences occurred in LDL-Ch (
p < 0.01) (
Table 4).
Table 5 presents HR, RER and VO
2 values after the mixed and ketogenic diets during the exercise protocol (rest, 10 min, 45 min, 90 min and max effort). A two-way repeated measures ANOVA and
post hoc tests revealed a statistically significant effect at rest on the respiratory exchange ratio (
F = 18.22, η
2 = 0.601,
p = 0.001), during the 45 min of exercise (
F = 10.16, η
2 = 0.442,
p = 0.001) (90 min (
F = 10.05, η
2 = 0.435,
p = 0.001)), after the ketogenic diet. No statistically significant differences were observed during maximal effort (
F = 1.22, η
2 = 0.101,
p = 0.065).
Table 6 presents the values of the biochemical variables under analysis. A two-way repeated measures ANOVA revealed a statistically significant effect of exercise on insulin (
F = 10.70, η
2 = 0.452,
p = 0.015), glucose (
F = 17.43, η
2 = 0.591,
p = 0.001) and cortisol (
F = 17.21, η
2 = 0.587,
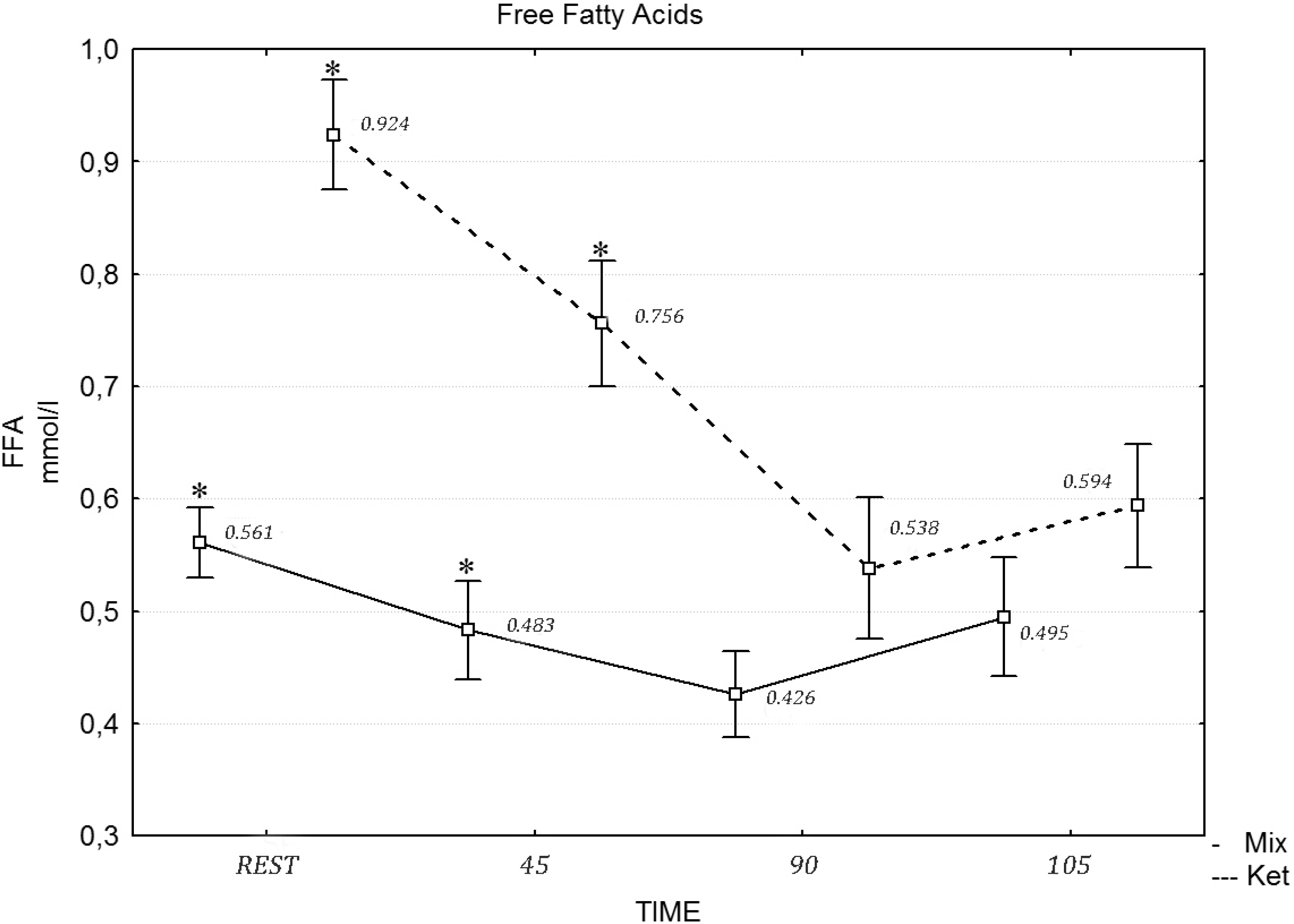
p = 0.001), while the differences in the FFA concentration after the mixed and ketogenic diets were statistically significant. The
post hoc tests revealed that the concentration of FFA differed at rest (
F = 12.13, η
2 = 0.491,
p = 0.021), during the 45 min of exercises (
F = 12.22, η
2 = 0.501,
p = 0.012), the 90th min (
F = 19.21, η
2 = 0.658,
p = 0.001) and after the maximum effort (
F = 22.23, η
2 = 0.747,
p = 0.001), following the ketogenic diet (
Figure 1). A two-way repeated measures ANOVA revealed no statistically significant effect of diet intervention on the testosterone (
F = 1.14, η
2 = 0.087,
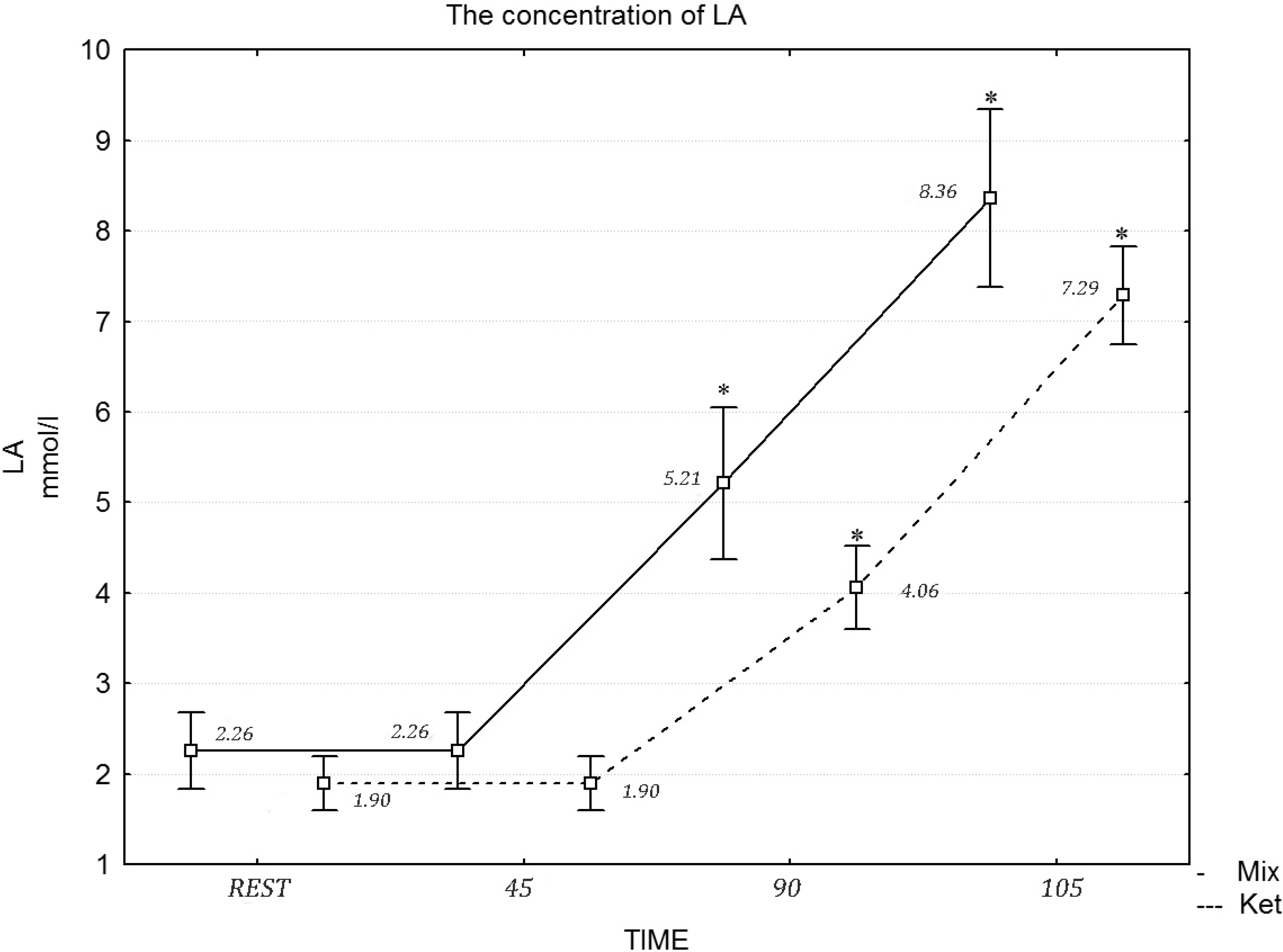
p = 0.061) concentration at rest and during the exercise protocol. Similarly, a two-way repeated measures ANOVA revealed a statistically significant effect of the diet in the 90 min of exercise (
F = 21.11, η
2 = 0.717,
p = 0.001) and during the maximum effort in LA concentration (
F = 20.03, η
2 = 0.697,
p = 0.001) (
Figure 2).
Figure 1.
The concentration of free fatty acids (FFA) during the exercise protocol, after a mixed and ketogenic diet. * Statistical significance with p < 0.05.
Figure 1.
The concentration of free fatty acids (FFA) during the exercise protocol, after a mixed and ketogenic diet. * Statistical significance with p < 0.05.
A two-way repeated measures ANOVA revealed no statistically significant effect of the diet intervention on creatine kinase (CK) and lactate dehydrogenase (LDH) activity.
Table 7 presents the values of physiological variables under analysis. A two-way repeated measures ANOVA revealed statistically significant differences between the moderate and small size effects of mixed and ketogenic diets on VO
2max (
F = 23.70, η
2 = 0.751,
p = 0.001), VO
2LT (
F = 17.43, η
2 = 0.592,
p = 0.012), LT work load (
F = 14.21, η
2 = 0.548,
p = 0.015) and max work load (
F = 10.11, η
2 = 0. 491,
p = 0.037).
Post hoc tests revealed a statistically significant effect of diet on VO
2max (
p = 0.001), VO
2LT (
p = 0.001), LT work load (
p = 0.001) and maximal work load (
p = 0.012).
Table 4.
Lipid and lipoprotein profiles during the mixed and ketogenic diets at rest and during the exercise protocol.
Table 4.
Lipid and lipoprotein profiles during the mixed and ketogenic diets at rest and during the exercise protocol.
| Variables | Rest | 45 min | 90 min | Max Effort |
|---|
| Mix | Ket | p | Mix | Ket | p | Mix | Ket | P | Mix | Ket | p |
|---|
| Triglycerides (mg/dL) | 117.21 ± 10.11 | 90.11 ± 8.75 | 0.002 * | 108.24 ± 8.23 | 110.23 ± 8.34 | 0.041 | 125.11 ± 9.33 | 129.96 ± 9.41 | 0.058 | 129.86 ± 9.44. | 112.34 ± 8.51 | 0.001 * |
| Total cholesterol (mg/dL) | 188.34 ± 16.22 | 215.34 ± 19.54 | 0.001 * | 196.31 ± 16.32 | 223.61 ± 20.12 | 0.001 * | 190.78 ± 17.34 | 230.93 ± 20.15 | 0.001 * | 191.67 ± 17.57 | 226.11 ± 20.45 | 0.001 * |
| High density lipoproteins (mg/dL) | 96.51 ± 7.12 | 117.20 ± 9.14 | 0.002 * | 99.12 ± 7.09 | 115.28 ± 9.10 | 0.002 * | 92.45 ± 7.031 | 118.34 ± 9.45 | 0.001 * | 91.89 ± 7.01 | 119.97 ± 9.51 | 0.001 * |
| Low density lipoproteins (mg/dL) | 69.12 ± 4.21 | 74.58 ± 5.12 | 0.461 | 73.31 ± 5.11 | 80.78 ± 6.45 | 0.049 | 72.28 ± 5.09 | 86.21 ± 6.75 | 0.068 | 72.22 ± 5.02 | 83.43 ± 6.56 | 0.593 |
Table 5.
Heart rate (HR), respiratory exchange ratio (RER) and VO2 values after a mixed and ketogenic diet during the exercise protocol.
Table 5.
Heart rate (HR), respiratory exchange ratio (RER) and VO2 values after a mixed and ketogenic diet during the exercise protocol.
| Variable | Rest | 10 min | 45 min | 90 min | Max Effort |
|---|
| Mix | Ket | Mix | Ket | Mix | Ket | Mix | Ket | Mix | Ket |
|---|
| HR | 72 ± 5 | 75 ± 6 | 150 ± 3 | 150 ± 3 | 158 ± 4 | 161 ± 5 | 167 ± 5 | 169 ± 5 | 187 ± 6 | 185 ± 6 |
| VO2 | 7.20 ± 1.22 | 9.40 ± 1.41 | 35.37 ± 3.45 | 41.25 ± 4.22 | 37.50 ± 3.64 | 44.25 ± 4.56 | 40.87 ± 4.11 | 44.00 ± 4.41 | 51.00 ± 4.87 | 50.00 ± 4.85 |
| RER | 0.88 ± 0.04 | 0.76 ± 0.01 | 0.86 ± 0.04 | 0.78 ± 0.02 | 0.85 ± 0.04 | 0.79 ± 0.02 | 0.84 ± 0.03 | 0.79 ± 0.02 | 0.97 ± 0.05 | 0.94 ± 0.05 |
Table 6.
Values of biochemical variables (insulin (Ins), glucose (Glu), creatine kinase and lactate dehydrogenase activity), as well as testosterone (T) and cortisol (Cor) concentration after a mixed and ketogenic diet at rest and during the exercise protocol.
Table 6.
Values of biochemical variables (insulin (Ins), glucose (Glu), creatine kinase and lactate dehydrogenase activity), as well as testosterone (T) and cortisol (Cor) concentration after a mixed and ketogenic diet at rest and during the exercise protocol.
| Variables | Rest | 45 min | 90 min | Max Effort |
|---|
| Mix | Ket | Mix | Ket | Mix | Ket | Mix | Ket |
|---|
| Ins (U/L) | 19.21 ± 0.81 | 9.87 ± 0.45 | 6.02 ± 0.31 | 4.25 ± 0.22 | 5.45 ± 0.25 | 4.97 ± 0.29 | 9.89 ± 0.45 | 5.63 ± 0.29 |
| Glu (mg/dL) | 91.26 ± 4.11 | 91.32 ± 4.13 | 106.11 ± 4.98 | 98.61 ± 4.22 | 89.78 ± 4.01 | 90.04 ± 4.07 | 121.67 ± 5.14 | 119.41 ± 5.08 |
| CK (U/L) | 126.32 ± 10.22 | 119.45 ± 9.74 | 158.12 ± 11.21 | 129.11 ± 10.31 | 160.76 ± 13.24 | 139.34 ± 10.58 | 178.12 ± 15.45 | 140.07 ± 12.51 |
| LDH (U/L) | 321.26 ± 30.14 | 262.23 ± 24.24 | 349.56 ± 32.17 | 267.56 ± 24.52 | 359.65 ± 33.23 | 265.45 ± 24.45 | 439.76 ± 39.56 | 311.21 ± 27.61 |
| T (ng/L) | 6.12 ± 0.4 | 5.86 ± 0.3 | 8.78 ± 0.6 | 7.21 ± 0.5 | 9.38 ± 0.7 | 8.08 ± 0.6 | 7.91 ± 0.5 | 8.14 ± 0.6 |
| Cor (nmol/L) | 649 ± 62 | 553 ± 49 | 389 ± 29 | 435 ± 33 | 495 ± 38 | 579 ± 51 | 650 ± 62 | 676 ± 65 |
Table 7.
Physiological variables (max work load, VO2max, VO2LT and LT work load) in off-road cyclists after a mixed (Mix) and ketogenic (Ket) diet.
Table 7.
Physiological variables (max work load, VO2max, VO2LT and LT work load) in off-road cyclists after a mixed (Mix) and ketogenic (Ket) diet.
| Variables | Mix | Ket | p |
|---|
| X | SD | X | SD |
|---|
| Max work load (W) | 362 | 16.09 | 350 | 14.60 | 0.037 |
| VO2max (mL/kg/min) | 56.02 | 3.50 | 59.40 | 3.10 | 0.001 |
| VO2LT (mL/kg/min) | 43.50 | 1.80 | 47.80 | 2.10 | 0.012 |
| LT work load (W) | 257 | 10.60 | 246 | 9.50 | 0.015 |
Figure 2.
The concentration of LA during the exercise protocol, after a mixed and ketogenic diet. * Statistical significance with p < 0.05.
Figure 2.
The concentration of LA during the exercise protocol, after a mixed and ketogenic diet. * Statistical significance with p < 0.05.
4. Discussion
Consuming a low carbohydrate ketogenic diet may be recommended to promote fat oxidation during exercise at moderate intensity and at rest. Fat loading may also slow down the rate of carbohydrate utilization and enhance endurance performance in long distance events lasting from 2 to 5 h. This is especially true during long-term ketogenic diets, where the body adapts to increased fat oxidation through enzymatic and endocrine changes [
34,
35]. The low carbohydrate, high fat diet applied in this study caused a four-fold elevation of pre-exercise β-hydroxybutyrate concentration and a two-fold increase in resting plasma FFA concentration. This indicates compliance with the prescribed ketogenic diet. Compared to a high carbohydrate diet, or a mixed diet with 50%–70% of energy coming from carbohydrate (CHO), a high fat diet with 70% of the calories derived from fat significantly increased the contribution of FFA to the total energy expenditure during moderate intensity exercise. This was observed during the first 90 min of the exercise protocol. During the last 15 min of exercise, when maximal intensity was introduced, FFA metabolism was inhibited by glycolysis, which was evidenced by significant increases in LA concentration. This phenomena was observed in the case of both diets, yet it was more pronounced in the case of the high fat diet [
21,
22].
The benefits of a ketogenic diet related to athletic performance may also be caused by changes in body mass and body composition. This may be of significance, not only in aerobic endurance sport disciplines, but also in sports that include weight class divisions and require body mass control and management [
11]. The ketogenic diet introduced in this research project stimulated favorable changes in body mass and body composition, as well as in the lipid and lipoprotein profiles. The most likely reason for such changes included the predominance of polyunsaturated fatty acids in such a diet [
34,
35,
36]. Further benefits of a high fat diet, with a significant intake of Ω-3 fatty acids, may be related to reduced post exercise muscle damage, which was observed by lower rest and exercise plasma CK and LDH activity in this research project [
37]. These differences were especially visible after the maximal effort phase.
During long endurance exercise at moderate intensity (between 50% and 70% VO
2max), a lower RER and lactate concentration are observed, yet a higher HR and VO
2 following a high fat diet, compared to a mixed or high carbohydrate diet [
34]. A significantly lower respiratory exchange ratio at submaximal workloads after the ketogenic diet indicates increased lipid metabolism. The ketogenic diet applied in the present study resulted in lower plasma lactate concentrations at rest, during the moderate intensity continuous exercise and especially after the last 15 min of the exercise protocol performed with maximal effort. Important findings of the present study include a significant improvement in relative values of VO
2max and LT VO
2 after the ketogenic diet, which can be explained by reductions in body mass and fat mass and or greater oxygen uptake necessary to obtain the same energy yield as on a mixed diet due to increased fat oxidation or by enhanced sympathetic activation [
38,
39]. Previous investigations have also reported a shift in LT to higher workloads under conditions of glycogen store reductions, due to a low carbohydrate diet, fasting or exhausting exercise [
17]. This phenomenon has not been fully explained, and there is still a debate on whether a ketogenic diet induced a shift in the LT and whether a reduction in the maximal LA concentration depends on a decreased rate of glycolysis or an inhibited lactate efflux from working muscles due to reduced blood buffering capacity [
17]. Most research projects indicate a tendency towards lower blood pH and reduced blood base excess and bicarbonate levels after a ketogenic diet at rest; and especially after exercise with maximal intensity [
34]. Our research showed improvements in VO
2max and VO
2LT, yet the power output during work at maximal intensity was compromised on the ketogenic diet, which can be explained by lower muscle glycogen stores and the reduced activity of glycolytic enzymes due to the four-week diet intervention [
19,
40]. The increase in aerobic capacity following the ketogenic diet may also have been influenced by positive changes in the morphological characteristics of the off-road cyclists, where higher values of RBC, HCT and HGB were registered at rest and during the exercise protocol. This could be explained by the ergogenic effects of Ω-3 polyunsaturated fatty acids on erythrocyte membrane integrity, erythrocyte deformability and blood viscosity, factors that improve circulation and oxygen transport to working muscles [
39,
41].
The changes in the considered hormone concentrations induced by the ketogenic diet and the exercise protocol were similar to those reported previously in regard to insulin and cortisol, while lower values of testosterone after the high fat diet at rest and during the exercise protocol are difficult to explain. The alterations in insulin and cortisol concentrations due to the dietary intervention confirm the concept that the glucostatic mechanism controls the hormonal and metabolic responses to exercise. According to this concept, depletion of muscle and liver glycogen leads to the stimulation of lipolysis and glucose production, due to changes in the secretion of glucoregulatory hormones [
37]. The main limitation of this study includes a small number of subjects participating in the experiment, the use of electrical impedance for body composition analysis and the lack of a 2–3-day carbohydrate loading phase following the four-week ketogenic diet. This could confirm or reject the hypothesis regarding improved endurance performance and increased lipid metabolism after a ketogenic diet followed by carbohydrate loading [
41].