Dietary Pattern among Schoolchildren with Normal Nutritional Status in Navarre, Spain
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. Nutrition Survey
- 1)
- Milk and dairy products
- 2)
- Cereals and cereal products
- 3)
- Sweets, bakery and pastry
- 4)
- Fruits and natural juices
- 5)
- Fats and oils
- 6)
- Meat and derivatives
- 7)
- Eggs and derivatives
- 8)
- Vegetables and tubers
- 9)
- Legumes
- 10)
- Fishes
2.3. Nutrition Study
3. Results
3.1. Characteristics of the Sample
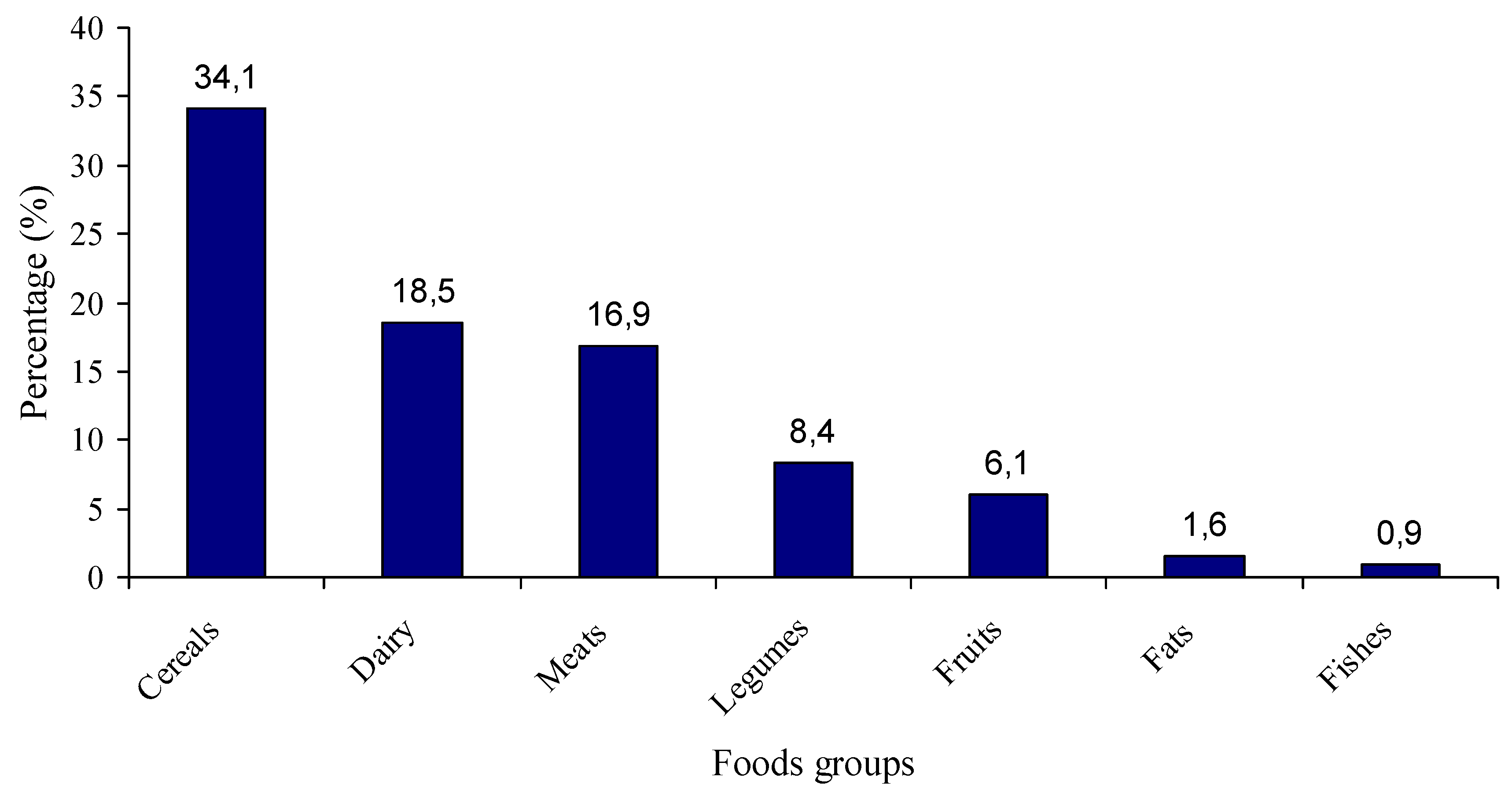
3.2. Consumption Frequencies
3.3. Nutrients and Calorie Intake
| Meal | Calorie intake (Kcal) | Calorie contribution (%) |
|---|---|---|
| M (CI 95%) | M (CI 95%) | |
| Breakfast | 323.5 (313.9–333.1) | 16.0 (15.5–16.5) |
| Mid-morning snack | 253.8 (242.2–265.4 | 11.9 (11.4–12.4) |
| Lunch | 734.2 (714.6–753.8) | 34.5 (33.7–35.4) |
| Afternoon snack | 298.1 (287.7–308.5) | 14.5 (14.0–15.0) |
| Dinner | 498.2 (480.3–516.1) | 23.5 (22.8–24.2) |
| Nutrients (recommended percentage) | Percentage (%) |
|---|---|
| Proteins (10%–15%) | 20.3 |
| Animal origin | 64.5 |
| Carbohydrates (50%–55%) | 48.8 |
| Total fat (30%–35%) | 30.9 |
| SFA (7%–10%) | 12.6 |
| MUFA (15%–20%) | 10.9 |
| PUFA (6%–10%) | 3.74 |
| Nutrients | Total group M (SD) | DRI |
|---|---|---|
| Calcium (mg) | 911.7 (174.1) | 1300 |
| Iron (mg) | 18.3 (6.0) | 8 |
| Iodine (μg) | 79.2 (22.6) | 120 |
| Magnesium (mg) | 311.8 (83.3) | 240 |
| Zinc (mg) | 10.5 (2.7) | 8 |
| Selenium (μg) | 125.8 (39.6) | 40 |
| Phosphorus (mg) | 1606.8 (294.0) | 1250 |
| Thiamine (mg) | 1.7 (0.5) | 0.9 |
| Riboflavin (mg) | 1.9 (0.5) | 0.9 |
| Niacin (mg) | 39.0 (7.6) | 12 |
| Vitamin B6 (mg) | 2.0 (0.6) | 1.0 |
| Folate (μg) | 334.6 (150.2) | 300 |
| Vitamin B12 (mg) | 5.8 (2.5) | 1.8 |
| Vitamin C (mg) | 54.3 (34.3) | 45 |
| Vitamin A (μg) | 463.6 (204.6) | 600 |
| Vitamin D (μg) | 88.0 (66.3) | 200 |
| Vitamin E (mg) | 3.8 (1.7) | 11 |
3.4. Percentage Contribution of Food Groups
| Food group | Dairy | Cereals | Meats | Eggs | Vegetab | Legumes | Fruits | Fishes | Sweets | Fat |
|---|---|---|---|---|---|---|---|---|---|---|
| Proteins | 19.7 | 20.8 | 38.0 | 2.0 | 0.5 | 11.5 | 0.7 | 2.9 | 3.9 | - |
| CH | 9.0 | 55.0 | 2.4 | - | 0.7 | 9.1 | 10.6 | - | 13.2 | - |
| Fat | 36.9 | 6.4 | 27.7 | 3.6 | 1.9 | - | 1.4 | 0.8 | 15.1 | 6.2 |
| SFA | 47.5 | 3.2 | 22.4 | 2.2 | - | - | - | - | 18.9 | 4.3 |
| MUFA | 32.4 | 3.3 | 33.5 | 4.8 | - | - | 1.8 | - | 14.5 | 7.8 |
| PUFA | 23.8 | 12.3 | 27.5 | 3.9 | 7.1 | - | 3.0 | 1.5 | 9.9 | 11.0 |
| Cholest | 20.2 | - | 42.9 | 21.4 | - | - | - | 5.4 | 8.5 | 1.6 |
| Fibre | - | 36.5 | - | - | 2.4 | 37.8 | 16.9 | - | 6.4 | - |
| Food group | Dairy | Cereals | Meats | Eggs | Vegetab | Legumes | Fruits | Fishes | Sweets | Fat |
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | 71.8 | 11.6 | 2.4 | 1.0 | 4.9 | - | 1.4 | - | 5.9 | - |
| Fe | 2.7 | 30.2 | 25.1 | 1.9 | 1.5 | 23.1 | 6.6 | 1.5 | 7.2 | - |
| I | 43.7 | 16.6 | 15.5 | 2.6 | 2.2 | - | 2.6 | 8.6 | 6.8 | 1.4 |
| Mg | 20.9 | 26.8 | 14.0 | - | 1.6 | 25.5 | 4.7 | 2.4 | 3.3 | - |
| Zn | 19.8 | 15.0 | 43.5 | 2.76 | 14.2 | - | 2.1 | - | 1.8 | - |
| Se | 5.1 | 62.4 | 14.2 | 1.2 | 4.3 | - | 2.1 | 7.1 | 3.6 | - |
| P | 35.0 | 15.2 | 25.5 | 2.2 | 14.0 | - | 1.6 | 2.2 | 4.4 | - |
| Food group | Dairy | Cereals | Meats | Eggs | Vegetab | Legumes | Fruits | Fishes | Sweets | Fat |
|---|---|---|---|---|---|---|---|---|---|---|
| B1 | 11.2 | 27.0 | 29.6 | 1.3 | 1.3 | 17.1 | 5.3 | 1.3 | 6.6 | - |
| B2 | 41.3 | 19.4 | 19.9 | 3.5 | 7.0 | - | 3.5 | 1.0 | 5.0 | - |
| Niacin | 10.3 | 25.6 | 42.5 | 1.4 | 9.8 | - | 0.7 | 5.7 | 3.9 | - |
| B6 | 9.9 | 21.3 | 38.0 | 1.0 | 1.9 | 17.6 | 5.9 | 1.9 | 2.8 | - |
| Folate | 9.4 | 30.4 | 5.3 | 3.4 | 4.3 | 34.9 | 4.8 | 1.2 | 6.3 | - |
| B12 | 38.3 | 4.1 | 38.3 | 9.4 | - | - | - | 77 | 2.3 | - |
| C | 9.4 | 18.4 | 4.7 | - | 13.5 | - | 54.0 | - | - | - |
| A | 47.6 | 11.9 | - | 9.7 | 14.1 | - | 2.4 | - | 7.2 | 7.0 |
| D | 11.0 | 43.1 | - | 17.2 | - | - | - | - | 8.8 | 19.9 |
| E | 11.4 | 3.2 | 6.5 | 8.4 | 4.3 | 24.6 | 20.8 | 2.5 | 6.5 | 11.2 |
4. Discussion
Conflicts of Interest
References
- Serra-Majem, L.; Roman, B.; Estruch, R. Scientific evidence of interventions using the Mediterranean diet: A systematic review. Nutr. Rev. 2006, 64, S27–S47. [Google Scholar] [CrossRef]
- Helsing, E. Traditional diets and disease patterns of the mediterranean, circa 1960. Am. J. Clin. Nutr. 1995, 61, 1329–1337. [Google Scholar]
- Willet, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulou, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402–1406. [Google Scholar]
- Trichopoulou, A.; Lagiou, P. Healthy traditional mediterranean diet: An expression of culture, history, and lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Bes-Rastrollo, M.; Martinez-Gonzalez, M.A.; Serra-Majem, L. Adherence to a mediterranean dietary pattern and weight gain in a follow-up study: The SUN cohort. Int. J. Obes. 2006, 30, 350–358. [Google Scholar]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a mediterranean diet and survival in a greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Tur, J.A.; Serra-Majem, L.; Romaguera, D.; Pons, A. Does the diet of the Balearic population, a mediterranean type diet, still provide adequate antioxidant nutrient intakes? Eur. J. Nutr. 2005, 44, 204–213. [Google Scholar] [CrossRef]
- Moreno, L.A.; Sarria, A.; Popkin, B.M. The nutrition transition in Spain: A european Mediterranean country. Eur. J. Clin. Nutr. 2002, 56, 992–1003. [Google Scholar] [CrossRef]
- Tur, J.A.; Romaguera, D.; Pons, A. Food consumption patterns in a mediterranean region: Does the mediterranean diet still exist? Ann. Nutr. Metab. 2004, 48, 193–201. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Ferrara, L.A.; Raimondi, S.; d´Episcopo, L.; Guida, L.; Dello Russo, A.; Marotta, T. Olive oil and reduced need for antihypertensive medications. Arch. Intern. Med. 2000, 160, 837–842. [Google Scholar] [CrossRef]
- Kris-Etherton, P.; Eckel, R.H.; Howard, B.V.; Jeor, S.; Bazzarre, T.L. AHA Science Advisory: Lyon diet heart study. Benefits of a mediterranean-style, national cholesterol education program/american heart association step I dietary pattern on cardiovascular disease. Circulation 2001, 103, 1823–1825. [Google Scholar] [CrossRef]
- Hu, F.B. The Mediterranean diet and mortality–olive oil and beyond. N. Engl. J. Med. 2003, 348, 2595–2596. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Naska, A.; Antoniou, A.; Friel, S.; Trygg, K.; Turrini, A. Vegetable and fruit: The evidence in their favour and the public health perspective. Int. J. Vitam. Nutr. Res. 2003, 73, 63–69. [Google Scholar] [CrossRef]
- Estruch, R.; Martinez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Gil, A.; Ortega, R.M.; Maldonado, J. Wholegrain cereals and bread: A duet of the Mediterranean diet for the prevention of chronic diseases. Public Health Nutr. 2011, 14, 2316–2322. [Google Scholar] [CrossRef]
- Van den Brandt, P.A. The impact of a Mediterranean diet and healthy lifestyle on premature mortality in men and women. Am. J. Clin. Nutr. 2011, 94, 913–920. [Google Scholar] [CrossRef]
- Kontou, N.; Psaltopoulou, T.; Panagiotakos, D.; Dimopoulos, M.A.; Linos, A. The mediterranean diet in cancer prevention: A review. J. Med. Food 2011, 14, 1065–1078. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef]
- SUVIMAX. Portions Alimentaires. Manuel Photos Pourl’estimation des Quantités; Polytechnica: Paris, France, 2002. [Google Scholar]
- Programa de cálculo nutricional CESNID 1.0 (CD-ROM). In Centre d’Ensenyament Superior de Nutrició i Dietética; Universitat de Barcelona-McGraw-Hill: Barcelona, Spain, 2003.
- Dietary References Intakes: Macronutrients, Elements and vitamins. Food and Nutrition Board, Institute of Medicine, National Academy of Sciences. 2004. Available online: http://www.nap.edu25 (accessed on 26 August 2013).
- Martínez, M.A.; Alonso, A.; Egües, N. Sistemas de evaluación del consumo de alimentos. In Nutrición Aplicada y Dietoterapia; Muñoz, M., Aranceta, J., García-Jalón, I., Eds.; EUNSA: Pamplona, Spain, 2004; pp. 67–82. [Google Scholar]
- Reilly, J.J.; Dorosty, A.R.; Emmett, P.M. Identification of the obese child: Adequacy of the body mass index for clinical practice and epidemiology. Int. J. Obes. 2000, 24, 1623–1627. [Google Scholar] [CrossRef]
- Marrodán-Serrano, M.D.; Mesa-Santurino, M.S.; Alba-Díaz, J.A.; Ambrosio-Soblechero, B.; Barrio-Caballero, P.A.; Drak-Hernández, L.; Gallardo-Yepes, M.; Lermo-Castelar, J.; Rosa-Rosa, J.M.; González-Montero de Espinosa, M. Obesity screening: Updated criteria and their clinical and populational validity. An. Pediatr. (Barc) 2006, 65, 5–14. [Google Scholar] [CrossRef]
- Calañas-Continente, A.; Arrizabalaga, J.; Caixàs, A.; Cordido, F. Diagnostic and therapeutic recommendations for overweight and obesity during adolescence. Med. Clin. (Barc) 2010, 135, 265–273. [Google Scholar] [CrossRef]
- Rodriguez-Artalejo, F.; Banegas, J.R.; Graciani, M.A.; Hernández-Vecino, R.; Rey-Calero, J. Food and nutrient consumption in Spain in the period 1940–1988. Analysis of its consistency with the Mediterranean diet. Med. Clin. (Barc) 1996, 106, 161–168. [Google Scholar]
- Iturbe, A.; Emparanza, J.; Perales, A. Dietary pattern of adolescents in Guipuzcoa. An. Esp. Pediatr. 1999, 50, 471–478. [Google Scholar]
- Serra-Majem, L.; García-Closas, R.; Ribas, L.; Pérez-Rodrigo, C.; Aranceta, J. Food patterns of spanish schoolchildren and adolescents: The enKid study. Public Health Nutr. 2001, 4, 1433–1438. [Google Scholar]
- Durá-Travé, T. Energy and nutrient intake in compulsory high school students. An. Esp. Pediatr. 2001, 54, 547–554. [Google Scholar] [CrossRef]
- Aranceta, J. Spanish food patterns. Public Health Nutr. 2001, 4, 1399–1402. [Google Scholar]
- Sánchez-Villegas, A.; Martinez, J.A.; De Irala, J.; Martínez-González, M.A. Determinants of the adherence to an «a priori» defined Mediterranean dietary pattern. Eur. J. Nutr. 2002, 41, 249–257. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, Youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar]
- Mariscal-Arcas, M.; Rivas, A.; Velasco, J.; Ortega, M.; Caballero, A.M.; Olea-Serrano, F. Evaluation of the Mediterranean Diet Quality Index (KIDMED) in children and adolescents in Southern Spain. Public Health Nutr. 2009, 12, 1408–1412. [Google Scholar] [CrossRef]
- Velasco, J.; Mariscal-Arcas, M.; Rivas, A.; Caballero, M.L.; Hernández-Elizondo, J.; Olea-Serrano, F. Assessment of the diet of school children from Granada and influence of social factors. Nutr. Hosp. 2009, 24, 193–199. [Google Scholar]
- Ayechu, A.; Durá, T. Quality of dietary habits (adherence to a mediterranean diet) in pupils of compulsory secondary education. An. Sist. Sanit. Navar. 2010, 33, 35–42. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Durá-Travé, T.; Gallinas-Victoriano, F. Dietary Pattern among Schoolchildren with Normal Nutritional Status in Navarre, Spain. Nutrients 2014, 6, 1475-1487. https://doi.org/10.3390/nu6041475
Durá-Travé T, Gallinas-Victoriano F. Dietary Pattern among Schoolchildren with Normal Nutritional Status in Navarre, Spain. Nutrients. 2014; 6(4):1475-1487. https://doi.org/10.3390/nu6041475
Chicago/Turabian StyleDurá-Travé, Teodoro, and Fidel Gallinas-Victoriano. 2014. "Dietary Pattern among Schoolchildren with Normal Nutritional Status in Navarre, Spain" Nutrients 6, no. 4: 1475-1487. https://doi.org/10.3390/nu6041475
APA StyleDurá-Travé, T., & Gallinas-Victoriano, F. (2014). Dietary Pattern among Schoolchildren with Normal Nutritional Status in Navarre, Spain. Nutrients, 6(4), 1475-1487. https://doi.org/10.3390/nu6041475




