Inhibitory Effect of High Temperature- and High Pressure-Treated Red Ginseng on Exercise-Induced Oxidative Stress in ICR Mouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of High Temperature- and High Pressure-Treated Red Ginseng Extract
2.3. Experimental Animals
| (n = 8) | Exercise | Type of Diet |
|---|---|---|
| CON 1 | X | AIN-93G diet |
| E 2 | O | AIN-93G diet |
| E + CRG 3 | O | AIN-93G diet + CRG (100 mg/kg BW) |
| E + HRG 4 | O | AIN-93G diet + HRG (100 mg/kg BW) |
| Formula | g/kg |
|---|---|
| Casein | 200 |
| Sucrose | 100 |
| Corn starch | 397.486 |
| l-cystein | 3 |
| Cellulose | 50 |
| Mineral mix, AIN-93G-MX (94046) | 35 |
| Vitamin mix AIN-93-VX (94047) | 10 |
| Choline Bitartrate | 2.5 |
| TBHQ 1, antioxidant | 0.014 |
| 94045 AIN-93G Purified Diet (Harlan) | |
| Day | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| speed (m/min) | 5 | 10 | 15 | 20 | 20 |
| time (min) | 5 | 10 | 15 | 20 | 20 |
| slope (°) | 7 | 7 | 7 | 7 | 7 |
2.4. Tissue Extraction for Enzyme Activity
2.5. Total Protein Assay
2.6. Blood Analysis
2.7. Determination of Hepatic Malondialdehyde (MDA)
2.8. Determination of Muscular Glycogen
2.9. Measurement of Enzyme Activity in Gastrocnemius Tissue
2.10. RNA Isolation and Semi-Quantitative RT-PCR
| Item | Direction | Sequence(5′-3′) |
|---|---|---|
| GAPDH 1 | F 2 | 5′-CAAGGTCATCCATGACAACT-3′ |
| R 3 | 5′-GGCCATCCACAGTCTTCTGG-3′ | |
| Cu/Zn SOD | F | 5′-CAGCATGGGTTCCACGTCCA-3′ |
| R | 5′-CACATTGGCCACACCGTCCT-3′ | |
| MRF4 | F | 5′-CTGACTGGGCAGTCGGGTG-3′ |
| R | 5′-ATGGACCTTTTTGAAACTGG-3′ |
2.11. Statistical Analysis
3. Results
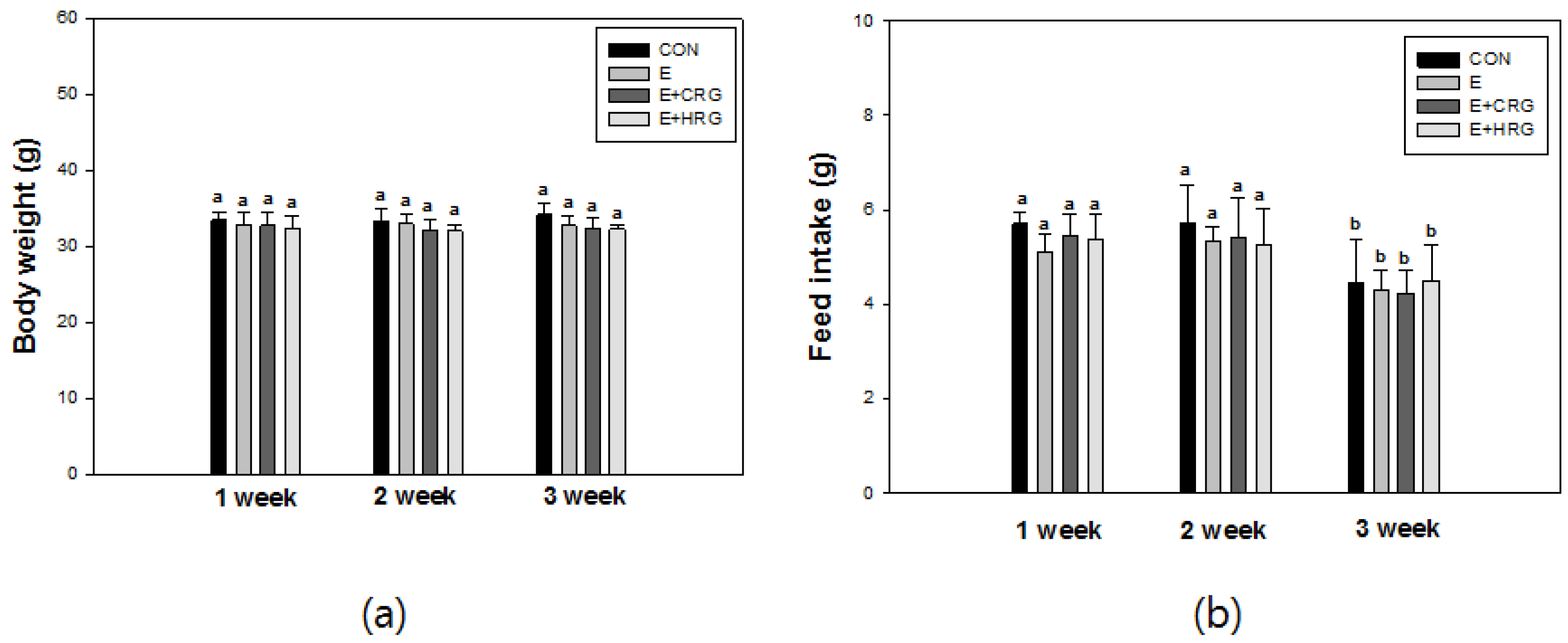
3.1. Weight Gain and Feed Efficiency
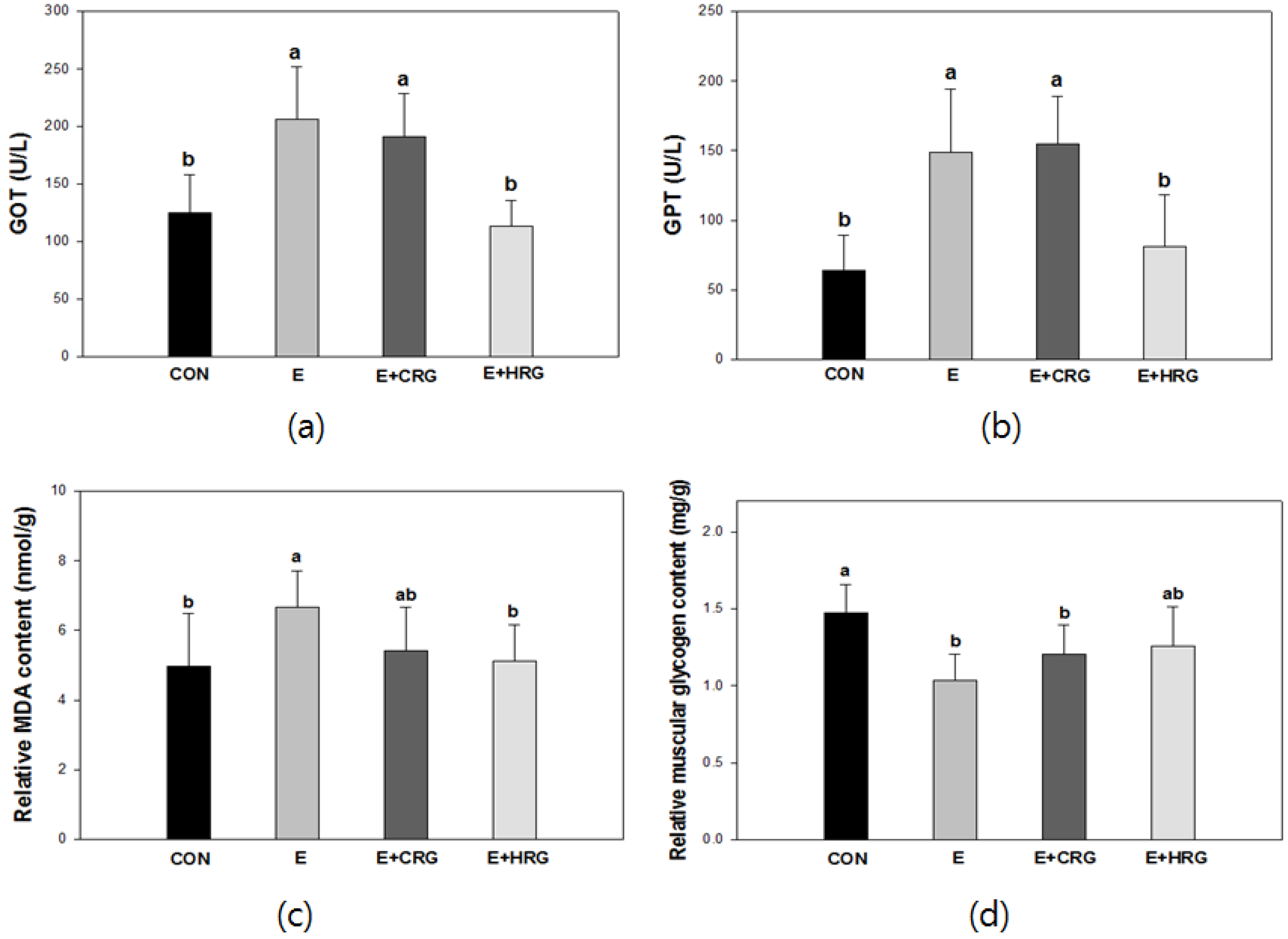
3.2. GOT, GPT, MDA, and Glycogen Analysis in Blood and Tissues
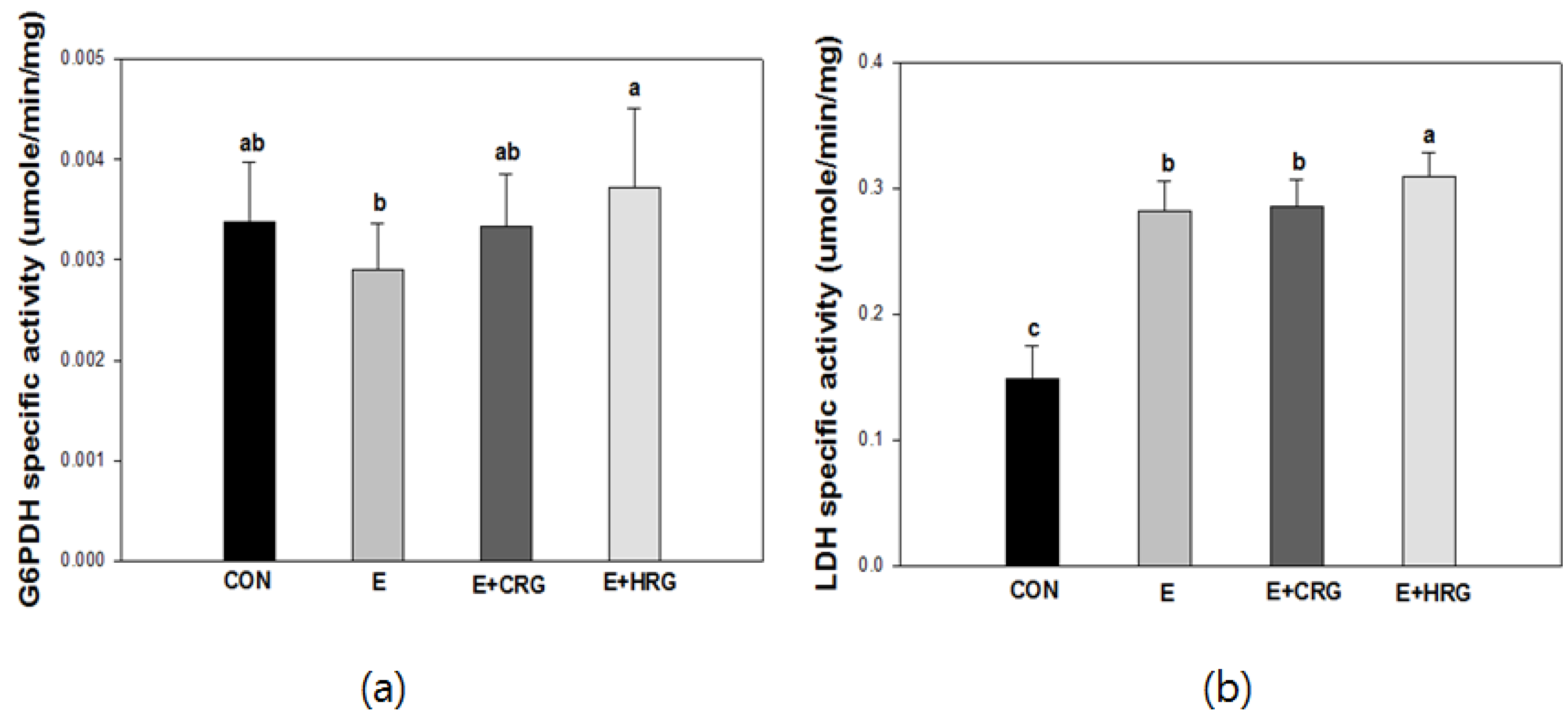
3.3. G6PDH and LDH Enzyme Activities in Gastrocnemius Tissue
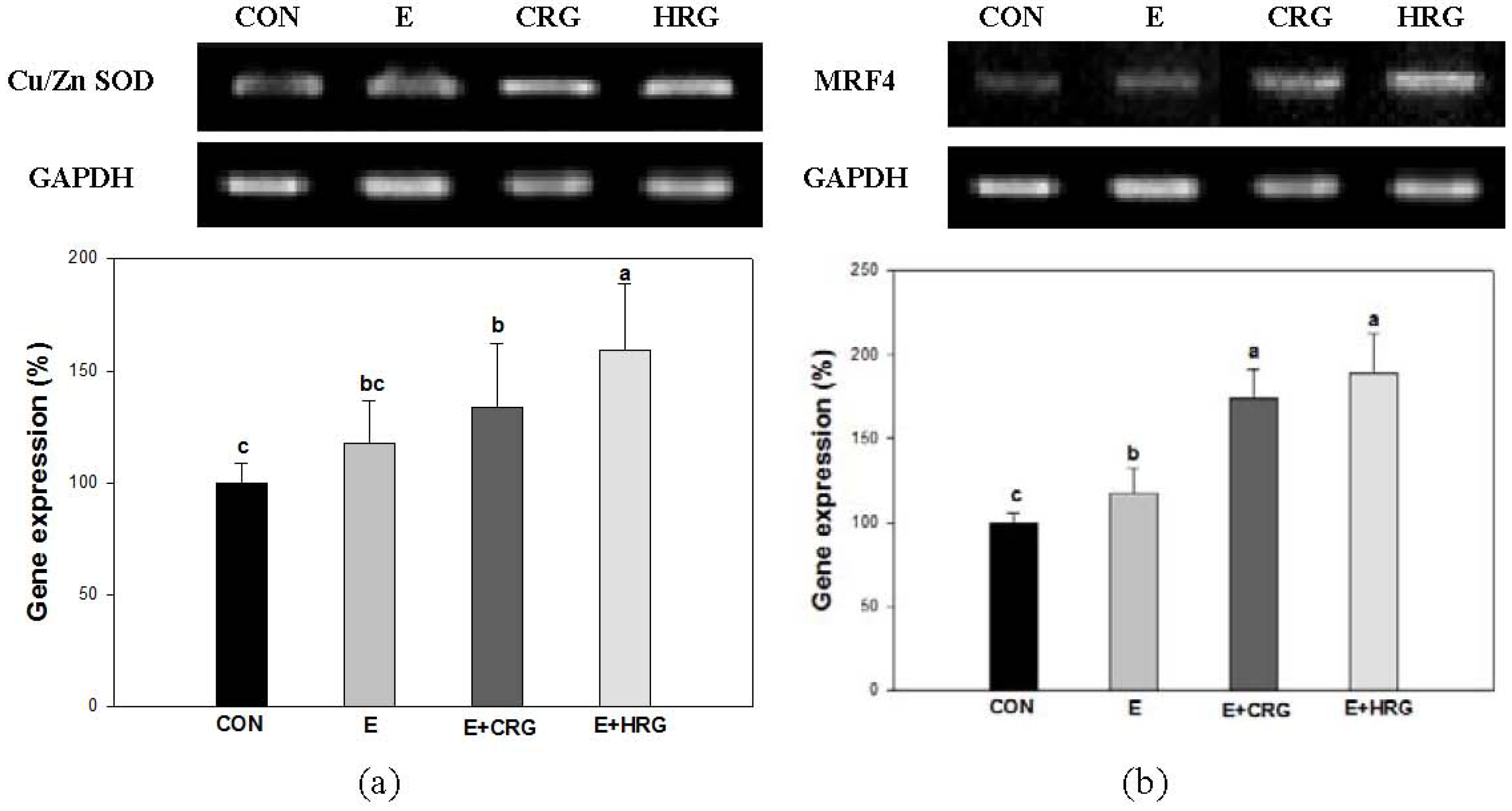
3.4. Cu/Zn-SOD and MRF4 mRNA Expression
4. Discussion
5. Conclusions
Conflicts of Interest
References
- Leeuwenburgh, C.; Heinecke, J.W. Oxidative stress and antioxidants in exercise. Curr. Med. Chem. 2001, 8, 829–838. [Google Scholar] [CrossRef]
- Manjamalai, A.; Grace, V.B. Antioxidant activity of essential oils from Wedelia chinensis (Osbeck) in vitro and in vivo lung cancer bearing C57BL/6 mice. Asian Pac. J. Cancer Prev. 2012, 13, 3065–3071. [Google Scholar] [CrossRef]
- Kim, E.; Rhee, D. Anti-oxidative properties of ginseng. J. Ginseng Res. 2009, 33, 1–7. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Effects of α-lipoic acid and l-carnosine supplementation on antioxidant activities and lipid profiles in rats. Nutr. Res. Pract. 2011, 5, 421–428. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Kim, B.G.; Choi, S.Y.; Kim, M.R.; Suh, H.J.; Park, H.J. Changes of ginsenosides in Korean red ginseng (Panax ginseng) fermented by Lactobacillus plantarum M1. Process Biochem. 2010, 45, 1319–1324. [Google Scholar] [CrossRef]
- Hong, Y.J.; Kim, N.; Lee, K.; Hee Sonn, C.; Eun Lee, L.; Tae Kim, S.; Ho Baeq, I.; Lee, K.M. Korean red ginseng (Panax ginseng) ameliorates type 1 diabetes and restores immune cell compartments. J. Ethnopharmacol. 2012, 144, 224–233. [Google Scholar]
- Kim, S.N.; Ha, Y.W.; Shin, H.; Son, S.H.; Wu, S.J.; Kim, Y.S. Simultaneous quantification of 14 ginsenosides in Panax ginseng CA Meyer (Korean red ginseng) by HPLC-ELSD and its application to quality control. J. Pharm. Biomed. Anal. 2007, 45, 164–170. [Google Scholar] [CrossRef]
- An, Y.R. Anti-Obesity Effect Study of Red Ginseng in HFD Induced Mouse Model by Gene Expression Profiling Analysis. Master’s Thesis, The graduate School Hanyang University, 2010. [Google Scholar]
- Sumiyoshi, M.; Sakanaka, M.; Kimura, Y. Effects of red ginseng extract on allergic reactions to food in Balb/c mice. J. Ethnopharmacol. 2010, 132, 206–212. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.Y. Korean red ginseng stimulates insulin release from isolated rat pancreatic islets. J. Ethnopharmacol. 2008, 120, 190–195. [Google Scholar] [CrossRef]
- Hong, H.D.; Kim, Y.C.; Rho, J.H.; Kim, K.T.; Lee, Y.C. Changes on physicochemical properties of Panax ginseng C.A. Meyer during repeated steaming process. J. Ginseng Res. 2007, 31, 222–229. [Google Scholar] [CrossRef]
- Jung, K.H.; Hong, H.D.; Cho, C.W.; Lee, M.Y.; Choi, U.K.; Kim, Y.C. Phenolic acid composition and antioxidative activity of red ginseng prepared by high temperature and high pressure process. Korean J. Food Nutr. 2012, 25, 827–832. [Google Scholar] [CrossRef]
- Yoon, B.R.; Lee, Y.J.; Hong, H.D.; Lee, Y.C.; Kim, Y.C.; Rhee, Y.K.; Kim, K.T.; Lee, O.H. Inhibitory effects of Panax ginseng C.A. Mayer treated with high temperature and high pressure on oxidative stress. Korean J. Food Nutr. 2012, 25, 800–806. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Seifter, S.; Dayton, S.; Novic, B.; Muntwyler, E. The estimation of glycogen with the anthrone reagent. Arch. Biochem. 1950, 25, 191–200. [Google Scholar]
- Deutsch, J. Glucose-6-phosphate dehydrogenase. Methods Enzym. Anal. 1983, 3, 190–196. [Google Scholar]
- Narang, S. Lactate dehydrogenase of Biomphalaria glabrata (say, 1818) (mollusca: Pulmonata)—I. Physico-chemical characterization of lactate dehydrogenase of various tissues. Comp. Biochem. Physiol. 1974, 47, 641–655. [Google Scholar]
- Statistical Analysis System, version 9.2; SAS Institute Inc.: Cary, NC, USA, 1988.
- Kim, P.; Park, J.H.; Kwon, K.J.; Kim, K.C.; Kim, H.J.; Lee, J.M.; Kim, H.Y.; Han, S.H.; Shin, C.Y. Effects of Korean red ginseng extracts on neural tube defects and impairment of social interaction induced by prenatal exposure to valproic acid. Food Chem. Toxicol. 2012, 51, 288–296. [Google Scholar]
- Jang, M.; Lee, M.J.; Kim, C.S.; Cho, I.H. Korean red ginseng extract attenuates 3-Nitropropionic acid-induced huntington’s-like symptoms. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Park, D.; Lee, H.; Yoon, M. Korean red ginseng (Panax ginseng) prevents obesity by inhibiting angiogenesis in high fat diet-induced obese C57BL/6J mice. Food Chem. Toxicol. 2012, 53, 402–408. [Google Scholar]
- Yoo, D.G.; Kim, M.C.; Park, M.K.; Song, J.M.; Quan, F.S.; Park, K.M.; Cho, Y.K.; Kang, S.M. Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J. Med. Food 2012, 15, 855–862. [Google Scholar] [CrossRef]
- Dong, G.Z.; Jang, E.J.; Kang, S.H.; Cho, I.J.; Park, S.D.; Kim, S.C.; Kim, Y.W. Red ginseng abrogates oxidative stress via mitochondria protection mediated by LKB1-AMPK pathway. BMC Complement. Altern. Med. 2013, 13, 64. [Google Scholar] [CrossRef]
- Metivier, G.; Gauthier, R. Effects of acute physical exercise on some serum enzymes in healthy male subjects between the ages of 40 and 64 years. Enzyme 1985, 33, 25–33. [Google Scholar]
- Yoshida, Y.; Itoh, N.; Hayakawa, M.; Piga, R.; Cynshi, O.; Jishage, K.i.; Niki, E. Lipid peroxidation induced by carbon tetrachloride and its inhibition by antioxidant as evaluated by an oxidative stress marker, HODE. Toxicol. Appl. Pharm. 2005, 208, 87–97. [Google Scholar] [CrossRef]
- Hawas, U.W.; Soliman, G.M.; Abou, E.K.L.; Farrag, A.; Mahmoud, K.; Leon, F. A new flavonoid C-glycoside from Solanum elaeagnifolium with hepatoprotective and curative activities against paracetamol-induced liver injury in mice. Z. Naturforsch. 2013, 68, 19–28. [Google Scholar] [CrossRef]
- Pederson, B.A.; Cope, C.R.; Irimia, J.M.; Schroeder, J.M.; Thurberg, B.L.; DePaoli-Roach, A.A.; Roach, P.J. Mice with elevated muscle glycogen stores do not have improved exercise performance. Biochem. Biophys. Res. Commun. 2005, 331, 491–496. [Google Scholar] [CrossRef]
- Hassan, A.F.; Kamal, M.M. Effect of exercise training and anabolic androgenic steroids on hemodynamics, glycogen content, angiogenesis and apoptosis of cardiac muscle in adult male rats. Int. J. Health Sci. 2013, 7, 47–60. [Google Scholar]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar]
- Yilmaz, S.; Beytut, E.; Erişir, M.; Ozan, S.; Aksakal, M. Effects of additional vitamin E and selenium supply on G6PDH activity in rats treated with high doses of glucocorticoid. Neurosci. Lett. 2006, 393, 85–89. [Google Scholar] [CrossRef]
- Richardson, R.S.; Noyszewski, E.A.; Leigh, J.S.; Wagner, P.D. Lactate efflux from exercising human skeletal muscle: Role of intracellular PO2. J. Appl. Physiol. 1998, 85, 627–634. [Google Scholar]
- Xu, J.; Li, Y. Effects of salidroside on exhaustive exercise-induced oxidative stress in rats. Mol. Med. Rep. 2012, 6, 1195–1198. [Google Scholar]
- Kan, N.W.; Huang, W.C.; Lin, W.T.; Huang, C.Y.; Wen, K.C.; Chiang, H.M.; Huang, C.C.; Hsu, M.C. Hepatoprotective effects of lxora parviflora extract against exhaustive exercise-induced oxidative stress in mice. Molecules 2013, 18, 10721–10732. [Google Scholar] [CrossRef]
- Kumaran, V.S.; Arulmathi, K.; Kalaiselvi, P. Senescence mediated redox imbalance in cardiac tissue: Antioxidant rejuvenating potential of green tea extract. Nutrition 2009, 25, 847–854. [Google Scholar] [CrossRef]
- Ramesh, T.; Kim, S.W.; Sung, J.H.; Hwang, S.Y.; Sohn, S.H.; Yoo, S.K.; Kim, S.K. Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp. Gerontol. 2012, 47, 77–84. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.Y.; Kang, H.J.; Kim, O.Y.; Lee, J.H. Beneficial effects of Korean red ginseng on lymphocyte DNA damage, antioxidant enzyme activity, and LDL oxidation in healthy participants: A randomized, double-blind, placebo-controlled trial. Nutr. J. 2012, 11, 47–55. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, K.S.; Chang, M.J.; Sung, J.H. Effects of Panax ginseng extract on exercise-induced oxidative stress. J. Sports Med. Phys. Fit. 2005, 45, 178–182. [Google Scholar]
- Wang, Q.; Hebert, S.L.; Rich, M.M.; Kraner, S.D. Loss of synaptic vesicles from neuromuscular junctions in aged MRF4-null mice. Neuroreport 2011, 22, 185–189. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Spektor, T.M.; Rice, J.C.; Sattler, F.R.; Schroeder, E.T. Influence of hormone replacement therapy on eccentric exercise induced myogenic gene expression in postmenopausal women. J. Appl. Physiol. 2009, 107, 1381–1388. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, S.-Y.; Yoon, B.-R.; Lee, Y.-J.; Lee, J.S.; Hong, H.-D.; Lee, Y.-C.; Kim, Y.-C.; Cho, C.-W.; Kim, K.-T.; Lee, O.-H. Inhibitory Effect of High Temperature- and High Pressure-Treated Red Ginseng on Exercise-Induced Oxidative Stress in ICR Mouse. Nutrients 2014, 6, 1003-1015. https://doi.org/10.3390/nu6031003
Yu S-Y, Yoon B-R, Lee Y-J, Lee JS, Hong H-D, Lee Y-C, Kim Y-C, Cho C-W, Kim K-T, Lee O-H. Inhibitory Effect of High Temperature- and High Pressure-Treated Red Ginseng on Exercise-Induced Oxidative Stress in ICR Mouse. Nutrients. 2014; 6(3):1003-1015. https://doi.org/10.3390/nu6031003
Chicago/Turabian StyleYu, Seok-Yeong, Bo-Ra Yoon, Young-Jun Lee, Jong Seok Lee, Hee-Do Hong, Young-Chul Lee, Young-Chan Kim, Chang-Won Cho, Kyung-Tack Kim, and Ok-Hwan Lee. 2014. "Inhibitory Effect of High Temperature- and High Pressure-Treated Red Ginseng on Exercise-Induced Oxidative Stress in ICR Mouse" Nutrients 6, no. 3: 1003-1015. https://doi.org/10.3390/nu6031003
APA StyleYu, S.-Y., Yoon, B.-R., Lee, Y.-J., Lee, J. S., Hong, H.-D., Lee, Y.-C., Kim, Y.-C., Cho, C.-W., Kim, K.-T., & Lee, O.-H. (2014). Inhibitory Effect of High Temperature- and High Pressure-Treated Red Ginseng on Exercise-Induced Oxidative Stress in ICR Mouse. Nutrients, 6(3), 1003-1015. https://doi.org/10.3390/nu6031003






