Cocoa Polyphenols and Inflammatory Markers of Cardiovascular Disease
Abstract
:1. Introduction
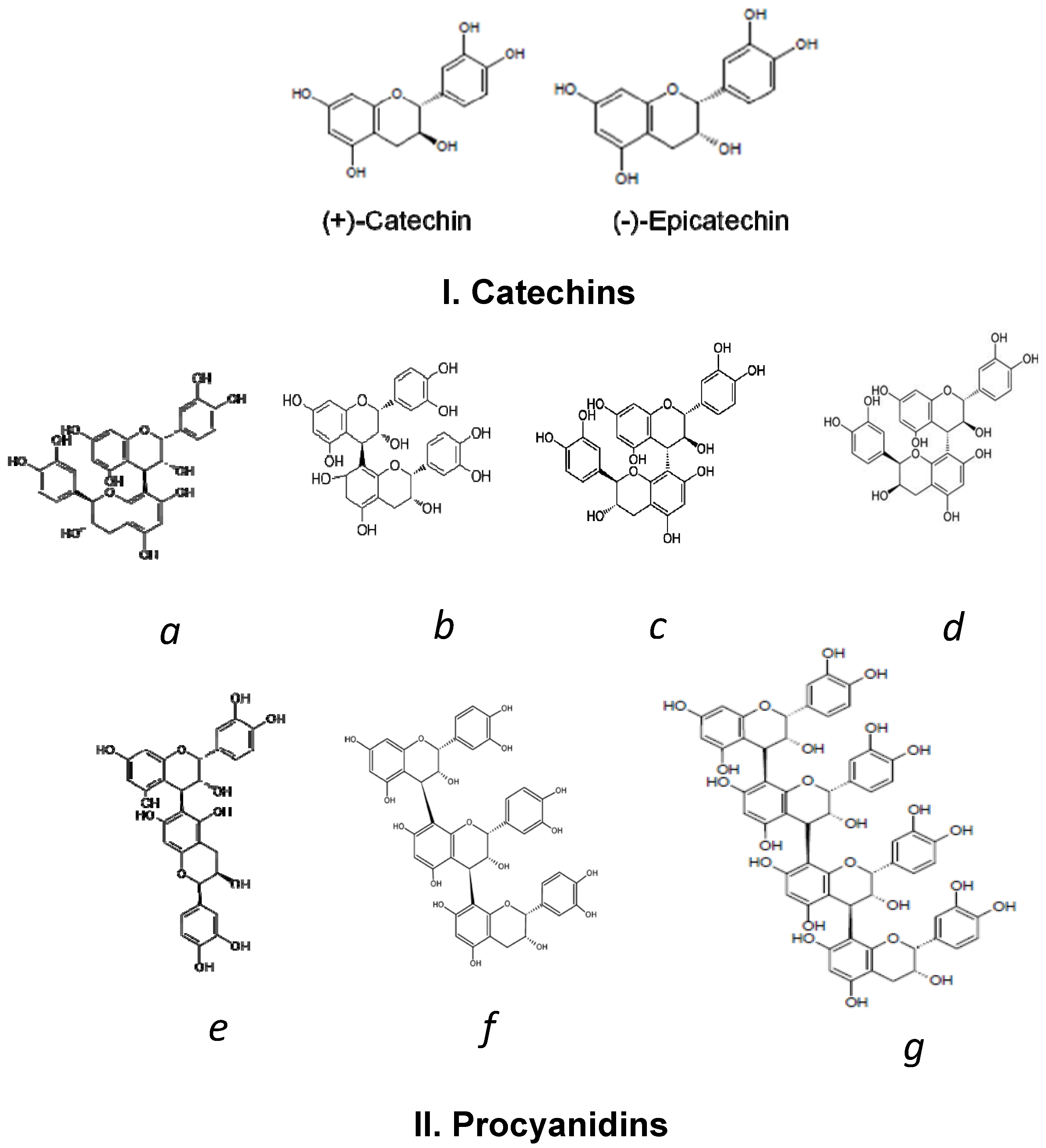
2. Cocoa Polyphenols
3. Bioavailability of Cocoa Polyphenols
4. Cocoa Polyphenols and CVD Inflammatory Markers
4.1. Human Studies
| Intervention | Comparison | Population | Markers of Dietary Compliance | Cocoa Consumption Impact | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cocoa Source (dose) | Type (time) | Study Design | Polyphenol Content | N | Subjects Status | ||||
| High- vs. Low-procyanidin chocolate (37 g/dose) | AI | CO | High-procyanidin chocolate: 148 mg tot. Pr | H vs. L | 10 | Healthy | 2 h plasma (BM) 6 h plasma (BM) | 2 h postprandial: | [89] |
| ↓leukotriene/prostacyclin ratio | |||||||||
| Low-procyanidin chocolate: 3.3 mg tot. Pr | ↓leukotrienes (C4 + D4 + E4) | ||||||||
| ↑prostacyclins | |||||||||
| Dark chocolate + cocoa beverage (36.9 g + 30.95 g/day) | MTI (6 weeks) | NC | Chocolate (daily): | Before/After | 25 | Healthy | Plasma (tot. PPh) | No effect: IL-1beta, IL-6, TNF-α, hs-CRP, P-selectin | [176] |
| 168.3 mg Pr | |||||||||
| Cocoa beverage (daily): | |||||||||
| 483.1 mg Pr | |||||||||
| Dark chocolate (41 g/day) | MTI (6 weeks) | PA | NA | Before/After DC vs. Ctrl group | 10 | Healthy | NA | ↓ICAM-1 | [193] |
| No effect: VCAM-1 and hs-CRP | |||||||||
| Chocolate and cocoa beverage (48 g chocolate + 18 g cocoa beverage/day) | AI (90 min) | PA PA | Flavanol group (daily): | Before/After Flavanol vs. Placebo (Ctrl) | 40 | Coronary artery disease (CAD) | NA | No effect (acute or chronic): ICAM-1, VCAM-1, E- and P-selectins and hs-CRP | [198] |
| 444 mg flavanols | |||||||||
| 107 mg epicatechin | |||||||||
| MTI (6 weeks) | Control group (daily): | ||||||||
| 19.6 mg flavanols | |||||||||
| 4.7 mg epicatechin | |||||||||
| Low- and High-flavanol cocoa beverages (36 g powder (18.8 g cocoa) per 240 mL W) | MTI (6 weeks) | PA | High-flavanol group: | Before/After H vs. L group | 32 | Postmenopausal hypercholesterolemic women | Fasting plasma (BM) | ↓sVCAM-1(High-flavanol group) No effect: ICAM-1; E- and P-selectins | [200] |
| 446 mg tot. flavanols | |||||||||
| Low-flavanol group: | |||||||||
| 43 mg tot. flavanols | |||||||||
| Dark chocolate 70% (100 g/day) | STI (7 days) | NC | Daily: 700 mg flavonoids | Before/After | 28 | Healthy | NA | ↓hs-CRP (females only, n = 19) | [196] |
| No effect: hs-CRP (common group) | |||||||||
| Dark chocolate (50 g/day × 2 times/day) | MTI (15 days) | CO | Dark chocolate (daily): | DC vs. WC (Ctrl) | 19 | Hypertensive prediabetic | NA | No effects: hs-CRP | [199] |
| 1008 mg tot. PPh | |||||||||
| 110.9 mg epicatechin | |||||||||
| 36.12 mg catechin | |||||||||
| White chocolate(daily): | |||||||||
| 0.13 g tot. PPh | |||||||||
| 0.04 mg catechin | |||||||||
| Cocoa beverage (31 g/150 mL W × 2 times/day | MTI (2 weeks) | CO | Flavanol beverage (daily): | Before/After Flavanol vs. Control beverage (Ctrl) | 20 | Hypertensive | Fasting plasma (BM) | No effect: TNF-α, IL-6, MCP-1, E-selectin, VCAM-1, and ICAM-1 | [201] |
| 451 mg tot. PPh | |||||||||
| 57 mg epicatechin | |||||||||
| 31 mg catechin | |||||||||
| 338 mg Pr | |||||||||
| Control beverage (daily): | |||||||||
| 14 mg tot. PPh | |||||||||
| 1 mg epicatechin | |||||||||
| 4 mg catechin | |||||||||
| 8 mg Pr | |||||||||
| Cocoa powder (20 g/250 mL M × 2 times/day) | MTI (4 weeks) | CO | Daily: 40.41 mg (+)-catechin | Cocoa (CM) vs. M (Ctrl) | 42 | CVD high risk | 24 h urine (BM) | ↓VLA-4, CD40, CD36 (monocytes) ↓P-selectin and ICAM-1 (serum) Non-significant changes: ↓VCAM-1 and MCP-1 No effect: hs-CRP, IL-6, E-selectin | [123] |
| 46.08 mg (−)-epicatechin | |||||||||
| 36.54 mg procyanidin B2 | |||||||||
| 495.2 mg tot.PPh | |||||||||
| 425.7 mg tot.Pr | |||||||||
| High- and Low-cocoa flavanol beverage (NA) | MTI (4 weeks) | CO | High-cocoa flavanol (daily): | Before/After H vs. L | 20 | Healthy | Fasting plasma (BM) 24 h urine (BM) | ↓CRP (High-cocoa flavanol group) | [194] |
| 494 mg tot. Flavanols | |||||||||
| Low-cocoa flavanol (daily): | |||||||||
| 23 mg tot. flavanols | |||||||||
| Different flavanol content cocoa beverages vs. Control beverage (28 g cocoa powder in W × 2 times/day) | STI (5 days) | CO | Cocoa beverages (daily): | L, M, H vs. Control beverage (Ctrl) | 20 | Obese healthy | NA | ↓CRP ↓IL-6 No effect: ICAM | [195] |
| 180 mg flavanols (Low) | |||||||||
| 400 mg flavanols (Medium) | |||||||||
| 900 mg flavanols (High) | |||||||||
| Control beverage (daily): | |||||||||
| 30 mg flavanols | |||||||||
| Dark chocolate 70% (25 g ×2 times/day) | MTI (4 weeks) | NC | Daily: 2135 mg PPh | Before/After | 20 | Hypertensive (excess body weight) | NA | Non-significant changes: | [202] |
| ↓ICAM-1 | |||||||||
| ↓VCAM-1, | |||||||||
| ↓E-selectin | |||||||||
| Dark chocolate 70% (100 g/day) | STI (7 days) | NC | 444 mg/kg catechin | Before/After | 15 | Normal weight obese women | NA | ↓IL-1Ra and No effect: IL-1α, IL-1β, IL-6, and TNF-α | [166] |
| 908 mg/kg epicatechin | |||||||||
| Cocoa product rich in fibre (15 g/200 mL M × 2 times/day) | MTI (4 weeks) | CO | 13·9 mg/g soluble PPh | Cocoa vs. M (Ctrl) | 24 20 | Healthy and Hypercholesterolemic subjects | NA | ↓IL-1β, IL-10 | [156] |
| No effect: IL-6, TNF-α, IL-8, | |||||||||
| MCP-1, VCAM and ICAM | |||||||||
| Cocoa powder (40 g/250 mL M or W) | AI (6 h) | CO | Daily: 40.41 mg (+)-catechin | Before/After Cocoa (CM+CW) vs. W (Ctrl) CM vs. CW | 18 | Healthy | 2 h plasma (BM) 6 h urine (BM) | ↓NF-κB ↓E-selectin ↓ICAM-1 No effect: VCAM-1 | [203] |
| 46.08 mg (−)-epicatechin | |||||||||
| 36.54 mg procyanidin B2 | |||||||||
| 495.2 mg tot.PPh | |||||||||
| 425.7 mg tot.Pr | |||||||||
4.2. Animal Models, in Vitro and Cell Culture Studies
| Model | Treatment (dose) | Outcomes | Ref. |
|---|---|---|---|
| Leukotrienes | |||
| (a) Isolated rabbit 15-LOX-1 | (a) Cocoa procyanidins: monomers to decamers (2.9 mg/mL) | Dose-dependent: (a) ↓15-LOX-1 activity | [215] |
| (b) Recombinant human platelet 12-LOX | (b) Epicatechin & procyanidin decamers | (b) ↓12-LOX activity | |
| Recombinant human 5-LOX | Cocoa epicatechin & procyanidins | ↓5-LOX activity | [216] |
| (10 μmol/L) | ↓Pro-inflammatory mediators (LTB4, LTC4, LTD4) | ||
| Pro-Inflammatory and Anti-Inflammatory Cytokines | |||
| PHA-stimulated PBMC | Cocoa procyanidins: monomers through decamers (25 μg/mL) | ↓IL-1β secretion (monomer to tetramer) | [217] |
| ↑IL-1β secretion (pentamer to decamer) | |||
| ↓IL-2 expression (pentamer to heptamer) | |||
| ↓IL-4 expression & secretion (pentamer to decamer) | |||
| Human PBMC | Cocoa procyanidins: monomers through decamers (25 μg/mL) | ↑IL-1β transcription & secretion (pentamers-decamers) | [218] |
| ↓IL-1β transcription & secretion (monomers-tetramers) | |||
| Resting and (PHA)-stimulated human PBMC | Cocoa procyanidins: monomers through decamers (25 μg/mL) | Resting PBMCs: | [219] |
| ↑IL-4 secretion (hexamer-decamer fraction) | |||
| PHA-stimulated PBMCs: | |||
| ↑IL-4 secretion (monomeric fraction) | |||
| ↓IL-4 secretion (hexamer-decamer fraction) | |||
| Resting and (PHA)-stimulated human PBMC | CFP fractions: monomers through decamers (25 μg/mL) | ↓ TNF-α (monomers and dimers) | [220] |
| ↑ TNF-α (tetramers through octamers) | |||
| PHA-stimulated PBMC | Cocoa flavanols and their related oligomers (25 μg/mL) | ↓ IL-5 release (oligomeric fractions: hexamers to decamers) | [221] |
| Rat NR8383 macrophages | Cocoa polyphenol extract (10–50 μg/mL tot. PPh) vs. epicatechin (30–60 μg/mL tot. PPh) | ↓ TNF-α, MCP-1, IL-1α, IL-6) | [222] |
| Murine EL4BOU6 lymphocytes | Cocoa extract (5–80 μg/mL tot. PPh) vs. epicatechin (60–120 μg/mL tot. PPh) | ↓IL-2 secretion | [223] |
| ↑IL-4 secretion | |||
| ↓T lymphocyte activation | |||
| Human PBMC | Cocoa flavanol fractions: Short (monomers-pentamers) | ↑IL-1β, IL-6, IL-10, TNF-α (long-chain fraction) | [224] |
| Long-chain (hexamers-decamers) (20 μg/mL) | |||
| LPS-stimulated human PBMC | Cocoa phenolic acids: (3,4-DHPPA, 3-HPA, | ↓TNF-α | [225] |
| 3,4-DHPAA, 3-HPAA, | ↓IL-6 | ||
| 4-HBA) (1 µM) | ↑IL-1β | ||
| NF-κB Activity | |||
| Jurkat T cells | Purified cocoa: [(−)-epicatechin (EC) and | ↓NF-κB | [226] |
| (+)-catechin (CT)] and a B dimeric procyanidin (DP-B) (1.7–17.2 µM) | ↓IL-2 transactivation | ||
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011.
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; de Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010, 121, e46–e215. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Halfon, N.; Hochstein, M. Life course health development: An integrated framework for developing health, policy, and research. Milbank Q. 2002, 80, 433–479. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Kuh, D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. Int. J. Epidemiol. 2002, 31, 285–293. [Google Scholar] [CrossRef]
- Wang, D.; He, Y.; Li, Y.; Luan, D.; Zhai, F.; Yang, X.; Ma, G. Joint association of dietary pattern and physical activity level with cardiovascular disease risk factors among Chinese men: A cross-sectional study. PLoS One 2013, 8, e66210. [Google Scholar]
- Chomistek, A.K.; Manson, J.E.; Stefanick, M.L.; Lu, B.; Sands-Lincoln, M.; Going, S.B.; Garcia, L.; Allison, M.A.; Sims, S.T.; LaMonte, M.J.; et al. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: Results from the Women’s Health Initiative. J. Am. Coll. Cardiol. 2013, 61, 2346–2354. [Google Scholar] [CrossRef]
- Chomistek, A.K.; Cook, N.R.; Flint, A.J.; Rimm, E.B. Vigorous-intensity leisure-time physical activity and risk of major chronic disease in men. Med. Sci. Sports Exerc. 2012, 44, 1898–1905. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Maghsoudi, Z.; Shirani, F.; Azadbakht, L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases-incidence: A systematic review and meta-analysis on observational prospective studies. Nutrition 2013, 29, 611–618. [Google Scholar] [CrossRef]
- He, M.; van Dam, R.M.; Rimm, E.; Hu, F.B.; Qi, L. Whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation 2010, 121, 2162–2168. [Google Scholar] [CrossRef]
- De Moura, F.F.; Lewis, K.D.; Falk, M.C. Applying the FDA definition of whole grains to the evidence for cardiovascular disease health claims. J. Nutr. 2009, 139, 2220S–2226S. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Steffen, L.M.; Loehr, L.R.; Rosamond, W.D.; Folsom, A.R. Incident heart failure is associated with lower whole-grain intake and greater high-fat dairy and egg intake in the Atherosclerosis Risk in Communities (ARIC) study. J. Am. Diet. Assoc. 2008, 108, 1881–1887. [Google Scholar] [CrossRef]
- Dauchet, L.; Ferrieres, J.; Arveiler, D.; Yarnell, J.W.; Gey, F.; Ducimetiere, P.; Ruidavets, J.B.; Haas, B.; Evans, A.; Bingham, A.; et al. Frequency of fruit and vegetable consumption and coronary heart disease in France and Northern Ireland: The PRIME study. Br. J. Nutr. 2004, 92, 963–972. [Google Scholar] [CrossRef]
- Dauchet, L.; Montaye, M.; Ruidavets, J.B.; Arveiler, D.; Kee, F.; Bingham, A.; Ferrieres, J.; Haas, B.; Evans, A.; Ducimetiere, P.; et al. Association between the frequency of fruit and vegetable consumption and cardiovascular disease in male smokers and non-smokers. Eur. J. Clin. Nutr. 2010, 64, 578–586. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar]
- Bhupathiraju, S.N.; Tucker, K.L. Greater variety in fruit and vegetable intake is associated with lower inflammation in Puerto Rican adults. Am. J. Clin. Nutr. 2011, 93, 37–46. [Google Scholar] [CrossRef]
- Clifton, P.M. Effect of grape seed extract and quercetin on cardiovascular and endothelial parameters in high-risk subjects. J. Biomed. Biotechnol. 2004, 2004, 272–278. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef]
- George, T.W.; Niwat, C.; Waroonphan, S.; Gordon, M.H.; Lovegrove, J.A. Effects of chronic and acute consumption of fruit- and vegetable-puree-based drinks on vasodilation, risk factors for CVD and the response as a result of the eNOS G298T polymorphism. Proc. Nutr. Soc. 2009, 68, 148–161. [Google Scholar] [CrossRef]
- Duthie, S.J.; Jenkinson, A.M.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.; Yap, L.S.; Christen, P.; Duthie, G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Franke, A.A.; Cooney, R.V.; Henning, S.M.; Custer, L.J. Bioavailability and antioxidant effects of orange juice components in humans. J. Agric. Food Chem. 2005, 53, 5170–5178. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Laniewska, I.; Millo, B.; Dluzniewski, M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis 2007, 194, e179–e184. [Google Scholar] [CrossRef]
- Source: UNCTAD Based on the Data from International Cocoa Organization, Quaterly Bulletin of Cocoa Statistics. Available online: http://www.unctad.info/en/Infocomm/Beverages/Cocoa2/Market/ (accessed on 10 August 2013).
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef]
- Vinson, J.A.; Proch, J.; Zubik, L. Phenol antioxidant quantity and quality in foods: Cocoa, dark chocolate, and milk chocolate. J. Agric. Food Chem. 1999, 47, 4821–4824. [Google Scholar] [CrossRef]
- Lea, A.G.H. The Procyanidins of Cocoa. In Proceedings of the American Chemical Society Meeting, Denver, CO, USA, 5–10 April 1987.
- Lea, A.G.H.; Ford, G.D. The Polyphenols of Cocoa Part I: Isolation and Characterisation of Procyanidins from Unfermented Beans; The Cadbury Schweppes Group: London, UK, 1988. [Google Scholar]
- Lea, P.J.; Ford, G.D. Characterisation of the Polyphenols of Cocoa; Bioflavour: Glasgow, UK, 1990. [Google Scholar]
- Niemenak, N.; Rohsius, C.; Elwers, S.; Ndoumou, D.O.; Lieberei, R. Comaparative study of different cocoa (Theobroma cacao L.) clones in terms of their phenolics and anthocyanins contents. J. Food Compos. Anal. 2006, 19, 612–619. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Shirley, J.R.; Donovan, J.L. Antioxidants in chocolate. Lancet 1996, 348, 834. [Google Scholar] [CrossRef]
- Adamson, G.E.; Lazarus, S.A.; Mitchell, A.E.; Prior, R.L.; Cao, G.; Jacobs, P.H.; Kremers, B.G.; Hammerstone, J.F.; Rucker, R.B.; Ritter, K.A.; et al. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J. Agric. Food Chem. 1999, 47, 4184–4188. [Google Scholar] [CrossRef]
- Buijsse, B.; Feskens, E.J.; Kok, F.J.; Kromhout, D. Cocoa intake, blood pressure, and cardiovascular mortality: The Zutphen Elderly Study. Arch. Intern. Med. 2006, 166, 411–417. [Google Scholar]
- Janszky, I.; Mukamal, K.J.; Ljung, R.; Ahnve, S.; Ahlbom, A.; Hallqvist, J. Chocolate consumption and mortality following a first acute myocardial infarction: The Stockholm Heart Epidemiology Program. J. Intern. Med. 2009, 266, 248–257. [Google Scholar] [CrossRef]
- Ried, K.; Sullivan, T.R.; Fakler, P.; Frank, O.R.; Stocks, N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2012, 8, CD008893. [Google Scholar]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Jia, L.; Liu, X.; Bai, Y.Y.; Li, S.H.; Sun, K.; He, C.; Hui, R. Short-term effect of cocoa product consumption on lipid profile: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2010, 92, 218–225. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar]
- Visioli, F.; Bernaert, H.; Corti, R.; Ferri, C.; Heptinstall, S.; Molinari, E.; Poli, A.; Serafini, M.; Smit, H.J.; Vinson, J.A.; et al. Chocolate, lifestyle, and health. Crit. Rev. Food Sci. Nutr. 2009, 49, 299–312. [Google Scholar] [CrossRef]
- Corti, R.; Flammer, A.J.; Hollenberg, N.K.; Luscher, T.F. Cocoa and cardiovascular health. Circulation 2009, 119, 1433–1441. [Google Scholar] [CrossRef]
- Rimbach, G.; Melchin, M.; Moehring, J.; Wagner, A.E. Polyphenols from cocoa and vascular health—A critical review. Int. J. Mol. Sci. 2009, 10, 4290–4309. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Packard, R.R.; Libby, P. Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clin. Chem. 2008, 54, 24–38. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammatory mechanisms in atherosclerosis. J. Thromb. Haemost. 2009, 7, 328–331. [Google Scholar] [CrossRef]
- Willerson, J.T.; Ridker, P.M. Inflammation as a cardiovascular risk factor. Circulation 2004, 109, II2–II10. [Google Scholar] [CrossRef]
- Kaplan, R.C.; Frishman, W.H. Systemic inflammation as a cardiovascular disease risk factor and as a potential target for drug therapy. Heart Dis. 2001, 3, 326–332. [Google Scholar] [CrossRef]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Harwood, M.L.; Ziegler, G.R.; Hayes, J.E. Tolerance for high flavanol cocoa powder in semisweet chocolate. Nutrients 2013, 5, 2258–2267. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on polyphenols in theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef]
- Porter, L.J.; Ma, Z.; Chan, B.G. Cacao procyanidins: Major flavonoids and identification of some minor metabolites. Phytochemistry 1991, 30, 1657–1663. [Google Scholar] [CrossRef]
- Hurst, W.J.; Glinski, J.A.; Miller, K.B.; Apgar, J.; Davey, M.H.; Stuart, D.A. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J. Agric. Food Chem. 2008, 56, 8374–8378. [Google Scholar] [CrossRef]
- Lamuela-Raventos, R.M.; Andres-Lacueva, C.; Permanyer, J.; Izquierdo-Pulido, M. More antioxidants in cocoa. J. Nutr. 2001, 131, 834–835. [Google Scholar]
- Sanchez-Rabaneda, F.; Jauregui, O.; Casals, I.; Andres-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventos, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Qin, Y.Z.; Holt, R.R.; Lazarus, S.A.; Ensunsa, J.L.; Hammerstone, J.F.; Schmitz, H.H.; Keen, C.L. Stability of flavan-3-ols epicatechin and catechin and related dimeric procyanidins derived from cocoa. J. Agric. Food Chem. 2002, 50, 1700–1705. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Hammerstone, J.F.; Lazarus, S.A.; Mitchell, A.E.; Rucker, R.; Schmitz, H.H. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 1999, 47, 490–496. [Google Scholar] [CrossRef]
- Tanaka, T.; Kondou, K.; Kouno, I. Oxidation and epimerization of epigallocatechin in banana fruits. Phytochemistry 2000, 53, 311–316. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Hammerstone, J.F.; Lazarus, S.A.; Schmitz, H.H.; Keen, C.L. Stabilizing effect of ascorbic acid on flavan-3-ols and dimeric procyanidins from cocoa. J. Agric. Food Chem. 2003, 51, 828–833. [Google Scholar] [CrossRef]
- Kelm, M.A.; Johnson, J.C.; Robbins, R.J.; Hammerstone, J.F.; Schmitz, H.H. High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J. Agric. Food Chem. 2006, 54, 1571–1576. [Google Scholar] [CrossRef]
- Clapperton, J.; Lockwood, R.; Romanczyk, L.; Hammerstone, J.F. Contribution of genotype to cocoa (Theobroma cacao L.) flavour. Trop. Agric. 1994, 71, 303–308. [Google Scholar]
- Azizah, O.; Amin, I.; Nawalyah, A.G.; Ilham, A. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007, 100, 1523–1530. [Google Scholar] [CrossRef]
- Cooper, K.A.; Campos-Gimenez, E.; Jimenez Alvarez, D.; Nagy, K.; Donovan, J.L.; Williamson, G. Rapid reversed phase ultra-performance liquid chromatography analysis of the major cocoa polyphenols and inter-relationships of their concentrations in chocolate. J. Agric. Food Chem. 2007, 55, 2841–2847. [Google Scholar] [CrossRef]
- Kim, H.; Keeney, P.G. (−)-Epicatechin content in fermented and unfermented cocoa beans. J. Food Sci. 1984, 49, 1090–1092. [Google Scholar] [CrossRef]
- Robinson, T.; Ranalli, A.W.; Phillips, A.W. Changes in cocoa tannins during processing. J. Agric. Food Chem. 1961, 9, 295–298. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Monagas, M.; Khan, N.; Izquierdo-Pulido, M.; Urpi-Sarda, M.; Permanyer, J.; Lamuela-Raventos, R.M. Flavanol and flavonol contents of cocoa powder products: Influence of the manufacturing process. J. Agric. Food Chem. 2008, 56, 3111–3117. [Google Scholar] [CrossRef]
- Kyi, T.M.; Wan Ramli, W.D.; Abu Bakar, M.; Mohd. Wahid, S.; Abdul Amir, H.K.; Meor Zainal, M.T. The kinetics of polyphenol degradation during the drying of malaysian cocoa beans. Int. J. Food Sci. Technol. 2005, 40, 323–331. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef]
- Hurst, W.J.; Krake, S.H.; Bergmeier, S.C.; Payne, M.J.; Miller, K.B.; Stuart, D.A. Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem. Cent. J. 2011, 5, 53. [Google Scholar] [CrossRef]
- Hii, C.L.; Law, C.L.; Suzannah, S.; Misnawi; Cloke, M. Polyphenols in cocoa (Theobroma cacao L.). Asian J. Food Agro Ind. 2009, 2, 702–722. [Google Scholar]
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. Food Chem. 2006, 54, 4057–4061. [Google Scholar] [CrossRef]
- De Brito, E.S.; Garcia, N.H.P.; Gallao, M.I.; Cortelazzo, A.L.; Fevereiro, P.S.; Braga, M.R. Structural and chemical changes in cocoa (Theobroma Cacao) during fermentation, drying and roasting. J. Sci. Food Agric. 2000, 81, 281–288. [Google Scholar]
- Cooper, K.A.; Donovan, J.L.; Waterhouse, A.L.; Williamson, G. Cocoa and health: A decade of research. Br. J. Nutr. 2008, 99, 1–11. [Google Scholar]
- Miller, K.B.; Stuart, D.A.; Smith, N.L.; Lee, C.Y.; McHale, N.L.; Flanagan, J.A.; Ou, B.; Hurst, W.J. Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States. J. Agric. Food Chem. 2006, 54, 4062–4068. [Google Scholar] [CrossRef]
- Miller, K.B.; Hurst, W.J.; Flannigan, N.; Ou, B.; Lee, C.Y.; Smith, N.; Stuart, D.A. Survey of commercially available chocolate- and cocoa-containing products in the United States. 2. Comparison of flavan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao. J. Agric. Food Chem. 2009, 57, 9169–9180. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar]
- Donovan, J.L.; Crespy, V.; Oliveira, M.; Cooper, K.A.; Gibson, B.B.; Williamson, G. (+)-Catechin is more bioavailable than (−)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Radic. Res. 2006, 40, 1029–1034. [Google Scholar] [CrossRef]
- Kofink, M.; Papagiannopoulos, M.; Galensa, R. (−)-Catechin in cocoa and chocolate: Occurrence and analysis of an atypical flavan-3-ol enantiomer. Molecules 2007, 12, 1274–1288. [Google Scholar] [CrossRef]
- Natsume, M.; Osakabe, N.; Yamagishi, M.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000, 64, 2581–2587. [Google Scholar] [CrossRef]
- Andrés-Lacueva, C.; Lamuela-Raventós, R.M.; Jáuregui, O.; Casals, I.; Izquierdo-Pulido, M.; Permanyer, J. An LC method for the analysis of cocoa phenolics. LC GC Eur. 2000, 12, 902–904. [Google Scholar]
- Bonvehi, J.S.; Coll, F.V. Evaluation of the bitterness and astringency of polyphenolic comppunds in cocoa powder. Food Chem. 1997, 60, 365–370. [Google Scholar] [CrossRef]
- Stahl, L.; Miller, K.B.; Apgar, J.; Sweigart, D.S.; Stuart, D.A.; McHale, N.; Ou, B.; Kondo, M.; Hurst, W.J. Preservation of cocoa antioxidant activity, total polyphenols, flavan-3-ols, and procyanidin content in foods prepared with cocoa powder. J. Food Sci. 2009, 74, C456–C461. [Google Scholar] [CrossRef]
- Emmanuel Ohene Afoakwa. Chocolate Science and Technology; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Arranz, S.; Valderas-Martinez, P.; Chiva-Blanch, G.; Casas, R.; Urpi-Sarda, M.; Lamuela-Raventos, R.M.; Estruch, R. Cardioprotective effects of cocoa: Clinical evidence from randomized clinical intervention trials in humans. Mol. Nutr. Food Res. 2013, 57, 936–947. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Corcoran, M.P.; McKay, D.L.; Blumberg, J.B. Flavonoid basics: Chemistry, sources, mechanisms of action, and safety. J. Nutr. Gerontol. Geriatr. 2012, 31, 176–189. [Google Scholar] [CrossRef]
- Schramm, D.D.; Wang, J.F.; Holt, R.R.; Ensunsa, J.L.; Gonsalves, J.L.; Lazarus, S.A.; Schmitz, H.H.; German, J.B.; Keen, C.L. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am. J. Clin. Nutr. 2001, 73, 36–40. [Google Scholar]
- Rein, D.; Lotito, S.; Holt, R.R.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G. Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status. J. Nutr. 2000, 130, 2109S–2114S. [Google Scholar]
- Holt, R.R.; Schramm, D.D.; Keen, C.L.; Lazarus, S.A.; Schmitz, H.H. Chocolate consumption and platelet function. JAMA 2002, 287, 2212–2213. [Google Scholar] [CrossRef]
- Pearson, D.A.; Paglieroni, T.G.; Rein, D.; Wun, T.; Schramm, D.D.; Wang, J.F.; Holt, R.R.; Gosselin, R.; Schmitz, H.H.; Keen, C.L. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res. 2002, 106, 191–197. [Google Scholar] [CrossRef]
- Steinberg, F.M.; Holt, R.R.; Schmitz, H.H.; Keen, C.L. Cocoa procyanidin chain length does not determine ability to protect LDL from oxidation when monomer units are controlled. J. Nutr. Biochem. 2002, 13, 645–652. [Google Scholar] [CrossRef]
- Holt, R.R.; Lazarus, S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. Procyanidin dimer B2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2002, 76, 798–804. [Google Scholar]
- Richelle, M.; Tavazzi, I.; Enslen, M.; Offord, E.A. Plasma kinetics in man of epicatechin from black chocolate. Eur. J. Clin. Nutr. 1999, 53, 22–26. [Google Scholar]
- Mullen, W.; Borges, G.; Donovan, J.L.; Edwards, C.A.; Serafini, M.; Lean, M.E.; Crozier, A. Milk decreases urinary excretion but not plasma pharmacokinetics of cocoa flavan-3-ol metabolites in humans. Am. J. Clin. Nutr. 2009, 89, 1784–1791. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Leveques, A.; Giuffrida, F.; Romanov-Michailidis, F.; Viton, F.; Barron, D.; Duenas-Paton, M.; Gonzalez-Manzano, S.; Santos-Buelga, C.; Williamson, G.; et al. Elucidation of (−)-epicatechin metabolites after ingestion of chocolate by healthy humans. Free Radic. Biol. Med. 2012, 53, 787–795. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Yasuda, A.; Natsume, M.; Takizawa, T.; Nakamura, T.; Terao, J. Bioavailability of (−)-epicatechin upon intake of chocolate and cocoa in human volunteers. Free Radic. Res. 2000, 33, 635–641. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Leveques, A.; Rein, M.; Teml, A.; Schafer, C.; Hofmann, U.; Li, H.; Schwab, M.; Eichelbaum, M.; Williamson, G. Intestinal absorption, metabolism, and excretion of (−)-epicatechin in healthy humans assessed by using an intestinal perfusion technique. Am. J. Clin. Nutr. 2013, 98, 924–933. [Google Scholar] [CrossRef]
- Wang, J.F.; Schramm, D.D.; Holt, R.R.; Ensunsa, J.L.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J. Nutr. 2000, 130, 2115S–2119S. [Google Scholar]
- Deprez, S.; Mila, I.; Huneau, J.F.; Tome, D.; Scalbert, A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid. Redox Signal. 2001, 3, 957–967. [Google Scholar] [CrossRef]
- Spencer, J.P.; Schroeter, H.; Shenoy, B.; Srai, S.K.; Debnam, E.S.; Rice-Evans, C. Epicatechin is the primary bioavailable form of the procyanidin dimers B2 and B5 after transfer across the small intestine. Biochem. Biophys. Res. Commun. 2001, 285, 588–593. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Momma, T.Y.; Heiss, C.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radic. Biol. Med. 2011, 50, 237–244. [Google Scholar] [CrossRef]
- Ritter, C.; Zimmermann, B.F.; Galensa, R. Chiral separation of (+)/(−)-catechin from sulfated and glucuronidated metabolites in human plasma after cocoa consumption. Anal. Bioanal. Chem. 2010, 397, 723–730. [Google Scholar] [CrossRef]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Remesy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef]
- Donovan, J.L.; Manach, C.; Faulks, R.M.; Kroon, P.A. Absorption and Metabolism of Plant Secondary Metabolites. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 303–351. [Google Scholar]
- Ottaviani, J.I.; Momma, T.Y.; Kuhnle, G.K.; Keen, C.L.; Schroeter, H. Structurally related (−)-epicatechin metabolites in humans: Assessment using de novo chemically synthesized authentic standards. Free Radic. Biol. Med. 2012, 52, 1403–1412. [Google Scholar] [CrossRef]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011, 55, S44–S55. [Google Scholar] [CrossRef]
- Stoupi, S.; Williamson, G.; Drynan, J.W.; Barron, D.; Clifford, M.N. A comparison of the in vitro biotransformation of (−)-epicatechin and procyanidin B2 by human faecal microbiota. Mol. Nutr. Food Res. 2010, 54, 747–759. [Google Scholar]
- Rios, L.Y.; Gonthier, M.P.; Remesy, C.; Mila, I.; Lapierre, C.; Lazarus, S.A.; Williamson, G.; Scalbert, A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am. J. Clin. Nutr. 2003, 77, 912–918. [Google Scholar]
- Meng, X.; Sang, S.; Zhu, N.; Lu, H.; Sheng, S.; Lee, M.J.; Ho, C.T.; Yang, C.S. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Llorach, R.; Khan, N.; Monagas, M.; Rotches-Ribalta, M.; Lamuela-Raventos, R.; Estruch, R.; Tinahones, F.J.; Andres-Lacueva, C. Effect of milk on the urinary excretion of microbial phenolic acids after cocoa powder consumption in humans. J. Agric. Food Chem. 2010, 58, 4706–4711. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Llorach, R.; Lamuela-Raventos, R.M.; Jauregui, O.; Estruch, R.; Izquierdo-Pulido, M.; Andres-Lacueva, C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7258–7267. [Google Scholar]
- Appeldoorn, M.M.; Vincken, J.P.; Aura, A.M.; Hollman, P.C.; Gruppen, H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites. J. Agric. Food Chem. 2009, 57, 1084–1092. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Lamuela-Raventos, R.M.; Santos-Buelga, C.; Sacanella, E.; Castell, M.; Permanyer, J.; Andres-Lacueva, C. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal. Bioanal. Chem. 2009, 394, 1545–1556. [Google Scholar] [CrossRef]
- Depeint, F.; Gee, J.M.; Williamson, G.; Johnson, I.T. Evidence for consistent patterns between flavonoid structures and cellular activities. Proc. Nutr. Soc. 2002, 61, 97–103. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar]
- Llorach, R.; Urpi-Sarda, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Andres-Lacueva, C. Metabolomic fingerprint in patients at high risk of cardiovascular disease by cocoa intervention. Mol. Nutr. Food Res. 2013, 57, 962–973. [Google Scholar] [CrossRef]
- Llorach-Asuncion, R.; Jauregui, O.; Urpi-Sarda, M.; Andres-Lacueva, C. Methodological aspects for metabolome visualization and characterization: A metabolomic evaluation of the 24 h evolution of human urine after cocoa powder consumption. J. Pharm. Biomed. Anal. 2010, 51, 373–381. [Google Scholar] [CrossRef]
- Llorach, R.; Urpi-Sarda, M.; Jauregui, O.; Monagas, M.; Andres-Lacueva, C. An LC-MS-based metabolomics approach for exploring urinary metabolome modifications after cocoa consumption. J. Proteome Res. 2009, 8, 5060–5068. [Google Scholar] [CrossRef]
- Ito, H.; Gonthier, M.P.; Manach, C.; Morand, C.; Mennen, L.; Remesy, C.; Scalbert, A. Polyphenol levels in human urine after intake of six different polyphenol-rich beverages. Br. J. Nutr. 2005, 94, 500–509. [Google Scholar] [CrossRef]
- Monagas, M.; Khan, N.; Andres-Lacueva, C.; Casas, R.; Urpi-Sarda, M.; Llorach, R.; Lamuela-Raventos, R.M.; Estruch, R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2009, 90, 1144–1150. [Google Scholar] [CrossRef]
- Khan, N.; Monagas, M.; Andres-Lacueva, C.; Casas, R.; Urpi-Sarda, M.; Lamuela-Raventos, R.M.; Estruch, R. Regular consumption of cocoa powder with milk increases HDL cholesterol and reduces oxidized LDL levels in subjects at high-risk of cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 1046–1053. [Google Scholar]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Dore, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef]
- Dall’Asta, M.; Calani, L.; Tedeschi, M.; Jechiu, L.; Brighenti, F.; Del Rio, D. Identification of microbial metabolites derived from in vitro fecal fermentation of different polyphenolic food sources. Nutrition 2012, 28, 197–203. [Google Scholar]
- Prakash, S.; Rodes, L.; Coussa-Charley, M.; Tomaro-Duchesneau, C. Gut microbiota: Next frontier in understanding human health and development of biotherapeutics. Biol. Targets Ther. 2011, 5, 71–86. [Google Scholar]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Bolca, S.; van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef]
- Martin, F.P.; Montoliu, I.; Nagy, K.; Moco, S.; Collino, S.; Guy, P.; Redeuil, K.; Scherer, M.; Rezzi, S.; Kochhar, S. Specific dietary preferences are linked to differing gut microbial metabolic activity in response to dark chocolate intake. J. Proteome Res. 2012, 11, 6252–6263. [Google Scholar]
- Serafini, M.; Bugianesi, R.; Maiani, G.; Valtuena, S.; de Santis, S.; Crozier, A. Plasma antioxidants from chocolate. Nature 2003, 424, 1013. [Google Scholar] [CrossRef]
- Roura, E.; Andres-Lacueva, C.; Estruch, R.; Lourdes Mata Bilbao, M.; Izquierdo-Pulido, M.; Lamuela-Raventos, R.M. The effects of milk as a food matrix for polyphenols on the excretion profile of cocoa (−)-epicatechin metabolites in healthy human subjects. Br. J. Nutr. 2008, 100, 846–851. [Google Scholar]
- Roura, E.; Andres-Lacueva, C.; Estruch, R.; Mata-Bilbao, M.L.; Izquierdo-Pulido, M.; Waterhouse, A.L.; Lamuela-Raventos, R.M. Milk does not affect the bioavailability of cocoa powder flavonoid in healthy human. Ann. Nutr. Metab. 2007, 51, 493–498. [Google Scholar] [CrossRef]
- Schroeter, H.; Holt, R.R.; Orozco, T.J.; Schmitz, H.H.; Keen, C.L. Nutrition: Milk and absorption of dietary flavanols. Nature 2003, 426, 787–788. [Google Scholar]
- Keogh, J.B.; McInerney, J.; Clifton, P.M. The effect of milk protein on the bioavailability of cocoa polyphenols. J. Food Sci. 2007, 72, S230–S233. [Google Scholar] [CrossRef]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Kirkpatrick, N.J.; Polagruto, J.A.; Ensunsa, J.L.; Schmitz, H.H.; Keen, C.L. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003, 73, 857–869. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Oruna-Concha, M.J.; Kwik-Uribe, C.; Vidal, A.; Spencer, J.P. Influence of sugar type on the bioavailability of cocoa flavanols. Br. J. Nutr. 2012, 108, 2243–2250. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Bordenave, N.; Hamaker, B.R. Does flavor impact function? Potential consequences of polyphenol-protein interactions in delivery and bioactivity of flavan-3-ols from foods. Physiol. Behav. 2012, 107, 591–597. [Google Scholar] [CrossRef]
- Neilson, A.P.; Ferruzzi, M.G. Influence of formulation and processing on absorption and metabolism of flavan-3-ols from tea and cocoa. Annu. Rev. Food Sci. Technol. 2011, 2, 125–151. [Google Scholar] [CrossRef]
- Neilson, A.P.; George, J.C.; Janle, E.M.; Mattes, R.D.; Rudolph, R.; Matusheski, N.V.; Ferruzzi, M.G. Influence of chocolate matrix composition on cocoa flavan-3-ol bioaccessibility in vitro and bioavailability in humans. J. Agric. Food Chem. 2009, 57, 9418–9426. [Google Scholar] [CrossRef]
- Wan, Y.; Vinson, J.A.; Etherton, T.D.; Proch, J.; Lazarus, S.A.; Kris-Etherton, P.M. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am. J. Clin. Nutr. 2001, 74, 596–602. [Google Scholar]
- Rimbach, G.; Egert, S.; de Pascual-Teresa, S. Chocolate: (Un)healthy source of polyphenols? Genes Nutr. 2011, 6, 1–3. [Google Scholar] [CrossRef]
- Murphy, K.J.; Chronopoulos, A.K.; Singh, I.; Francis, M.A.; Moriarty, H.; Pike, M.J.; Turner, A.H.; Mann, N.J.; Sinclair, A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003, 77, 1466–1473. [Google Scholar]
- Martin, F.P.; Rezzi, S.; Pere-Trepat, E.; Kamlage, B.; Collino, S.; Leibold, E.; Kastler, J.; Rein, D.; Fay, L.B.; Kochhar, S. Metabolic effects of dark chocolate consumption on energy, gut microbiota, and stress-related metabolism in free-living subjects. J. Proteome Res. 2009, 8, 5568–5579. [Google Scholar] [CrossRef]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Keen, C.L. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr. Opin. Lipidol. 2002, 13, 41–49. [Google Scholar] [CrossRef]
- McCullough, M.L.; Chevaux, K.; Jackson, L.; Preston, M.; Martinez, G.; Schmitz, H.H.; Coletti, C.; Campos, H.; Hollenberg, N.K. Hypertension, the Kuna, and the epidemiology of flavanols. J. Cardiovasc. Pharmacol. 2006, 47, S103–S109. [Google Scholar] [CrossRef]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007, 85, 709–717. [Google Scholar]
- Osakabe, N.; Baba, S.; Yasuda, A.; Iwamoto, T.; Kamiyama, M.; Takizawa, T.; Itakura, H.; Kondo, K. Daily cocoa intake reduces the susceptibility of low-density lipoprotein to oxidation as demonstrated in healthy human volunteers. Free Radic. Res. 2001, 34, 93–99. [Google Scholar] [CrossRef]
- Sarria, B.; Martinez-Lopez, S.; Sierra-Cinos, J.L.; Garcia-Diz, L.; Mateos, R.; Bravo, L. Regular consumption of a cocoa product improves the cardiometabolic profile in healthy and moderately hypercholesterolaemic adults. Br. J. Nutr. 2014, 111, 122–134. [Google Scholar] [CrossRef]
- Fisher, N.D.; Hollenberg, N.K. Aging and vascular responses to flavanol-rich cocoa. J. Hypertens. 2006, 24, 1575–1580. [Google Scholar] [CrossRef]
- Heiss, C.; Finis, D.; Kleinbongard, P.; Hoffmann, A.; Rassaf, T.; Kelm, M.; Sies, H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J. Cardiovasc. Pharmacol. 2007, 49, 74–80. [Google Scholar]
- Heiss, C.; Kleinbongard, P.; Dejam, A.; Perre, S.; Schroeter, H.; Sies, H.; Kelm, M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2005, 46, 1276–1283. [Google Scholar] [CrossRef]
- Innes, A.J.; Kennedy, G.; McLaren, M.; Bancroft, A.J.; Belch, J.J. Dark chocolate inhibits platelet aggregation in healthy volunteers. Platelets 2003, 14, 325–327. [Google Scholar] [CrossRef]
- Balzer, J.; Rassaf, T.; Heiss, C.; Kleinbongard, P.; Lauer, T.; Merx, M.; Heussen, N.; Gross, H.B.; Keen, C.L.; Schroeter, H.; et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008, 51, 2141–2149. [Google Scholar] [CrossRef]
- Faridi, Z.; Njike, V.Y.; Dutta, S.; Ali, A.; Katz, D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2008, 88, 58–63. [Google Scholar]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Summary of American heart association diet and lifestyle recommendations revision 2006. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2186–2191. [Google Scholar] [CrossRef]
- Baba, S.; Natsume, M.; Yasuda, A.; Nakamura, Y.; Tamura, T.; Osakabe, N.; Kanegae, M.; Kondo, K. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J. Nutr. 2007, 137, 1436–1441. [Google Scholar]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Rissanen, T.H.; Virtanen, J.K.; Kaikkonen, J.; Nyyssonen, K.; Salonen, J.T. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radic. Biol. Med. 2004, 37, 1351–1359. [Google Scholar] [CrossRef]
- Di Renzo, L.; Rizzo, M.; Sarlo, F.; Colica, C.; Iacopino, L.; Domino, E.; Sergi, D.; de Lorenzo, A. Effects of dark chocolate in a population of Normal Weight Obese women: A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2257–2266. [Google Scholar]
- Steinberg, D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 1997, 272, 20963–20966. [Google Scholar] [CrossRef]
- Witztum, J.L.; Steinberg, D. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Investig. 1991, 88, 1785–1792. [Google Scholar] [CrossRef]
- Fraley, A.E.; Tsimikas, S. Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr. Opin. Lipidol. 2006, 17, 502–509. [Google Scholar] [CrossRef]
- Holvoet, P.; Lee, D.H.; Steffes, M.; Gross, M.; Jacobs, D.R., Jr. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA 2008, 299, 2287–2293. [Google Scholar] [CrossRef]
- Ishigaki, Y.; Oka, Y.; Katagiri, H. Circulating oxidized LDL: A biomarker and a pathogenic factor. Curr. Opin. Lipidol. 2009, 20, 363–369. [Google Scholar] [CrossRef]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Takizawa, T.; Terao, J.; Kondo, K. Catechins and their oligomers linked by C4→C8 bonds are major cacao polyphenols and protect low-density lipoprotein from oxidation in vitro. Exp. Biol. Med. 2002, 227, 51–56. [Google Scholar]
- Richelle, M.; Tavazzi, I.; Offord, E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J. Agric. Food Chem. 2001, 49, 3438–3442. [Google Scholar] [CrossRef]
- Kurosawa, T.; Itoh, F.; Nozaki, A.; Nakano, Y.; Katsuda, S.; Osakabe, N.; Tsubone, H.; Kondo, K.; Itakura, H. Suppressive effects of cacao liquor polyphenols (CLP) on LDL oxidation and the development of atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. Atherosclerosis 2005, 179, 237–246. [Google Scholar]
- Kurosawa, T.; Itoh, F.; Nozaki, A.; Nakano, Y.; Katsuda, S.; Osakabe, N.; Tsubone, H.; Kondo, K.; Itakura, H. Suppressive effect of cocoa powder on atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. J. Atheroscler. Thromb. 2005, 12, 20–28. [Google Scholar]
- Mathur, S.; Devaraj, S.; Grundy, S.M.; Jialal, I. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J. Nutr. 2002, 132, 3663–3667. [Google Scholar]
- Vita, J.A. Endothelial function and clinical outcome. Heart 2005, 91, 1278–1279. [Google Scholar] [CrossRef]
- Rein, D.; Paglieroni, T.G.; Wun, T.; Pearson, D.A.; Schmitz, H.H.; Gosselin, R.; Keen, C.L. Cocoa inhibits platelet activation and function. Am. J. Clin. Nutr. 2000, 72, 30–35. [Google Scholar]
- Monahan, K.D. Effect of cocoa/chocolate ingestion on brachial artery flow-mediated dilation and its relevance to cardiovascular health and disease in humans. Arch. Biochem. Biophys. 2012, 527, 90–94. [Google Scholar] [CrossRef]
- Sies, H.; Schewe, T.; Heiss, C.; Kelm, M. Cocoa polyphenols and inflammatory mediators. Am. J. Clin. Nutr. 2005, 81, 304S–312S. [Google Scholar]
- Fisher, N.D.; Hughes, M.; Gerhard-Herman, M.; Hollenberg, N.K. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J. Hypertens. 2003, 21, 2281–2286. [Google Scholar] [CrossRef]
- Heiss, C.; Dejam, A.; Kleinbongard, P.; Schewe, T.; Sies, H.; Kelm, M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 2003, 290, 1030–1031. [Google Scholar] [CrossRef]
- Grassi, D.; Necozione, S.; Lippi, C.; Croce, G.; Valeri, L.; Pasqualetti, P.; Desideri, G.; Blumberg, J.B.; Ferri, C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar]
- Fraga, C.G. Cocoa, diabetes, and hypertension: Should we eat more chocolate? A. J. Clin. Nutr. 2005, 81, 541–542. [Google Scholar]
- Flammer, A.J.; Hermann, F.; Sudano, I.; Spieker, L.; Hermann, M.; Cooper, K.A.; Serafini, M.; Luscher, T.F.; Ruschitzka, F.; Noll, G.; et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation 2007, 116, 2376–2382. [Google Scholar] [CrossRef]
- Holt, R.R.; Actis-Goretta, L.; Momma, T.Y.; Keen, C.L. Dietary flavanols and platelet reactivity. J. Cardiovasc. Pharmacol. 2006, 47, S187–S196. [Google Scholar]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar]
- Tracy, R.P. Inflammation in cardiovascular disease: Cart, horse, or both? Circulation 1998, 97, 2000–2002. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar]
- Di Giuseppe, R.; di Castelnuovo, A.; Centritto, F.; Zito, F.; de Curtis, A.; Costanzo, S.; Vohnout, B.; Sieri, S.; Krogh, V.; Donati, M.B.; et al. Regular consumption of dark chocolate is associated with low serum concentrations of C-reactive protein in a healthy Italian population. J. Nutr. 2008, 138, 1939–1945. [Google Scholar]
- Abraham, J.; Campbell, C.Y.; Cheema, A.; Gluckman, T.J.; Blumenthal, R.S.; Danyi, P. C-reactive protein in cardiovascular risk assessment: A review of the evidence. J. Cardiometab. Syndr. 2007, 2, 119–123. [Google Scholar] [CrossRef]
- Kurlandsky, S.B.; Stote, K.S. Cardioprotective effects of chocolate and almond consumption in healthy women. Nutr. Res. 2006, 26, 509–516. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Stote, K.S.; Clevidence, B.A.; Novotny, J.A.; Henderson, T.; Radecki, S.V.; Baer, D.J. Effect of cocoa and green tea on biomarkers of glucose regulation, oxidative stress, inflammation and hemostasis in obese adults at risk for insulin resistance. Eur. J. Clin. Nutr. 2012, 66, 1153–1159. [Google Scholar] [CrossRef]
- Hamed, M.S.; Gambert, S.; Bliden, K.P.; Bailon, O.; Singla, A.; Antonino, M.J.; Hamed, F.; Tantry, U.S.; Gurbel, P.A. Dark chocolate effect on platelet activity, C-reactive protein and lipid profile: A pilot study. South. Med. J. 2008, 101, 1203–1208. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Mumford, S.L.; Sjaarda, L.A. Failure to consider the menstrual cycle phase may cause misinterpretation of clinical and research findings of cardiometabolic biomarkers in premenopausal women. Epidemiol. Rev. 2014, 36, 71–82. [Google Scholar] [CrossRef]
- Farouque, H.M.; Leung, M.; Hope, S.A.; Baldi, M.; Schechter, C.; Cameron, J.D.; Meredith, I.T. Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: A randomized double-blind placebo-controlled study. Clin. Sci. 2006, 111, 71–80. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar]
- Wang-Polagruto, J.F.; Villablanca, A.C.; Polagruto, J.A.; Lee, L.; Holt, R.R.; Schrader, H.R.; Ensunsa, J.L.; Steinberg, F.M.; Schmitz, H.H.; Keen, C.L. Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J. Cardiovasc. Pharmacol. 2006, 47, S177–S186. [Google Scholar] [CrossRef]
- Muniyappa, R.; Hall, G.; Kolodziej, T.L.; Karne, R.J.; Crandon, S.K.; Quon, M.J. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am. J. Clin. Nutr. 2008, 88, 1685–1696. [Google Scholar] [CrossRef]
- Nogueira Lde, P.; Knibel, M.P.; Torres, M.R.; Nogueira Neto, J.F.; Sanjuliani, A.F. Consumption of high-polyphenol dark chocolate improves endothelial function in individuals with stage 1 hypertension and excess body weight. Int. J. Hypertens. 2012, 2012, 147321. [Google Scholar]
- Vazquez-Agell, M.; Urpi-Sarda, M.; Sacanella, E.; Camino-Lopez, S.; Chiva-Blanch, G.; Llorente-Cortes, V.; Tobias, E.; Roura, E.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; et al. Cocoa consumption reduces NF-κB activation in peripheral blood mononuclear cells in humans. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 257–263. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Roitman-Johnson, B.; Stampfer, M.J.; Allen, J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 1998, 351, 88–92. [Google Scholar] [CrossRef]
- Blann, A.D.; Seigneur, M.; Steiner, M.; Miller, J.P.; McCollum, C.N. Circulating ICAM-1 and VCAM-1 in peripheral artery disease and hypercholesterolaemia: Relationship to the location of atherosclerotic disease, smoking, and in the prediction of adverse events. Thromb. Haemost. 1998, 79, 1080–1085. [Google Scholar]
- Von der Thüsen, J.H.; Kuiper, J.; van Berkel, T.J.C.; Biessen, E.A.L. Interleukins in atherosclerosis: Molecular pathways and therapeutic potential. Pharmacol. Rev. 2003, 55, 133–166. [Google Scholar] [CrossRef]
- Thurberg, B.L.; Collins, T. The nuclear factor-κB/inhibitor of kappa B autoregulatory system and atherosclerosis. Curr. Opin. Lipidol. 1998, 9, 387–396. [Google Scholar] [CrossRef]
- Funk, C.D. Leukotriene inflammatory mediators meet their match. Sci. Transl. Med. 2011, 3, 66ps3. [Google Scholar]
- Riccioni, G.; Back, M. Leukotrienes as modifiers of preclinical atherosclerosis? Sci. World J. 2012, 2012, 490968. [Google Scholar]
- Gu, Y.; Yu, S.; Lambert, J.D. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur. J. Nutr. 2014, 53, 149–158. [Google Scholar] [CrossRef]
- Vinson, J.A.; Proch, J.; Bose, P.; Muchler, S.; Taffera, P.; Shuta, D.; Samman, N.; Agbor, G.A. Chocolate is a powerful ex vivo and in vivo antioxidant, an antiatherosclerotic agent in an animal model, and a significant contributor to antioxidants in the European and American Diets. J. Agric. Food Chem. 2006, 54, 8071–8076. [Google Scholar] [CrossRef]
- Desch, S.; Schmidt, J.; Kobler, D.; Sonnabend, M.; Eitel, I.; Sareban, M.; Rahimi, K.; Schuler, G.; Thiele, H. Effect of cocoa products on blood pressure: Systematic review and meta-analysis. Am. J. Hypertens. 2010, 23, 97–103. [Google Scholar] [CrossRef]
- Yakala, G.K.; Wielinga, P.Y.; Suarez, M.; Bunschoten, A.; van Golde, J.M.; Arola, L.; Keijer, J.; Kleemann, R.; Kooistra, T.; Heeringa, P. Effects of chocolate supplementation on metabolic and cardiovascular parameters in ApoE3L mice fed a high-cholesterol atherogenic diet. Mol. Nutr. Food Res. 2013, 57, 2039–2048. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Pérez-Cano, F.J.; Pérez-Berezo, T.; Castellote, C.; Franch, A.; Catell, M. A seven-day high cocoa diet decreces oxidant and inflammatory properties of peritoneal macrophages in rats. Proc. Nutr. Soc. 2010, 69, E253. [Google Scholar] [CrossRef]
- Schewe, T.; Sadik, C.; Klotz, L.O.; Yoshimoto, T.; Kuhn, H.; Sies, H. Polyphenols of cocoa: Inhibition of mammalian 15-lipoxygenase. Biol. Chem. 2001, 382, 1687–1696. [Google Scholar]
- Schewe, T.; Kuhn, H.; Sies, H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J. Nutr. 2002, 132, 1825–1829. [Google Scholar]
- Mao, T.; van de Water, J.; Keen, C.L.; Schmitz, H.H.; Gershwin, M.E. Cocoa procyanidins and human cytokine transcription and secretion. J. Nutr. 2000, 130, 2093S–2099S. [Google Scholar]
- Mao, T.K.; Powell, J.; van de Water, J.; Keen, C.L.; Schmitz, H.H.; Hammerstone, J.F.; Gershwin, M.E. The effect of cocoa procyanidins on the transcription and secretion of interleukin 1 beta in peripheral blood mononuclear cells. Life Sci. 2000, 66, 1377–1386. [Google Scholar] [CrossRef]
- Mao, T.K.; Powell, J.; van de water, J.; Keen, C.L.; Schmitz, H.H.; Gershwin, M.E. Effect of cocoa procyanidins on the secretion of interleukin-4 in peripheral blood mononuclear cells. J. Med. Food 2000, 3, 107–114. [Google Scholar] [CrossRef]
- Mao, T.K.; van de Water, J.; Keen, C.L.; Schmitz, H.H.; Gershwin, M.E. Modulation of TNF-alpha secretion in peripheral blood mononuclear cells by cocoa flavanols and procyanidins. Dev. Immunol. 2002, 9, 135–141. [Google Scholar] [CrossRef]
- Mao, T.K.; van de Water, J.; Keen, C.L.; Schmitz, H.H.; Gershwin, M.E. Effect of cocoa flavanols and their related oligomers on the secretion of interleukin-5 in peripheral blood mononuclear cells. J. Med. Food 2002, 5, 17–22. [Google Scholar] [CrossRef]
- Ramiro, E.; Franch, A.; Castellote, C.; Perez-Cano, F.; Permanyer, J.; Izquierdo-Pulido, M.; Castell, M. Flavonoids from Theobroma cacao down-regulate inflammatory mediators. J. Agric. Food Chem. 2005, 53, 8506–8511. [Google Scholar] [CrossRef]
- Ramiro, E.; Franch, A.; Castellote, C.; Andres-Lacueva, C.; Izquierdo-Pulido, M.; Castell, M. Effect of Theobroma cacao flavonoids on immune activation of a lymphoid cell line. Br. J. Nutr. 2005, 93, 859–866. [Google Scholar] [CrossRef]
- Kenny, T.P.; Keen, C.L.; Schmitz, H.H.; Gershwin, M.E. Immune effects of cocoa procyanidin oligomers on peripheral blood mononuclear cells. Exp. Biol. Med. 2007, 232, 293–300. [Google Scholar]
- Monagas, M.; Khan, N.; Andres-Lacueva, C.; Urpi-Sarda, M.; Vazquez-Agell, M.; Lamuela-Raventos, R.M.; Estruch, R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009, 102, 201–206. [Google Scholar] [CrossRef]
- Mackenzie, G.G.; Carrasquedo, F.; Delfino, J.M.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-κB activation at multiple steps in Jurkat T cells. FASEB J. 2004, 18, 167–169. [Google Scholar]
- Kroon, P.A.; Clifford, M.N.; Crozier, A.; Day, A.J.; Donovan, J.L.; Manach, C.; Williamson, G. How should we assess the effects of exposure to dietary polyphenols in vitro? Am. J. Clin. Nutr. 2004, 80, 15–21. [Google Scholar]
- Saliou, C.; Valacchi, G.; Rimbach, G. Assessing bioflavonoids as regulators of NF-κB activity and inflammatory gene expression in mammalian cells. Methods Enzymol. 2001, 335, 380–387. [Google Scholar] [CrossRef]
- Park, Y.C.; Rimbach, G.; Saliou, C.; Valacchi, G.; Packer, L. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-alpha secretion, and NF-κB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett. 2000, 465, 93–97. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef]
- Patel, K.R.; Andreadi, C.; Britton, R.G.; Horner-Glister, E.; Karmokar, A.; Sale, S.; Brown, V.A.; Brenner, D.E.; Singh, R.; Steward, W.P.; et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Science Transl. Med. 2013, 5, 205ra133. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khan, N.; Khymenets, O.; Urpí-Sardà, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cubillos, X.; Llorach, R.; Andres-Lacueva, C. Cocoa Polyphenols and Inflammatory Markers of Cardiovascular Disease. Nutrients 2014, 6, 844-880. https://doi.org/10.3390/nu6020844
Khan N, Khymenets O, Urpí-Sardà M, Tulipani S, Garcia-Aloy M, Monagas M, Mora-Cubillos X, Llorach R, Andres-Lacueva C. Cocoa Polyphenols and Inflammatory Markers of Cardiovascular Disease. Nutrients. 2014; 6(2):844-880. https://doi.org/10.3390/nu6020844
Chicago/Turabian StyleKhan, Nasiruddin, Olha Khymenets, Mireia Urpí-Sardà, Sara Tulipani, Mar Garcia-Aloy, María Monagas, Ximena Mora-Cubillos, Rafael Llorach, and Cristina Andres-Lacueva. 2014. "Cocoa Polyphenols and Inflammatory Markers of Cardiovascular Disease" Nutrients 6, no. 2: 844-880. https://doi.org/10.3390/nu6020844
APA StyleKhan, N., Khymenets, O., Urpí-Sardà, M., Tulipani, S., Garcia-Aloy, M., Monagas, M., Mora-Cubillos, X., Llorach, R., & Andres-Lacueva, C. (2014). Cocoa Polyphenols and Inflammatory Markers of Cardiovascular Disease. Nutrients, 6(2), 844-880. https://doi.org/10.3390/nu6020844





