Reduced Glutathione: A Radioprotector or a Modulator of DNA-Repair Activity?
Abstract
:Abbreviations
| GRX | glutaredoxin |
| MPG | mercaptopropionylglycine |
| BSO | buthionine sulfoximine |
| NHEJ | non-homologous end-joining |
| AP-1 | activator protein 1 |
| NF-κB | nuclear factor kappa B |
| Sp-1 | Specificity Protein 1 |
| APE1 | apurinic endonuclease 1 |
| NPSH | non-protein bound sulphydryls |
| MEA | β-mercaptoethylamine |
| AET | S-2-aminoethylisothiourea |
| TRX | thioredoxin |
| SSb | single-strand break |
| dsb | double-strand break |
1. Introduction
| Type of Reaction | GSH Acts as | Name of the Enzyme | Acts on | Citation |
|---|---|---|---|---|
| Enzymatic | electron donor | Glutathione peroxidase | various peroxides | [17] |
| Non-enzymatic | adduct (as in detoxification) | Glutathione S-transferase | xenobiotic compounds | [18] |
| building block of leukotriene (LT) | LTC4 synthase | conjugate LTA4 to form LTC4 and finally LTD4 | [19] | |
| antioxidant | reactive oxygen species | [20] | ||
| reducing agent | cytochrome C | [21] | ||
| hydrogen-donor | ribonucleotide | [22] | ||
| oxidative and nitrosative modifier | SH-group of GSH | [23] | ||
| oxidative modifier of proteins | Cys-SH of protein and GSH | [24] | ||
| reversible glutathionylation | GSH moiety of GRX that can be freed by another GSH | [25] |
2. Radioprotectors: Reducing Agents/Free Radical Scavengers
2.1. Reduced Glutathione as a Radioprotector
| Type of treatment and Parameters | Radiation and Dose | Dose of GSH Mode of Treatment | Results | Citation |
|---|---|---|---|---|
| Whole body radiation, weights and histologic appearance of tissues | X-rays; 8 Gy | 4 mg/g in mice subcutaneously | No protection of cellular damage, rapid regeneration of tissues | [51] |
| Chromosome aberrations in Tradescantia root tips | Co60-source; 50 Gy | 0.001–0.3 mM | Significant reduction | [52] |
| Frequency of sex-linked lethal and translocation in Drosophila melanogaster | X-rays; 20 Gy | 3.33 g/kg, injected | No protection | [53] |
| Marrow prophylaxis (C57BL × C3H) F1 mice | X-rays | 1.6 g/kg | Increment of LD50/30 from 7.25 to 9.5 Gy | [54] |
| Frequency of sex-linked lethal and translocation in Drosophila melanogaster | X-rays; 20 Gy | 1.65 mg/kg | Significant reduction of sex-linked lethal, slight reduction of translocation | [55] |
| Mitotic index and mitotic delay time in mammalian L-5 cells | X-rays; 2 Gy | 20 mM | Recovery rate of mitotic index facilitated but no effect on mitotic delay time | [56] |
| Reversion of his-dependent S. tiphimurium Ames test | - | 5–20 mM with and without liver/kidney S9-fraction | Increased number of revertants; positive for mutagenicity | [57] |
| Muntjac lymphocytes | X-rays; 2–4 Gy | 10–25 mM | Consistent protection of deletions; inconsistent protection of exchanges at 3 and 4 Gy | [35] |
| Short-term radiation lethality, adult male mice | X-rays; 4 Gy | 15 mg/kg | Cysteine, GSH & MPG less efficient radioprotectors than WR-2721 | [58] |
| Polychromatic erythrocytes in mouse bone marrow, peripheral blood; micronuclei | X-rays; 6 Gy | 400 mg/kg | Reduction in frequency of micronuclei induction | [33] |
2.2. Controversy on the Role of GSH as a Radioprotector
3. S-Glutathioylation of Proteins: A Crucial Modulating Factor
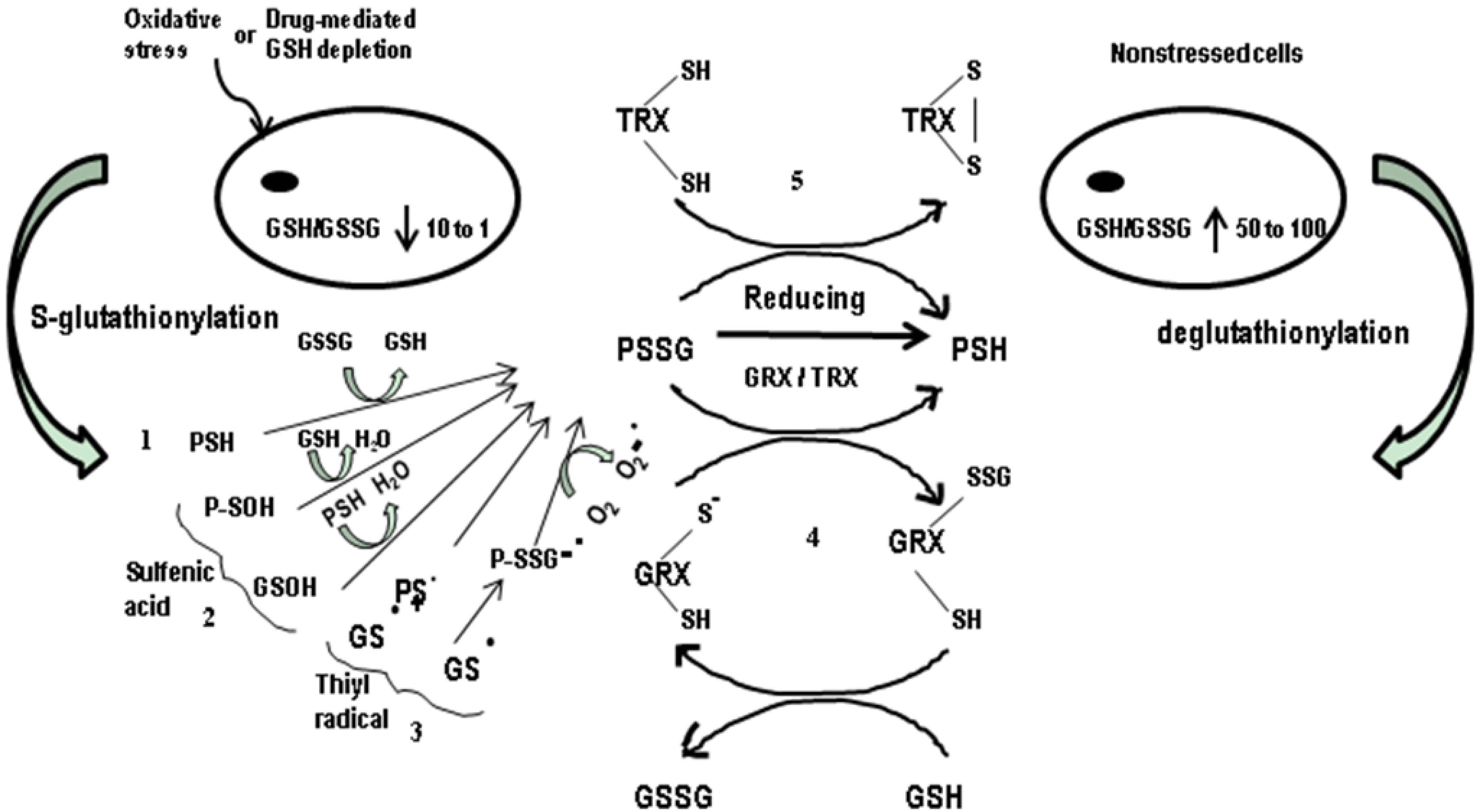
4. Probable Involvement of Cellular Glutathione in DNA-Repair
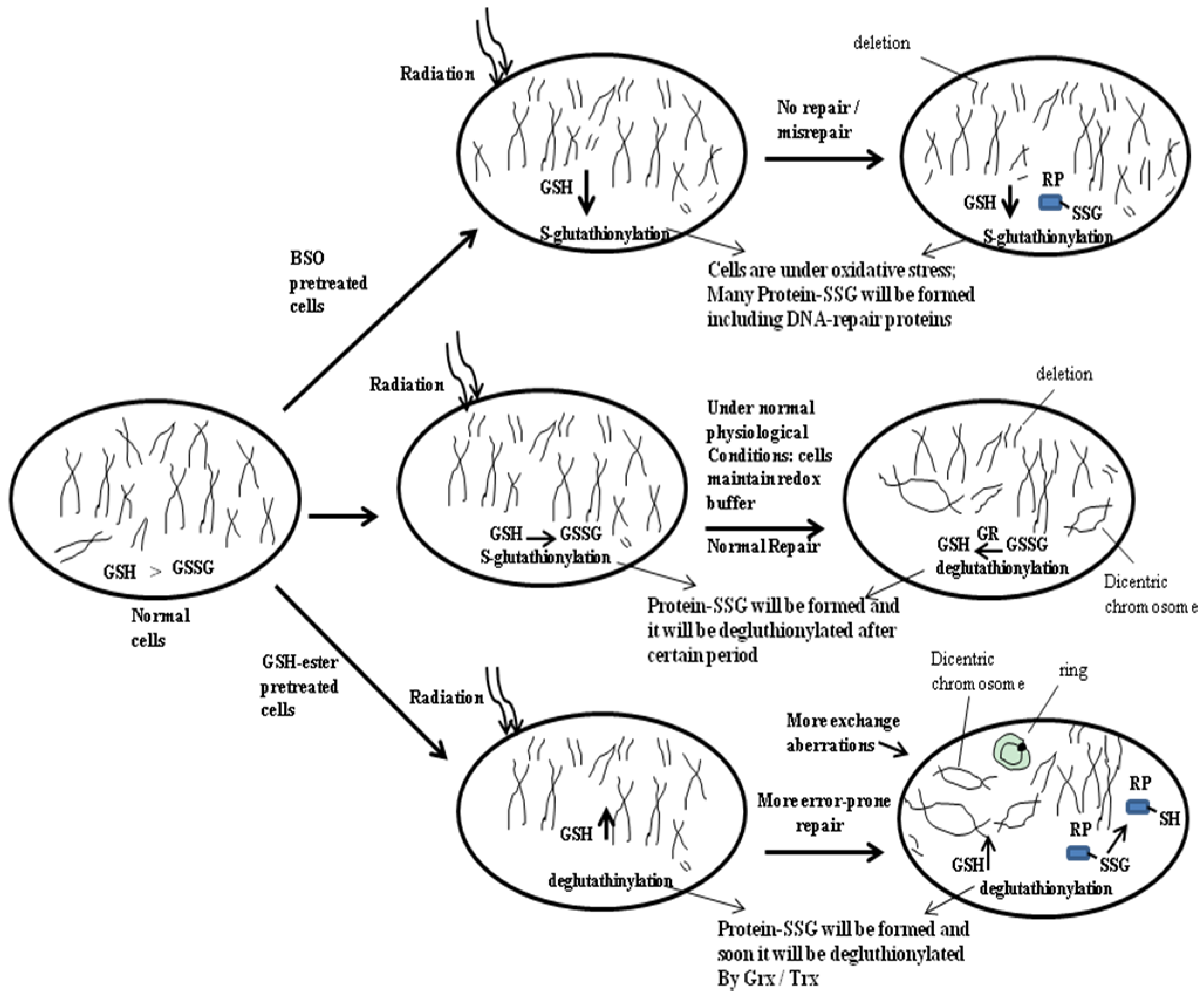
5. Glutathionylation and Its Biological Implications, Particularly in Cancer
6. Conclusions and Perspectives
Conflict of Interest
Acknowledgements
References
- Dickinson, D.A.; Forman, H.J. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002, 64, 1019–1026. [Google Scholar]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar]
- Astley, S.B.; Elliott, R.M.; Archer, D.B.; Southon, S. Evidence that dietary supplementation with carotenoids and carotenoid-rich foods modulates the DNA damage: Repair balance in human lymphocytes. Br. J. Nutr. 2004, 91, 63–72. [Google Scholar]
- Stahl, W.; van den Berg, H.; Arthur, J.; Bast, A.; Dainty, J.; Faulks, R.M.; Gartner, C.; Haenen, G.; Hollman, P.; Holst, B.; et al. Bioavailability and metabolism. Mol. Aspects Med. 2002, 23, 39–100. [Google Scholar] [CrossRef]
- Sáez, G.T.; Valls, V.; Muñiz, P.; Perez-Broseta, C.; Iradi, A.; Oliva, M.R.; Bannister, J.V.; Bannister, W.H. The role of glutathione in protection against DNA damage induced by rifamycin SV and copper(II) ions. Free Radic. Res. Commun. 1993, 19, 81–92. [Google Scholar] [CrossRef]
- Meyer, Y.; Buchanan, B.B.; Vignols, F.; Reichheld, J.P. Thioredoxins and glutaredoxins: Unifying elements in redox biology. Annu. Rev. Genet. 2009, 43, 335–367. [Google Scholar] [CrossRef]
- Awasthi, Y.C.; Chaudhary, P.; Vatsyayan, R.; Sharma, A.; Awasthi, S.; Sharma, R. Physiological and pharmacological significance of glutathione-conjugate transport. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 540–551. [Google Scholar] [CrossRef]
- Franco, R.; Cidlowski, J.A. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ. 2009, 16, 1303–1314. [Google Scholar] [CrossRef]
- Cotgreave, I.A. Analytical developments in the assay of intra-and extracellular GSH homeostasis: Specific protein S-glutathionylation, cellular GSH and mixed disulphide compartmentalisation and interstitial GSH redox balance. Biofactors 2003, 17, 269–277. [Google Scholar] [CrossRef]
- Chen, J.; Delannoy, M.; Odwin, S.; He, P.; Trush, M.A.; Yager, J.D. Enhanced mitochondrial gene transcript, ATP, bcl-2 protein levels, and altered glutathione distribution in ethinyl estradiol-treated cultured female rat hepatocytes. Toxicol. Sci. 2003, 75, 271–278. [Google Scholar] [CrossRef]
- Turella, P.; Cerella, C.; Filomeni, G.; Bullo, A.; DeMaria, F.; Ghibelli, L.; Ciriolo, M.R.; Cianfriglia, M.; Mattei, M.; Frederici, G.; Ricci, G.; Caccuri, A.M. Proapoptotic activity of new glutathione S-Transferase inhibitors. Cancer Res. 2005, 65, 3751–3761. [Google Scholar]
- Estrela, J.M.; Obrador, E.; Navarro, J.; Lasso De la Vega, M.C.; Pellicer, J.A. Elimination of Ehrlich tmours by ATP-induced growth inhibition, glutathione depletion and X-rays. Nat. Med. 1995, 1, 84–88. [Google Scholar]
- Suthanthiran, M.; Anderson, M.E.; Sharma, V.K.; Meister, A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc. Natl. Acad. Sci. USA 1990, 87, 3343–3347. [Google Scholar] [CrossRef]
- Dijkwel, P.A.; Wenink, P.W. Structural integrity of the nuclear matrix: Differential effects of thiol agents and metal chelators. J. Cell Sci. 1986, 84, 53–67. [Google Scholar]
- Klug, A.; Rhodes, D. Zinc fingers: A novel protein fold for nucleic acid recognition. Cold Spring Harb. Symp. Quant. Biol. 1987, 52, 473–482. [Google Scholar]
- Flohe, L. Selenium in mammalian spermiogenesis. Biol. Chem. 2007, 388, 987–995. [Google Scholar]
- Sau, A.; Pellizzari, T.F.; Valentino, F.; Federici, G.; Caccuri, A.M. Glutathione transferases and development of new principles to overcome drug resistance. Arch. Biochem. Biophys. 2010, 500, 116–122. [Google Scholar]
- Lam, B.K. Leukotriene C(4) synthase. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 111–116. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hampton, M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008, 45, 549–561. [Google Scholar] [CrossRef]
- Suto, D.; Sato, K.; Ohba, Y.; Yoshimura, T.; Fujii, J. Suppression of the pro-apoptotic function of cytochrome c by singlet oxygen via a haem redox state-independent mechanism. Biochem. J. 2005, 392, 399–406. [Google Scholar] [CrossRef]
- Holmgren, A. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 1989, 264, 13963–13966. [Google Scholar]
- Janssen-Heininger, Y.M.; Mossman, B.T.; Heintz, N.H.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; van der Vliet, A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008, 45, 1–17. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Milzani, A.; Gagliano, N.; Colombo, R.; Giustarini, D.; Rossi, R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid. Redox Signal. 2008, 10, 445–473. [Google Scholar] [CrossRef]
- Lillig, C.H.; Berndt, C.; Vergnolle, O.; Lönn, M.E.; Hudemann, C.; Bill, E.; Arne, H. Characterization of human glutaredoxin 2 as iron–sulfur protein: A possible role as redox Sensor. Proc. Natl. Acad. Sci. USA 2005, 102, 8168–8173. [Google Scholar]
- Altman, K.I.; Gerber, G.B.; Okada, S. Radiation Biochemistry; Academic Press: New York, NY, USA, 1970. [Google Scholar]
- Hutterman, J.; Kohnlein, W.; Teoule, R. Effects of Ionizing Radiation on DNA; Springer Verlag: Berlin, Germany, 1978. [Google Scholar]
- Xavier, S.; Yamada, K.; Samuni, A.M.; Samuni, A.; DeGraff, W.; Krishna, M.C.; Mitchel, J.B. Differential protection by nitroxides and hydroxylamines to radiation-induced and metal ion-catalyzed oxidative damage. Biochim. Biophys. Acta 2002, 1573, 109–120. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Pogozelski, W.K.; Tullius, T.D. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc. Natl. Acad. Sci. USA 1998, 95, 9538–9543. [Google Scholar]
- Ewing, D.; Walton, H.L. Radiation protection of in vitro mammalian cells: Effects of hydroxyl radical scavengers on the slopes and shoulders of survival curves. Radiat. Res. 1991, 126, 187–197. [Google Scholar] [CrossRef]
- Ewing, D.; Walton, H.L. Do ·OH scavenger secondary radicals protect by competing with oxygen for cellular target sites? Radiat. Res. 1991, 128, 29–36. [Google Scholar] [CrossRef]
- Biaglow, J.E.; Varnes, M.E.; Clark, E.P.; Epp, E.R. The role of thiols in cellular response to radiation and drugs. Radiat. Res. 1983, 95, 437–455. [Google Scholar]
- Mazur, L. Radioprotective effects of the thiols GSH and WR-2721 against X-ray-induction of micronuclei in erythroblasts. Mutat. Res. 2000, 468, 27–33. [Google Scholar] [CrossRef]
- Chaudhury, J.P.; Langendroff, H. Chemical radioprotection of mammalian chromosome in vivo: Radioprotection of rat bone marrow chromosomes with a single prophylactic dose of AET. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1968, 14, 463–467. [Google Scholar] [CrossRef]
- Chatterjee, A.; Jacob-Raman, M. Modifying effect of reduced glutathione on X-ray-induced chromosome aberrations and cell cycle delay. Mutat. Res. 1986, 175, 73–82. [Google Scholar]
- Meister, A. Selective modification of glutathione metabolism. Science 1983, 220, 472–477. [Google Scholar]
- Louie, K.G.; Behrens, B.C.; Kinsella, T.J.; Hamilton, T.C.; Grotzinger, K.R.; Mckoy, W.M.; Winker, M.A.; Ozols, R.F. Radiation survival parameters of antineoplastic drug-sensitive and -resistant human ovarian cancer cell lines and their modification by buthionine sulfoximine. Cancer Res. 1985, 45, 2110–2115. [Google Scholar]
- Chattopadhyay, A.; Deb, S.; Chatterjee, A. Modulation of the clastogenic activity of γ-irradiation in buthionine sulfoximine-mediated glutathione depleted mammalian cells. Int. J. Radiat. Biol. 1999, 75, 1283–1291. [Google Scholar]
- Fijo, T.; Bates, S. Strategies for reversing drug resistance. Oncogene 2003, 22, 7512–7523. [Google Scholar] [CrossRef]
- Révész, L.; Edgren, M.; Nishidai, T. Mechanisma of inherent radioprotection in mammalian cells. In Modification of Radiosensitivity in Cancer Treatment; Sugahara, T., Ueno, Y., Eds.; Academic Press: San Diego, CA, USA, 1984; pp. 13–29. [Google Scholar]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Holmgren, A. Glutathione dependent synthesis of deoxyribonucleotides. J. Biol. Chem. 1979, 254, 3622–3638. [Google Scholar]
- Révész, L.; Bergstrand, H.; Modig, H.G. Intrinsic non-protein sulphydryl levels and cellular radiosensitivity. Nature 1963, 198, 1275–1276. [Google Scholar]
- Prise, K.M.; Davies, S.; Stratford, M.R.L.; Michael, B.D. The role of non-protein suulphydryl in determining the chemical repair rates of free radical precursors of DNA damage and cell killing in Chinese hamster V79 cells. Int. J. Radiat. Biol. 1992, 62, 297–306. [Google Scholar] [CrossRef]
- Modig, H.G.; Edgren, M.; Révész, L. Release of thiolsfrom cellular mixed disulphides and its possible role in radiation protection. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1972, 22, 257–268. [Google Scholar]
- Gorin, G. Mercaptan-disulphide interchange and radioprotection. Prog. Biochem. Pharmacol. 1965, 1, 142–150. [Google Scholar]
- Larsson, A. 5-oxoprolinuria and other inborn errors related to the γ-glutamyl cycle. In Transport and Inherited Disease; Belton, N.R., Toothill, C., Eds.; MIT Press: Cambridge, MA, USA, 1981; pp. 277–294. [Google Scholar]
- Edgren, M.; Revesz, L.; Larsson, A. Induction and repair of single-strand DNA breaks after X-irradiation of human fibroblasts deficient in glutathione. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1981, 40, 355–363. [Google Scholar]
- Varnes, E.M.; Biaglow, J.E.; Koch, E.J.; Hall, E.J. Depletion of nonprotein thiols of hypoxic cells by misonidazole and metronidazole. In Radiation Sensitizers; Brady, L.W., Ed.; Masson: New York, NY, USA, 1980; pp. 121–134. [Google Scholar]
- Griffith, O.W.; Meister, A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-N-butyl homocysteine sulfoximine). J. Biol. Chem. 1979, 254, 7558–7560. [Google Scholar]
- Cronkite, E.P.; Brecher, G.; Chapman, W.H. Studies on the mechanism of the protective action of glutathione against whole body radiation. Mil. Surg. 1951, 109, 294–307. [Google Scholar]
- Mikaelsen, K. The protective effect of glutathione against radiation-induced chromosome aberrations. Science 1952, 116, 172–174. [Google Scholar]
- Mittler, S. The failure of sulphydryl compounds, AET, MEA, and glutathione to protect against X-ray-induced chromosome aberrations in male Drosophia. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1964, 8, 405–413. [Google Scholar] [CrossRef]
- Hollander, A.; Doherty, D.G. The background for modification of radiation damage by sulphur compounds. In Radiation Damage and Sulphydryl Compounds; International Atomic Energy Agency: Vienna, Austria, 1969; pp. 1–14. [Google Scholar]
- Jacob, M.; Ray-Chaudhury, S.P. Radioprotective effect of six chemicals against X-ray induced genetic damage in D. melanogaster. Mutat. Res. 1973, 18, 279–288. [Google Scholar] [CrossRef]
- Kawasaki, S. Protective effect of various thiol compounds on radiation-induced mitotic delay in cultured mammalian cells (L-5). Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1977, 32, 577–581. [Google Scholar] [CrossRef]
- Glatt, H.; Protic-Sabljic, M.; Oesch, F. Mutagenecity of glutathione and cysteine in the Ames Test. Science 1983, 220, 961–963. [Google Scholar]
- Maisin, J.R.; Albert, C.; Henry, A. Reduction of short-term radiation lethality by biological response modifiers given alone or in association with other chemical protectors. Radiat. Res. 1993, 135, 332–337. [Google Scholar] [CrossRef]
- Vos, O. Role of endogenous thiols in protection. Adv. Space Res. 1992, 12, 201–207. [Google Scholar] [CrossRef]
- Bump, E.A.; Brown, J.M. Role of glutathione in the radiation response of mammalian cells in vitro and in vivo. Pharmacol. Ther. 1990, 47, 117–136. [Google Scholar] [CrossRef]
- Fahey, R.C.; Vojnovic, B.; Michael, B.D. The effects of counter ion condensation and co-ion depletion upon the rates of chemical repair of poly(U) radicals by thiols. Int. J. Radiat. Biol. 1991, 59, 885–899. [Google Scholar] [CrossRef]
- Bump, E.A.; Ning, Y.Y.; Brown, J.M. Radiosensitization of hypoxic tumor cells by depletion of intracellular glutathione. Science 1982, 217, 544–545. [Google Scholar]
- Dussy, A.; Meggers, E.; Giese, B. Spontanepous cleavage of 4′-DNA radicals under aerobic conditions: Apparent discrepancy between trapping rates and cleavage products. J. Am. Chem. Soc. 1998, 120, 7399–7403. [Google Scholar] [CrossRef]
- Pujari, G.; Sarma, A.; Chatterjee, A. The influence of reduced glutathione on chromosome damage induced by X-rays or heavy-ion beams of different LET and on the interaction of DNA lesions induced by radiations and bleomycin. Mutat. Res. 2010, 696, 154–159. [Google Scholar] [CrossRef]
- Yang, Z.; Faustino, P.J.; Andrews, P.A.; Monastra, R.; Rasmussen, A.A.; Ellison, C.D.; Cullen, K.J. Decreased cisplatin/DNA adduct formation is associated with cisplatin resistance in human head and neck cancer cell lines. Cancer Chemother. Pharmacol. 2000, 46, 255–262. [Google Scholar]
- Hentze, H.; Schmitz, I.; Latta, M.; Krueger, A.; Krammer, P.H.; Wendel, A. Glutathione dependence of caspase-8 activation at the death-inducing signaling complex. J. Biol. Chem. 2002, 277, 5588–5595. [Google Scholar]
- Fratelli, M.; Gianazza, E.; Ghezzi, P. Redox proteomics: Identification and functional role of glutathionylated proteins. Expert Rev. Proteomics 2004, 1, 365–376. [Google Scholar] [CrossRef]
- Jacob, C.; Knight, I.; Winyard, P.G. Aspects of the biological redox chemistry of cysteine: From simple redox responses to sophisticated signalling pathways. Biol. Chem. 2006, 387, 1385–1397. [Google Scholar]
- Gallogly, M.M.; Mieyal, J.J. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr. Opin. Pharmacol. 2007, 7, 381–391. [Google Scholar]
- Adachi, T.; Pimentel, D.R.; Heibeck, T.; Hou, X.; Lee, Y.J.; Jiang, B.; Ido, Y.; Cohen, R.A. S-glutathionylation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 2004, 279, 29857–29862. [Google Scholar]
- Dafre, A.L.; Sies, H.; Akerboom, T. Protein S-thiolation and regulation of microsomal glutathione transferase activity by the glutathione redox couple. Arch. Biochem. Biophys. 1996, 332, 288–294. [Google Scholar]
- Mieyal, J.J.; Starke, D.W.; Gravina, S.A.; Dothey, C.; Chung, J.S. Thioltransferase in human red blood cells: Purification and properties. Biochemistry 1991, 30, 6088–6097. [Google Scholar] [CrossRef]
- Qanungo, S.; Starke, D.W.; Pai, H.V.; Mieyal, J.J.; Nieminen, A.L. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J. Biol. Chem. 2007, 282, 18427–18436. [Google Scholar]
- Holmgren, A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2000, 2, 811–820. [Google Scholar]
- Giles, N.M.; Giles, G.I.; Jacob, C. Multiple roles of cysteine in biocatalysis. Biochem. Biophys. Res. Commun. 2003, 300, 1–4. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; Martin, N.M.; Jackson, S.P.; Smith, G.C.; Ashwort, A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar]
- Wood, R.D.; Mitchell, M.; Lindahl, T. Human DNA repair genes. Mutat. Res. 2005, 577, 275–280. [Google Scholar] [CrossRef]
- Christmann, M.; Tomicic, M.T.; Roos, W.P.; Kaina, B. Mechanisms of human DNA repair: An update. Toxicology 2003, 193, 3–34. [Google Scholar]
- Rojas, E.; Valverde, M.; Kala, S.V.; Kala, G.; Lieberman, M.W. Accumulation of DNA damage in the organs of mice deficient in gamma-glutamyltranspeptidase. Mutat. Res. 2000, 447, 305–316. [Google Scholar] [CrossRef]
- Frankenberg-Schwager, M. Induction, repair and biological relevance of radiation-induced DNA lesions in eukaryotic cells. Radiat. Environ. Biophys. 1990, 29, 273–292. [Google Scholar] [CrossRef]
- Iliakis, G. The role of DNA double strand breaks in ionizing radiation-induced killing of eukaryotic cells. BioEssays 1991, 13, 641–648. [Google Scholar]
- Révész, L.; Edgren, M. Glutathione-dependent yield and repair of single-strand breaks in irradiated cells. Br. J. Cancer Suppl. 1984, 6, 55–60. [Google Scholar]
- Cornforth, M.N.; Bedford, J.S. Ionizing radiation damage and its early development in chromosomes. In DNA and Chromatin Damage Caused by Radiation: Advances in Radiation Biology; Lett, J.T., Sinclair, W.K., Eds.; Academic Press: London, UK, 1993; Volume 17, pp. 423–496. [Google Scholar]
- Xue, L.Y.; Friedman, L.R.; Oleinick, N.L. Repair of chromatin damage in glutathione depleted V-79 cells: Comparison of oxic and hypoxic conditions. Radiat. Res. 1988, 114, 11–27. [Google Scholar]
- Lai, G.; Ozols, R.; Young, R.; Hamilton, T. Effect of glutathione on DNA repair in cisplatin resistant human ovarian cancer cell lines. J. Natl. Cancer Inst. 1989, 81, 535–540. [Google Scholar] [CrossRef]
- Preston, R.J. DNA repair and chromosome alteration: Interactive effects radiation and chemicals. Prog. Mutat. Res. 1982, 4, 25–35. [Google Scholar]
- Dar, M.E.; Jorgensen, T.J. Deletion at short direct repeats and base substitutions are characteristic mutations for bleomycin-induced double- and single-strand breaks, respectively, in human shuttle vector system. Nucleic Acids Res. 1995, 23, 3224–3250. [Google Scholar] [CrossRef]
- Dutta, A.; Chakraborty, A.; Saha, A.; Ray, S.; Chatterjee, A. Interaction of radiation- and bleomycin-induced lesions and influence of glutathione level on the interaction. Mutagenesis 2005, 20, 329–335. [Google Scholar]
- Pendyala, L.; Perez, R.; Weinstein, A.; Zdanowicz, J.; Creaven, P.J. Effect of glutathione depletion on the cytotoxicity of cisplatin and iproplatin in a human melanoma cell line. Cancer Chemother. Pharmacol. 1997, 40, 38–44. [Google Scholar] [CrossRef]
- Ayene, I.S.; Biaglow, J.E.; Kachur, A.V.; Stamato, T.D.; Koch, C.J. Mutation in G6PD gene leads to loss of cellular control of protein glutathionylation: Mechanism and implication. J. Cell Biochem. 2008, 103, 123–135. [Google Scholar]
- Kim, H. DNA repair Ku proteins in gastric cancer cells and pancreatic acinar cells. Amino Acids 2008, 34, 195–202. [Google Scholar]
- Kim, Y.J.; Kim, D.; Illuzzi, J.L.; Delaplane, S.; Su, D.; Bernier, M.; Gross, M.L.; Georgiadis, M.M.; Wilson, D.M., III. S-glutathionylation of cysteine 99 in the APE1 protein impairs abasic endonuclease activity. J. Mol. Biol. 2011, 414, 313–326. [Google Scholar] [CrossRef]
- Sampathkumar, R.; Balasubramanyam, M.; Sudarslal, S.; Rema, M.; Mohan, V.; Balaram, P. Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangio-pathy. Clin. Biochem. 2005, 38, 892–899. [Google Scholar] [CrossRef]
- Sparaco, M.; Gaeta, L.M.; Santorelli, F.M.; Passarelli, C.; Tozzi, G.; Bertini, E.; Simonati, A.; Scaravilli, F.; Taroni, F.; Duyckaerts, C.; et al. Friedreich’s ataxia: Oxidative stress and cytoskeletal abnormalities. J. Neurol. Sci. 2009, 287, 111–118. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Baltimore, D. NF-kappa B: Ten years after. Cell 1996, 87, 13–20. [Google Scholar]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef]
- Cortés, S.M.; Rodríguez, F.V.; Sánchez, P.I.; Perona, R. The role of the NFkappaB signalling pathway in cancer. Clin. Transl. Oncol. 2008, 10, 143–147. [Google Scholar]
- Pineda-Molina, E.; Klatt, P.; Vazquez, J.; Marina, A.; García de Lacoba, M.; Perez-Sala, D.; Lamas, S. Glutathionylation of the p50 subunit of NF-kappaB: A mechanism for redox-induced inhibition of DNA binding. Biochemistry 2001, 40, 14134–14142. [Google Scholar]
- Klatt, P.; Molina, E.P.; De Lacoba, M.G.; Padilla, C.A.; Martinez-Galesteo, E.; Barcena, J.A.; Lamas, S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J. 1999, 13, 1481–1490. [Google Scholar]
- Velu, C.S.; Niture, S.K.; Doneanu, C.E.; Pattabiraman, N.; Srivenugopal, K.S. Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry 2007, 46, 7765–7780. [Google Scholar] [CrossRef]
- Huang, Z.; Pinto, J.T.; Deng, H.; Richie, J.P. Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem. Pharmacol. 2008, 75, 2234–2244. [Google Scholar]
- Sykes, M.C.; Mowbray, A.L.; Jo, H. Reversible glutathiolation of caspase-3 by glutaredoxin as a novel redox signaling mechanism in tumor necrosis factor-alpha-induced cell death. Circ. Res. 2007, 100, 152–154. [Google Scholar] [CrossRef]
- Friesen, C.; Kiess, Y.; Debatin, K.M. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ. 2004, 11 (Suppl. 1), S73–S85. [Google Scholar] [CrossRef]
- Meyer, E.B.; Wells, W.W. Thioltransferase overexpression increases resistance of MCF-7 cells to adriamycin. Free Radic. Biol. Med. 1999, 26, 770–776. [Google Scholar] [CrossRef]
- Daily, D.; Vlamis-Gardikas, A.; Offen, D.; Mittelmann, L.; Melamed, E.; Holmgren, A.; Barzilai, A. Glutaredoxin protects cerebellar granulae neurons from dopamine-induced apoptosis by dual activation of the ras-phosphoinositide 3-kinase and Jun N-terminal kinase pathway. J. Biol. Chem. 2001, 276, 21618–21626. [Google Scholar]
- Lillig, C.H.; Lönn, M.E.; Enoksson, M.; Fernandes, A.P.; Holmgren, A. Short interfering RNA-mediated silencing of glutaredoxin 2 increases the sensitivity of HeLa cells towards doxorubicin and phenylarsine oxide. Proc. Natl. Acad. Sci. USA 2004, 101, 13227–13232. [Google Scholar]
- Nagata, J.; Kijima, H.; Hatanaka, H.; Asai, S.; Miyachi, H.; Takagi, A.; Miwa, T.; Mine, T.; Yamazaki, H.; Nakamura, M.; Kado, T.; Scanlon, K.J.; Ueyama, Y. Reversal of cisplatin and multidrug resistance by ribozyme-mediated glutathione suppression. Biochem. Biophys. Res. Commun. 2001, 286, 406–413. [Google Scholar] [CrossRef]
- Bassi, L.; Carloni, M.; Meschini, R.; Fonti, E.; Palitti, F. X-irradiated human lymphocytes with unstable aberrations and their preferential elimination by p53/surviving-dependent apoptosis. Int. J. Radiat. Biol. 2003, 79, 943–954. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chatterjee, A. Reduced Glutathione: A Radioprotector or a Modulator of DNA-Repair Activity? Nutrients 2013, 5, 525-542. https://doi.org/10.3390/nu5020525
Chatterjee A. Reduced Glutathione: A Radioprotector or a Modulator of DNA-Repair Activity? Nutrients. 2013; 5(2):525-542. https://doi.org/10.3390/nu5020525
Chicago/Turabian StyleChatterjee, Anupam. 2013. "Reduced Glutathione: A Radioprotector or a Modulator of DNA-Repair Activity?" Nutrients 5, no. 2: 525-542. https://doi.org/10.3390/nu5020525
APA StyleChatterjee, A. (2013). Reduced Glutathione: A Radioprotector or a Modulator of DNA-Repair Activity? Nutrients, 5(2), 525-542. https://doi.org/10.3390/nu5020525




