The Gluten-Free Diet: Testing Alternative Cereals Tolerated by Celiac Patients
Abstract
:1. Introduction
2. Natural Varieties of Cereal and Pseudocereals Suitable for Patients with Celiac Disease
2.1. Wheat and Barley
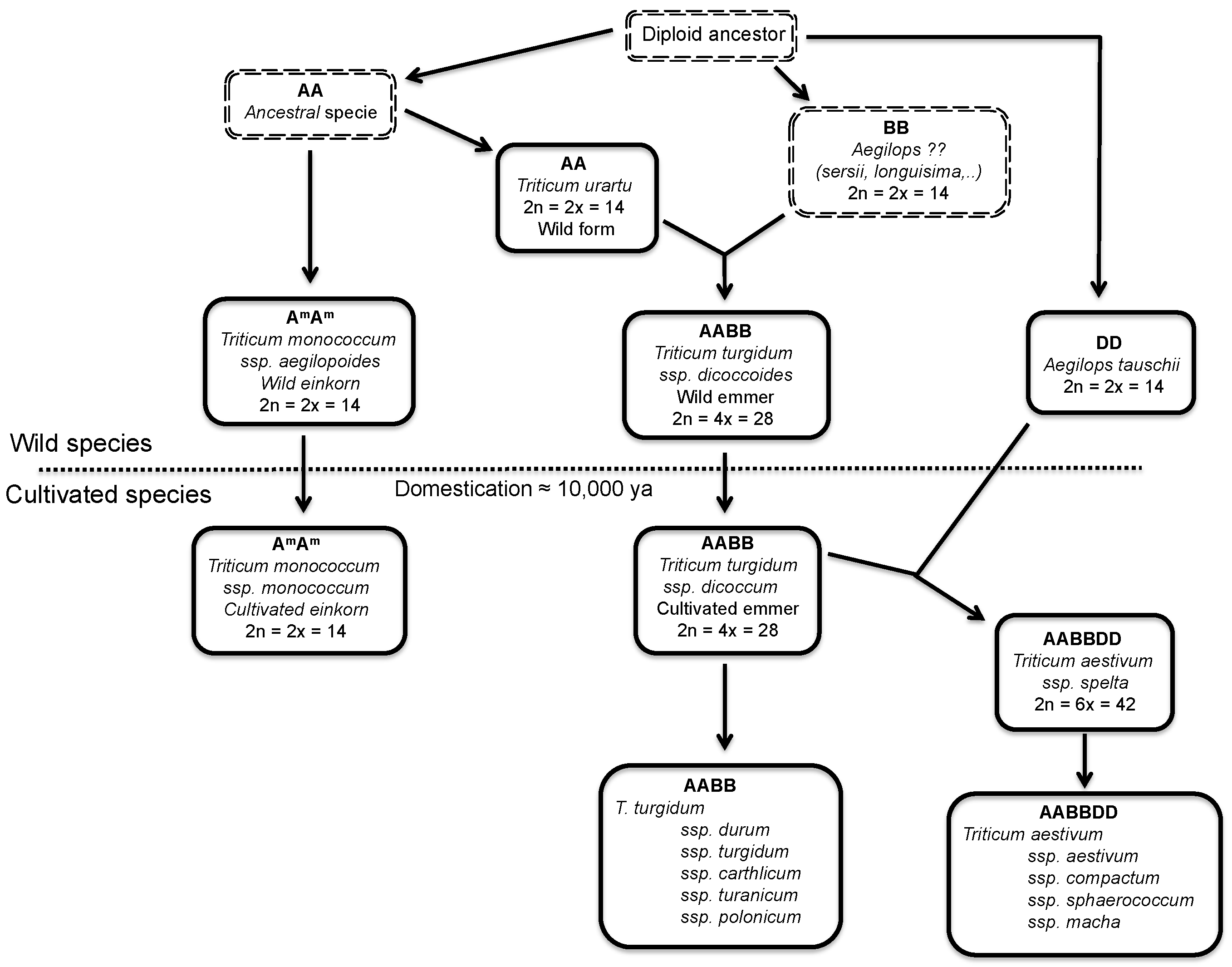
2.2. Oats
2.3. Other Cereals and Pseudocereals
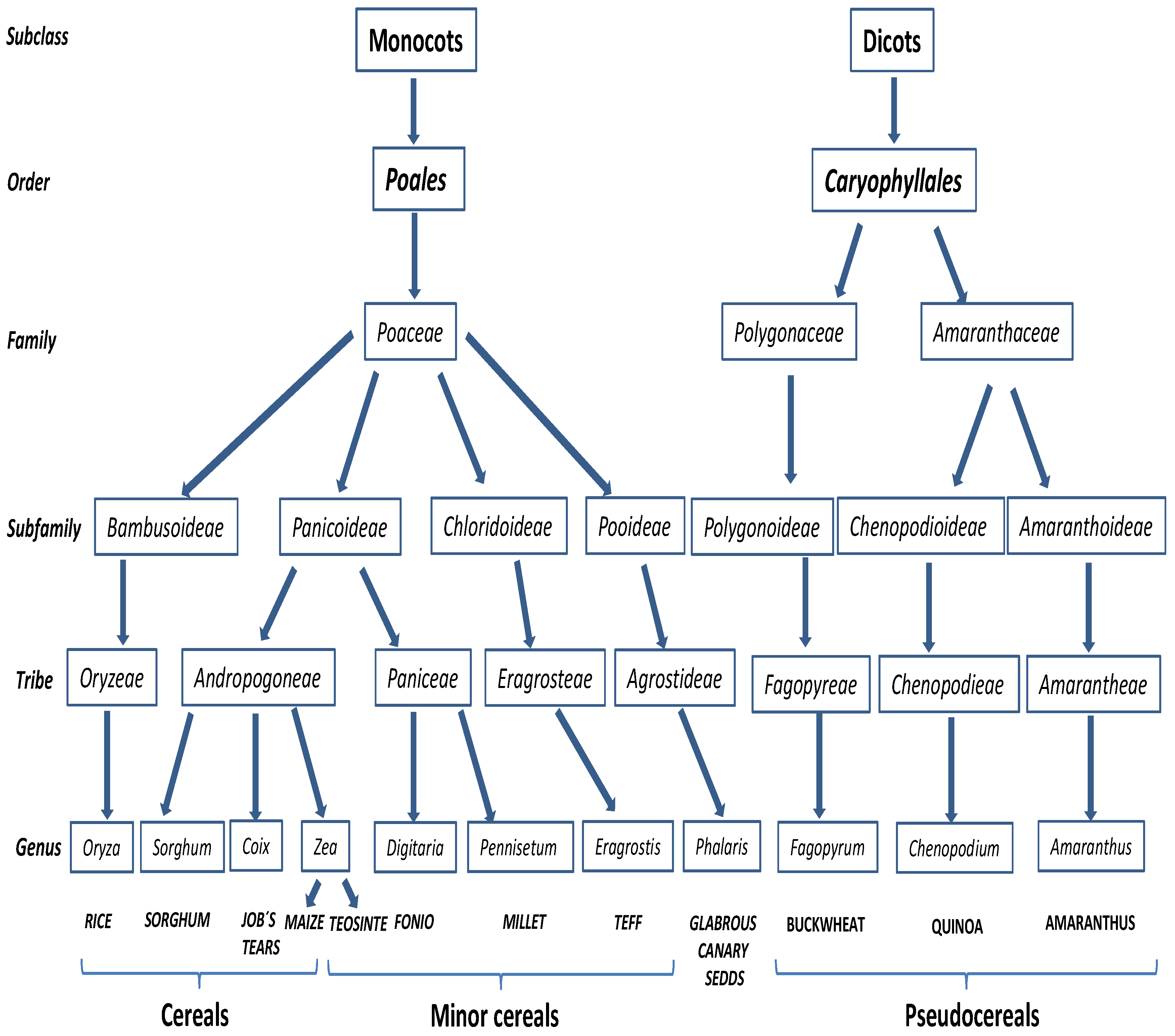
2.3.1. Rice, Maize and Sorghum
2.3.2. Minor Cereals
2.3.3. Pseudocereals
2.3.4. Other Cereals
3. Modified Harmless Cereal Varieties
3.1. Gluten Detoxification by Biotechnological Methods
3.2. Gluten Detoxification by Enzymatic Methods
3.2.1. Prolyl Endopeptidases
3.2.2. Germinating Cereals
3.2.3. Transamidation
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sollid, L.M. Coeliac disease: Dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2002, 2, 647–655. [Google Scholar] [CrossRef]
- Bernardo, D.; Peña, A.S. Developing strategies to improve the quality of life of patients with gluten intolerance in patients with and without coeliac disease. Eur. J. Intern. Med. 2012, 23, 6–8. [Google Scholar] [CrossRef]
- Immune Epitope Database and Analysis Resourse. Available online: http://www.iedb.org/ (accessed on 14 October 2013).
- Corrao, G.; Corazza, G.R.; Bagnardi, V.; Brusco, G.; Ciacci, C.; Cottone, M.; Sategna Guidetti, C.; Usai, P.; Cesari, P.; Pelli, M.A.; et al. Mortality in patients with coeliac disease and their relatives: A cohort study. Lancet 2001, 358, 356–361. [Google Scholar] [CrossRef]
- Rashtak, S.; Murray, J.A. Review article: Coeliac disease, new approaches to therapy. Aliment. Pharmacol. Ther. 2012, 35, 768–781. [Google Scholar] [CrossRef]
- Feldman, M.; Levy, A.A. Genome evolution in allopolyploid wheat—A revolutionary reprogramming followed by gradual changes. J. Genet. Genomics 2009, 36, 511–518. [Google Scholar] [CrossRef]
- Marietta, E.V.; Murray, J.A. Testing the safety of alternative wheat species and cultivars for consumption by celiac patients. Am. J. Clin. Nutr. 2012, 96, 1247–1248. [Google Scholar] [CrossRef]
- Van Slageren, M.W. Wild wheats: A Monograph of Aegilops L. and Amblyopyrum (Jaub. & Spach) Eig (Poaceae); Wageningen Agriculture University: Wageningen, The Netherlands, 1994. [Google Scholar]
- Auricchio, S.; de Ritis, G.; de Vincenzi, M.; Occorsio, P.; Silano, V. Effects of gliadin-derived peptides from bread and durum wheats on small intestine cultures from rat fetus and coeliac children. Pediatr. Res. 1982, 16, 1004–1010. [Google Scholar] [CrossRef]
- Van Herpen, T.W.; Goryunova, S.V.; van der Schoot, J.; Mitreva, M.; Salentijn, E.; Vorst, O.; Schenk, M.F.; van Veelen, P.A.; Koning, F.; van Soest, L.J.; et al. Alpha-Gliadin genes from the A, B, and D genomes of wheat contain different sets of celiac disease epitopes. BMC Genomics 2006, 10, 7–11. [Google Scholar]
- Spaenij-Dekking, L.; Kooy-Winkelaar, Y.; van Veelen, P.; Drijfhout, J.W.; Jonker, H.; van Soest, L.; Smulders, M.J.; Bosch, D.; Gilissen, L.J.; Koning, F. Natural variation in toxicity of wheat: Potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology 2005, 129, 797–806. [Google Scholar] [CrossRef]
- Molberg, O.; Uhlen, A.K.; Jensen, T.; Flaete, N.S.; Fleckenstein, B.; Arentz-Hansen, H.; Raki, M.; Lundin, K.E.; Sollid, L.M. Mapping of gluten T-cell epitopes in the bread wheat ancestors: Implications for celiac disease. Gastroenterology 2005, 128, 393–401. [Google Scholar] [CrossRef]
- Pizzuti, D.; Buda, A.; D’Odorico, A.; D’Incà, R.; Chiarelli, S.; Curioni, A.; Martines, D. Lack of intestinal mucosal toxicity of Triticum monococcum in coeliac disease patients. Scand. J. Gastroenterol. 2006, 41, 1305–1311. [Google Scholar] [CrossRef]
- Vincentini, O.; Maialetti, F.; Gazza, L.; Silano, M.; Dessi, M.; de Vincenzi, M.; Pogna, N.E. Environmental factors of coeliac disease: Cytotoxicity of hulled wheat species Triticum monococcum, T. turgidum ssp. dicoccum and T. aestivum ssp. spelta. J. Gastroenterol. Hepatol. 2007, 22, 1816–1822. [Google Scholar] [CrossRef]
- Gianfrani, C.; Maglio, M.; Rotondi Aufiero, V.; Camarca, A.; Vocca, I.; Iaquinto, G.; Giardullo, N.; Pogna, N.; Troncone, R.; Auricchio, S.; et al. Immunogenicity of monococcum wheat in celiac patients. Am. J. Clin. Nutr. 2012, 96, 1339–1345. [Google Scholar] [CrossRef]
- Tanner, G.J.; Howitt, C.A.; Forrester, R.I.; Campbell, P.M.; Tye-Din, J.A.; Anderson, R.P. Dissecting the T-cell response to hordeins in coeliac disease can develop barley with reduced immunotoxicity. Aliment. Pharmacol. Ther. 2010, 32, 1184–1191. [Google Scholar] [CrossRef]
- Tanner, G.J.; Blundell, M.J.; Colgrave, M.L.; Howitt, C.A. Quantification of hordeins by ELISA: The correct standard makes a magnitude of difference. PLoS One 2013, 8, e56456. [Google Scholar]
- Comino, I.; Real, A.; Gil-Humanes, J.; Pistón, F.; de Lorenzo, L.; Moreno, M.L.; López-Casado, M.Á.; Lorite, P.; Cebolla, A.; Torres, M.I.; et al. Significant differences in coeliac immunotoxicity of barley varieties. Mol. Nutr. Food. Res. 2012, 56, 1697–1707. [Google Scholar] [CrossRef]
- Suttie, J.M.; Reynolds, S.G. Fodder oats: A world overview, 2004. Food and Agriculture Organization of the United Nations (FAO). Available online: http://www.fao.org/docrep/008/y5765e/y5765e00.htm (accessed on 16 October 2012).
- Eppendorfer, W.H. Nutritive value of oat and rye grain protein as influenced by nitrogen and amino acid composition. J. Sci. Food Agric. 2006, 28, 152–156. [Google Scholar] [CrossRef]
- Pulido, O.; Gillespie, Z.; Zarkadas, M.; Dubois, S.; Vavasour, E.; Rashid, M.; Switzer, C.; Godefroy, S.B. Introduction of oats in the diet of individuals with coeliac disease: A systematic review. Adv. Food Nutr. Res. 2009, 57, 235–285. [Google Scholar] [CrossRef]
- Codex Alimentarius International Food Standars. Available online: http://www.codexalimentarius.net/web/more_info.jsp?id_sta=291 (accessed on 28 August 2013).
- COMMISSION REGULATION (EC) No 41/2009 of 20 January 2009 Concerning the Composition and Labelling of Foodstuffs Suitable for People Intolerant to Gluten. 2009. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:016:0003:0005:EN:PDF (accessed on 21 January 2009).
- Arentz-Hansen, H.; Fleckenstein, B.; Molberg, Ø.; Scott, H.; Koning, F.; Jung, G.; Roepstorff, P.; Lundin, K.E.; Sollid, L.M. The molecular basis for oat intolerance in patients with coeliac disease. PLoS Med. 2004, 1, 84–92. [Google Scholar]
- Silano, M.; di Benedetto, R.; Maialetti, F.; de Vincenzi, A.; Calcaterra, R.; Cornell, H.J.; de Vincenzi, M. Avenins from different cultivars of oats elicit response by coeliac peripheral lymphocytes. Scand. J. Gastroenterol. 2007, 42, 1302–1305. [Google Scholar] [CrossRef]
- Maglio, M.; Mazzarella, G.; Barone, M.V.; Gianfrani, C.; Pogna, N.; Gazza, L.; Stefanile, R.; Camarca, A.; Colicchio, B.; Nanayakkara, M.; et al. Immunogenicity of two oat varieties, in relation to their safety for celiac patients. Scand. J. Gastroenterol. 2011, 46, 1194–1205. [Google Scholar] [CrossRef]
- Comino, I.; Real, A.; de Lorenzo, L.; Cornell, H.; López-Casado, M.Á.; Barro, F.; Lorite, P.; Torres, M.I.; Cebolla, A.; Sousa, C. Diversity in oat potential immunogenicity: Basis for the selection of oat varieties with no toxicity in celiac disease. Gut 2011, 60, 915–922. [Google Scholar] [CrossRef]
- Real, A.; Comino, I.; de Lorenzo, L.; Merchán, F.; Gil-Humanes, J.; Giménez, M.J.; López-Casado, M.Á.; Torres, M.I.; Cebolla, Á.; Sousa, C.; et al. Molecular and immunological characterization of gluten proteins isolated from oat cultivars that differ in toxicity for celiac disease. PLoS One 2012, 7, e48365. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Stewart, J.A.; Dromey, J.A.; Beissbarth, T.; van Heel, D.A.; Tatham, A.; Henderson, K.; Mannering, S.I.; Gianfrani, C.; Jewell, D.P.; et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med. 2010, 2, 41ra51. [Google Scholar] [CrossRef]
- Rosell, C.M.; Marco, C. Rice. In Gluten Free Cereal Products and Beverages; Arendt, E.K., dal Bello, F., Eds.; Elsevier Science: London, UK, 2008; pp. 81–100. [Google Scholar]
- Boye, J.I.; Achouri, A.; Raymond, N.; Cleroux, C.; Weber, D.; Koerner, T.B.; Hucl, P.; Patterson, C.A. Analysis of Glabrous canary seeds by ELISA, Mass spectrometry, and western blotting for the absence of cross-reactivity with major plant food allergens. J. Agric. Food Chem. 2013, 61, 6102–6112. [Google Scholar] [CrossRef]
- Accomando, S.; Albino, C.; Montaperto, D.; Amato, G.M.; Corsello, G. Multiple food intolerance or refractory celiac sprue? Dig. Liver Dis. 2006, 38, 784–785. [Google Scholar] [CrossRef]
- Cabrera-Chávez, F.; Iamett, S.; Miriani, M.; Calderón de la Barca, A.M.; Mamone, G.; Bonomi, F. Maize prolamins resistant to peptic-tryptic digestion maintain immune-recognition by IgA from some celiac disease patients. Plant Foods. Hum. Nutr. 2012, 67, 24–30. [Google Scholar] [CrossRef]
- Darewicz, M.; Dziuba, J.; Minkiewicz, P. Computational characterization and identification of peptides for in silico detection of potentially celiac-toxic proteins. Food Sci. Technol. Int. 2007, 13, 125–133. [Google Scholar] [CrossRef]
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945. [Google Scholar]
- Ciacci, C.; Maiuri, L.; Caporaso, N.; Bucci, C.; del Giudice, L.; Rita Massardo, D.; Pontieri, P.; di Fonzo, N.; Bean, S.R.; Ioerger, B.; et al. Celiac disease: in vitro and in vivo safety and palatability of wheat-free sorghum food products. Clin. Nutr. 2007, 26, 799–805. [Google Scholar] [CrossRef]
- Pontieri, P.; Mamone, G.; de Caro, S.; Tuinstra, M.R.; Roemer, E.; Okot, J.; de Vita, P.; Ficco, D.B.; Alifano, P.; Pignone, D.; et al. Sorghum, a healthy and gluten-free food for celiac patients as demonstrated by genome, biochemical, and immunochemical analyses. J. Agric. Food. Chem. 2013, 61, 2565–2571. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef]
- Arendt, E.K.; Dal Bello, F. Gluten-Free Cereal, Products and Beverages (Food Science and Technology); Academic press: London, UK, 2011. [Google Scholar]
- Ballabio, C.; Uberti, F.; di Lorenzo, C.; Brandolini, A.; Penas, E.; Restani, P. Biochemical and immunochemical characterization of different varieties of amaranth (Amaranthus L. ssp.) as a safe ingredient for gluten-free products. J. Agric. Food. Chem. 2011, 59, 12969–12974. [Google Scholar]
- Ranhotra, G.S.; Gelroth, J.A.; Glaser, B.K.; Lorenz, K.J.; Johnson, D.L. Composition and protein nutritional quality of quinoa. Cereal. Chem. 1993, 70, 303–305. [Google Scholar]
- Repo-Carrasco, R.; Esponiza, C.; Jacobsen, S.-E. Nutritional value and use of the Andean crops: Quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Abugoch, J.L.E. Quinoa (Chenopodium quinoa Wild.): Composition, Chemistry, Nutritional, and Functional Properties. In Advances in Food and Nutrition Research; Taylor, S.L., Ed.; Academic Press: Lincoln, NE, USA, 2009; Volume 58, pp. 1–31. [Google Scholar]
- Gonzalez, J.A.; Konishi, Y.; Bruno, M.; Valoy, M.; Prado, F.E. Interrelationships among seed yield, total protein and amino acid composition of ten quinoa (Chenopodium quinoa) cultivars from two different agroecological regions. J. Sci. Food Agric. 2012, 92, 1222–1229. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Ellis, H.J.; Suligoj, T.; Herencia, L.I.; Ciclitira, P.J. Variable activation of immune response by quinoa (Chenopodium quinoa Wild.) prolamins in celiac disease. Am. J. Clin. Nutr. 2012, 96, 337–344. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Zannini, E.; Arendt, E.K. Germination of oat and quinoa and evaluation of the malts as gluten free baking ingredients. Plant Foods Hum. Nutr. 2013, 68, 90–95. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Silano, M.; Luchetti, R.; Carratu, B.; Boniglia, C.; Pogna, N.E. Agglutinating activity of alcohol-soluble proteins from quinoa seed flour in celiac disease. Plant Foods Hum. Nutr. 1999, 54, 93–100. [Google Scholar] [CrossRef]
- Berti, C.; Ballabio, C.; Restani, P.; Porrini, M.; Bonomi, F.; Iametti, S. Immunochemical and molecular properties of proteins in Chenopodium quinoa. Cereal Chem. 2004, 81, 275–277. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Skrabanja, V.; Kreft, I.; Golob, T.; Modic, M.; Ikeda, S.; Ikeda, K.; Kreft, S.; Bonafaccia, G.; Knapp, M.; Kosmelj, K. Nutrient content in buckwheat milling fractions. Cereal Chem. 2004, 81, 172–176. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Gambelli, L.; Fabjan, N.; Kreft, I. Trace elements in flour and bran from common and Tartary buckwheat. Food Chem. 2003, 83, 1–5. [Google Scholar] [CrossRef]
- Ikeda, K. Buckwheat: Composition, chemistry and processing. Adv. Food Nutr. Res. 2002, 44, 395–434. [Google Scholar] [CrossRef]
- Stember, R.H. Buckwheat allergy. Allergy Asthma Proc. 2006, 27, 393–395. [Google Scholar] [CrossRef]
- Wieslander, G. Review on buckwheat allergy. Allergy 1996, 51, 661–665. [Google Scholar]
- Panda, R.; Taylor, S.L.; Goodman, R.E. Development of a Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) for detection of buckwheat residues in food. J. Food Sci. 2010, 75, 110–117. [Google Scholar] [CrossRef]
- Watanabe, Y. Overview of Plant RNAi. In Methods in Molecular Biology; Kodama, H., Komamine, A., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 744, pp. 1–11. [Google Scholar]
- Becker, D.; Wieser, H.; Koehler, P.; Folck, A.; Mühling, K.H.; Zörb, C. Protein composition and techno-functional properties of transgenic wheat with reduced α-gliadin content obtained by RNA interference. J. Appl. Bot. Food Qual. 2012, 85, 23. [Google Scholar]
- Gil-Humanes, J.; Pistón, F.; Hernando, A.; Álvarez, J.B.; Shewry, P.R.; Barro, F. Silencing of γ-gliadins by RNA interference (RNAi) in bread wheat. J. Cereal Sci. 2008, 48, 565–568. [Google Scholar] [CrossRef]
- Pistón, F.; Gil-Humanes, J.; Rodríguez-Quijano, M.; Barro, F. Down-Regulating γ-gliadins in bread wheat leads to non-specific increases in other gluten proteins and has no major effect on dough gluten strength. PLoS One 2011, 6, e24754. [Google Scholar]
- Altenbach, S.B.; Allen, P.V. Transformation of the US bread wheat “Butte 86” and silencing of omega-5 gliadin genes. GM Crops 2011, 2, 66–73. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Pistón, F.; Tollefsen, S.; Sollid, L.M.; Barro, F. Effective shutdown in the expression of celiac disease-related wheat gliadin T-cell epitopes by RNA interference. Proc. Natl. Acad. Sci. USA 2010, 107, 17023–17028. [Google Scholar]
- Wen, S.; Wen, N.; Pang, J.; Langen, G.; Brew-Appiah, R.A.T.; Mejias, J.H.; Osorio, C.; Yang, M.; Gemini, R.; Moehs, C.P.; et al. Structural genes of wheat and barley 5-methylcytosine DNA glycosylases and their potential applications for human health. Proc. Natl. Acad. Sci. USA 2012, 109, 20543–20548. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Pistón, F.; Shewry, P.R.; Tosi, P.; Barro, F. Suppression of gliadins results in altered protein body morphology in wheat. J. Exp. Bot. 2011, 62, 4203–4213. [Google Scholar] [CrossRef]
- Valdés, I.; García, E.; Llorente, M.; Méndez, E. Innovative approach to low-level gluten determination in foods using a novel sandwich enzyme-linked immunosorbent assay protocol. Eur. J. Gastroenterol. Hepatol. 2003, 15, 465–747. [Google Scholar]
- Codex Alimentarius International Food Standars. Available online: http://www.codexalimentarius.org (accessed on 28 August 2013).
- Wieser, H.; Koehler, P.; Folck, A.; Becker, D. Characterization of Wheat with Strongly Reduced α-Gliadin Content. In Gluten Proteins; Lookhart, L.G., Ng, W.P.K., Eds.; AACC International: St Paul, MN, USA, 2006; pp. 13–16. [Google Scholar]
- Van den Broeck, H.C.; Gilissen, L.J.W.J.; Smulders, M.J.M.; van der Meer, I.M.; Hamer, R.J. Dough quality of bread wheat lacking alpha-gliadins with celiac disease epitopes and addition of celiac-safe avenins to improve dough quality. J. Cereal Sci. 2011, 53, 206–216. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Auty, M.; Arendt, E.K.; Gallagher, E. Baking properties and microstructure of pseudocereal flours in gluten-free bread formulations. Eur. Food Res. Technol. 2010, 230, 437–445. [Google Scholar] [CrossRef]
- Frazer, A.C.; Fletcher, R.F.; Ross, C.A.C.; Shaw, B.; Sammons, H.G.; Schneider, R. Gluten-Induce enteropathy the effect of partially digested gluten. Lancet 1959, 2, 252–255. [Google Scholar]
- Van de Kamer, J.H.; Weijers, H.A. Celiac disease. V. Some experiments on the cause of the harmful effect of wheat gliadin. Acta Pediatr. Scand. 1955, 44, 465–469. [Google Scholar] [CrossRef]
- Shan, L.; Molberg, Ø.; Parrot, I.; Hausch, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef]
- Di Cagno, R.; de Angelis, M.; Lavermicocca, P.; de Vincenzi, M.; Giovanini, C.; Faccia, M.; Gobbetti, M. Proteolysis by sourdough lactic acid bacteria: Effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl. Environ. Microbiol. 2002, 68, 623–633. [Google Scholar] [CrossRef]
- Gerez, C.L.; Font de Valdez, G.; Rollan, G.C. Functionality of lactic acid bacteria peptidase activities in the hydrolysis of gliadin-like fragments. Appl. Microbiol. 2008, 47, 427–432. [Google Scholar] [CrossRef]
- Di Cagno, R.; de Angelis, M.; Auricchio, S.; Greco, L.; Clarke, C.; de Vincenzi, M.; Giovanini, C.; D’Archivio, M.; Landolfo, F.; Parrilli, G.; et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl. Environ. Microbiol. 2004, 70, 1088–1096. [Google Scholar] [CrossRef]
- Hartmann, G.; Koehler, P.; Wieser, H. Rapid degradation of gliadin peptides toxic for celiac disease patients by proteases from germinating cereals. J. Cereal Sci. 2006, 44, 368–371. [Google Scholar] [CrossRef]
- Kiyosaki, T.; Matsumoto, I.; Asakura, T.; Funaki, J.; Kuroda, M.; Misaka, T.; Arai, S.; Abe, K. Gliadain, a gibberellin-inducible cysteine proteinase occurring in germinating seeds of wheat, Triticum aestivum L., specifically digests gliadin and is regulated by intrinsic cystatins. FEBS J. 2007, 274, 1908–1917. [Google Scholar] [CrossRef]
- Michalcova, E.; Potoka, E.; Chmelova, D.; Ondrejovic, M. Study of wheat protein degradation during germination. J. Microbiol. Biotechnol. 2012, 1, 1439–1447. [Google Scholar]
- Stenman, S.M.; Lindfors, K.; Venäläinen, J.I.; Hautala, A.; Mänistö, P.T.; Garcia-Horsman, J.A.; Kaukorvita-Norja, A.; Auriola, S.; Mauriala, T.; Mäki, M.; et al. Degradation of celiac disease-inducing rye secalin by germinating cereal enzymes: Diminishing toxic effects in intestinal epithelial cells. Clin. Exp. Immunol. 2010, 161, 242–249. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P. Detoxification of gluten by means of enzymatic treatment. J. AOAC Int. 2012, 95, 356–363. [Google Scholar] [CrossRef]
- Van de Wal, Y.; Kooy, Y.; van Veelen, P.A.; Pena, S.; Mearin, L.; Papadopoulus, G.; Koning, F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 1998, 15, 1585–1588. [Google Scholar]
- Skovbjerg, H.; Koch, C.; Anthonsen, D.; Sjoestroem, H. Deamidation and cross-linking of gliadin peptides by transglutaminases and the relation to celiac disease. Biochim. Biophys. Acta 2004, 1690, 220–230. [Google Scholar] [CrossRef]
- Stamnaes, J.; Fleckenstein, B.; Sollid, L.M. The propensity for deamidation and transamidation of peptides by transglutaminase 2 is dependent on substrate affinity and reaction conditions. Biochim. Biophys. Acta 2008, 1784, 1804–1811. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Umezawa, Y.; Nio, N.; Kubota, K. Comparison of deamidation activity of transglutaminases. J. Cereal Sci. 2001, 66, 25–29. [Google Scholar]
- Nonaka, M.; Sawa, A.; Matsuura, Y.; Motoki, M.; Nio, N. Deamidation of several food proteins using free and immobilized Ca2+-independent microbial transglutaminase. Biosci. Biotechnol. Biochem. 1996, 60, 532–533. [Google Scholar] [CrossRef]
- Gianfrani, C.; Siciliano, R.A.; Facchiano, A.M.; Camarca, A.; Mazzeo, M.F.; Costantini, S.; Salvati, V.M.; Maurano, F.; Mazzarella, G.; Iaquinto, G.; et al. Transamidation of wheat flour inhibits the response to gliadin of intestinal T cells in celiac disease. Gastroenterology 2007, 133, 780–789. [Google Scholar] [CrossRef]
- Mazzarella, G.; Salvati, V.M.; Laquinto, G.; Stefanile, R.; Capobianco, F.; Luongo, D.; Bergamo, P.; Maurano, F.; Giardullo, N.; Malamisura, B.; et al. Reintroduction of gluten following flour transamidation in adult celiac patients: A randomized, controlled clinical study. Clin. Dev. Immunol. 2012, 2012, 329150. [Google Scholar]
- Pasternack, R.; Marx, S.; Jordan, D. Prolamin-Reduced Beverages and Methods for the Preparation Thereof. Int. Pat. WO 2006051093 A1, 18 May 2006. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Comino, I.; De Lourdes Moreno, M.; Real, A.; Rodríguez-Herrera, A.; Barro, F.; Sousa, C. The Gluten-Free Diet: Testing Alternative Cereals Tolerated by Celiac Patients. Nutrients 2013, 5, 4250-4268. https://doi.org/10.3390/nu5104250
Comino I, De Lourdes Moreno M, Real A, Rodríguez-Herrera A, Barro F, Sousa C. The Gluten-Free Diet: Testing Alternative Cereals Tolerated by Celiac Patients. Nutrients. 2013; 5(10):4250-4268. https://doi.org/10.3390/nu5104250
Chicago/Turabian StyleComino, Isabel, María De Lourdes Moreno, Ana Real, Alfonso Rodríguez-Herrera, Francisco Barro, and Carolina Sousa. 2013. "The Gluten-Free Diet: Testing Alternative Cereals Tolerated by Celiac Patients" Nutrients 5, no. 10: 4250-4268. https://doi.org/10.3390/nu5104250
APA StyleComino, I., De Lourdes Moreno, M., Real, A., Rodríguez-Herrera, A., Barro, F., & Sousa, C. (2013). The Gluten-Free Diet: Testing Alternative Cereals Tolerated by Celiac Patients. Nutrients, 5(10), 4250-4268. https://doi.org/10.3390/nu5104250




