Effect of Diets Supplemented with Different Sources of Astaxanthin on the Gonad of the Sea Urchin Anthocidaris crassispina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Maintenance
2.2. Experimental Diets
2.2.1. Astaxanthin Sources
2.2.2. Diet Preparation
| Compositions (%) | Basal Diet | HP1 | HP2 | CZ1 | CZ2 | AST1 | AST2 |
|---|---|---|---|---|---|---|---|
| Dried fish meal | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Dried agar | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Dried S. hemiphyllum | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Dried Flour | 50.0 | 47.5 | 49.4 | 19.7 | 42.4 | 50.0 | 50.0 |
| Dried H. pluvialis 1 | – | 2.5 | 0.6 | – | – | – | – |
| Dried C. zofingiensis 2 | – | – | – | 30.3 | 7.6 | – | – |
| Synthetic astaxanthin | – | – | – | – | – | 0.04 | 0.01 |
2.3. Analysis
2.3.1. Determination of Gonad Index
2.3.2. Determination of Carotenoid Concentrations
2.4. Statistical Analysis
3. Results
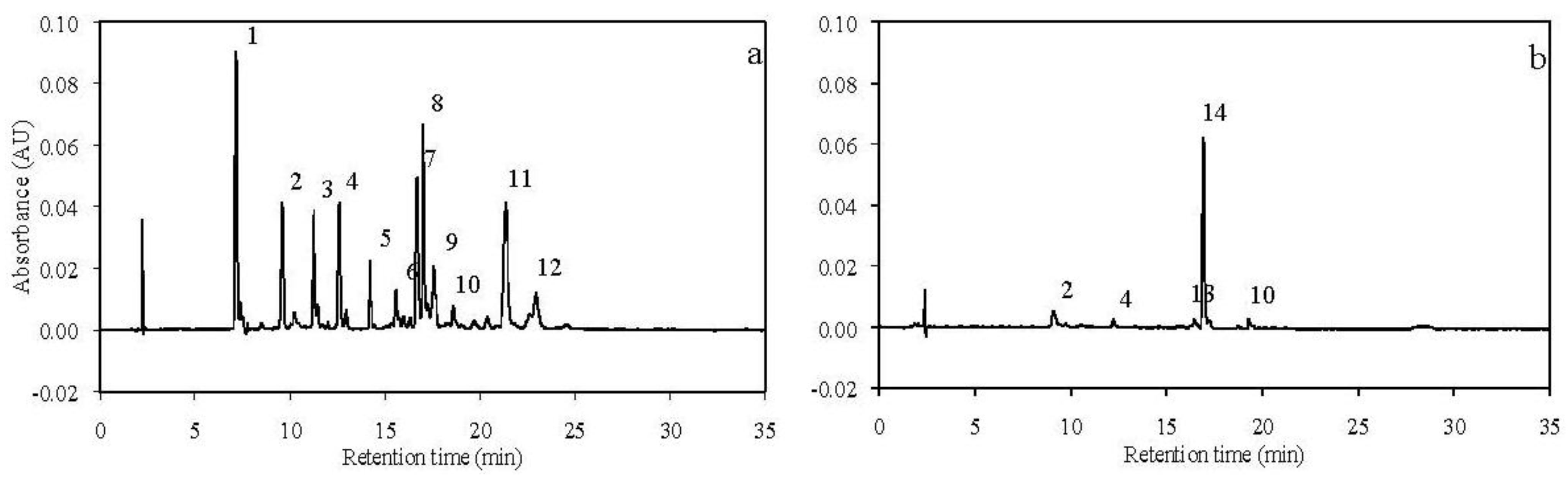
3.1. Carotenoids in Experimental Diets
| Carotenoids | Basal Diet | HP1 | HP2 | CZ1 | CZ2 | AST1 | AST2 |
|---|---|---|---|---|---|---|---|
| Astaxanthins | – | 380.59 | 95.92 | 387.27 | 97.87 | 385.04 | 92.13 |
| free | ND | 10.47 ± 0.07 | 2.42 ± 0.03 | 8.77 ± 1.00 | 2.25 ± 0.00 | 385.04 ± 14.60 | 92.13 ± 4.19 |
| monoesters | ND | 183.16 ± 10.92 | 46.27 ± 1.52 | 62.24 ± 2.40 | 15.72 ± 1.01 | ND | ND |
| diesters | ND | 186.96 ± 14.42 | 47.23 ± 1.83 | 316.26 ± 3.55 | 79.90 ± 3.70 | ND | ND |
| Lutein | 0.56 ± 0.11 | 18.15 ± 3.07 | 4.71 ± 0.13 | 40.23 ± 2.95 | 6.80 ± 1.03 | ND | ND |
| Fucoxanthin | 86.34 ± 9.85 | 80.74 ± 4.47 | 83.90 ± 3.25 | 80.53 ± 1.12 | 84.77 ± 0.92 | 85.53 ± 1.43 | 84.34 ± 2.48 |
| Canthaxanthin | ND | 10.88 ± 1.38 | 2.87 ± 0.19 | 142.23 ± 3.90 | 21.82 ± 2.50 | ND | ND |
| β-Carotene | 8.93 ± 0.09 | 15.60 ± 0.01 | 8.10 ± 0.05 | 14.22 ± 0.19 | 8.20 ± 0.00 | 8.67 ± 0.08 | 8.34 ± 0.00 |
| Isocryptoxanthin | ND | ND | ND | ND | ND | ND | ND |
| Echinenone | ND | ND | ND | ND | ND | ND | ND |
3.2. Carotenoids in the Gonads of the Sea Urchin A. crassispina
| Carotenoids | Initial | Basal Diet | HP1 | HP2 | CZ1 | CZ2 | AST1 | AST2 |
|---|---|---|---|---|---|---|---|---|
| Astaxanthin | ND | ND e | 3.01 ± 0.06 a | 1.09 ± 0.05 b | 0.26 ± 0.01 d | 0.15 ± 0.01 d | 2.93 ± 0.04 a | 0.61 ± 0.01 c |
| monoester | ND | ND | ND | ND | ND | ND | ND | ND |
| diester | ND | ND | ND | ND | ND | ND | ND | ND |
| Lutein | ND | ND | ND | ND | ND | ND | ND | ND |
| Fucoxanthin | 0.21 ± 0.01 | ND | ND | ND | ND | ND | ND | ND |
| Canthaxanthin | 1.84 ± 0.05 | ND d | 1.09 ± 0.49 c | 0.46 ± 0.01 cd | 7.48 ± 0.35 a | 2.79 ± 0.42 b | ND d | ND d |
| Isocryptoxanthin | 1.82 ± 0.04 | 0.60 ± 0.02 c | 0.71 ± 0.01 bc | 0.95 ± 0.24 ab | 0.48 ± 0.06 c | 0.67 ± 0.02 bc | 0.51 ± 0.01 c | 1.08 ± 0.07 a |
| Echinenone | 45.96 ± 1.31 | 12.7 ± 1.72 b | 23.52 ± 0.12 a | 21.77 ± 0.12 a | 15.41 ± 2.23 a | 14.11 ± 0.24 b | 15.34 ± 0.76 a | 9.56 ± 1.87 b |
| β-carotene | 3.61 ± 0.28 | 0.89 ± 0.04 de | 1.96 ± 0.01 a | 1.69 ± 0.06 b | 0.91 ± 0.03 d | 0.56 ± 0.05 f | 1.27 ± 0.05 c | 0.68 ± 0.14 ef |
3.3. The Gonad Index of Sea Urchins
| Initial | Basal Diet | HP1 | HP2 | CZ1 | CZ2 | AST1 | AST2 | |
|---|---|---|---|---|---|---|---|---|
| Diameter (mm) | 37.8 ± 1.4 | 38.2 ± 1.2 | 38.3 ± 1.1 | 38.8 ± 1.0 | 38.3 ± 0.7 | 38.4 ± 0.7 | 38.3 ± 0.3 | 38.3 ± 0.6 |
| Total weight (g) | 29.9 ± 0.3 | 30.3 ± 2.8 | 30.7 ± 2.5 | 32.3 ± 2.2 | 31.0 ± 1.7 | 32.0 ± 1.7 | 30.2 ± 0.6 | 30.7 ± 1.4 |
| Gonad weight (g) | 1.6 ± 0.6 | 2.6 ± 0.4 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.3 ± 0.3 | 2.8 ± 0.3 | 3.2 ± 0.3 | 3.5 ± 0.3 |
| Gonad index (%) | 5.2 ± 1.2 | 8.5 ± 0.8 | 11.2 ± 0.8 | 10.9 ± 1.0 | 10.7 ± 0.7 | 8.7 ± 0.9 | 10.5 ± 0.8 | 11.2 ± 0.9 |
4. Discussion

5. Conclusions
Conflict of Interest
References
- Freeman, S.M.; Rogers, S.I. A new analytical approach to the characterisation of macro-epibenthic habitats: Linking species to the environment. Estuar. Coast. Shelf Sci. 2003, 56, 749–764. [Google Scholar] [CrossRef]
- Ding, J.; Chang, Y.Q.; Wang, C.H.; Cao, X.B. Evaluation of the growth and heterosis of hybrids among three commercially important sea urchins in China: Strongylocentrotus nudus, S. intermedius and Anthocidaris crassispina. Aquaculture 2007, 272, 273–280. [Google Scholar]
- Chen, G.Q.; Xiang, W.Z.; Lau, C.C.; Peng, J.; Qiu, J.W.; Chen, F.; Jiang, Y. A comparative analysis of lipid and carotenoid composition of the gonads of Anthocidaris crassispina, Diadema setosum and Salmacis sphaeroides. Food Chem. 2010, 120, 973–977. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Olave, S.; Otaiza, R.; Lawrence, A.L.; Bustos, E. Enhancement of gonad production in the sea urchin Loxechinus albus in Chile fed extruded feeds. J. World Aquac. Soc. 1997, 28, 91–96. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F.; Whittick, A. The effect of an artificial diet on the biochemical composition of the gonads of the sea urchin (Strongylocentrotus droebachiensis). Food Chem. 2002, 79, 461–472. [Google Scholar] [CrossRef]
- Robinson, S.M.C.; Castell, J.D.; Kennedy, E.J. Developing suitable color in the gonads of cultured sea urchins (Strongylocentrotus droebachiensis). Aquaculture 2002, 206, 289–303. [Google Scholar] [CrossRef]
- Griffiths, M.G.; Perrott, P. Seasonal changes in the carotenoids of the sea urchin Strongylocentrotus droebachiensis. Comp. Biochem. Phys. 1976, 55, 435–441. [Google Scholar]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Hávardsson, B.; Imsland, A.K.; Christiansen, R. The effect of astaxanthin in feed and environmental temperature on carotenoid concentration in the gonads of green sea urchin Strongylocentrotus droebachiensis Muller. J. World Aquac. Soc. 1999, 30, 208–218. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health promoting effects of astaxanthin, a high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Boussiba, S.; Bing, W.; Yuan, J.P.; Zarka, A.; Chen, F. Changes in pigment profile in the green alga Haematococcus pluvialis exposed to environmental stresses. Biotechnol. Lett. 1999, 21, 601–604. [Google Scholar] [CrossRef]
- Yuan, J.P.; Chen, F. Puriffication of trans-astaxanthin from a high-yielding astaxanthin ester-producing strain of the microalga Haematococcus pluvialis. Food Chem. 2000, 68, 443–448. [Google Scholar] [CrossRef]
- Mann, V.; Harker, M.; Pecker, I.; Hirschberg, J. Metabolic engineering of astaxanthin production in tobacco flowers. Nat. Biotechnol. 2000, 18, 888–892. [Google Scholar] [CrossRef]
- Ip, P.F.; Wong, K.H.; Chen, F. Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process Biochem. 2004, 39, 1761–1766. [Google Scholar] [CrossRef]
- Ip, P.F.; Chen, F. Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem. 2005, 40, 733–738. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J. Growth-Associated biosynthesis of astaxanthin in heterotrophic Chlorella zofingiensis (Chlorophyta). World J. Microbiol. Biotechnol. 2008, 24, 1915–1922. [Google Scholar] [CrossRef]
- McLaughlin, G.; Kelly, M.S. Effect of artificial diets containing carotenoid-rich microalgae on gonad growth and color in the sea urchin Psammechinus miliaris (Gmelin). J. Shellfish Res. 2001, 20, 377–382. [Google Scholar]
- Shpigel, M.; McBride, S.C.; Marciano, S.; Ron, S.; Ben-Amotz, A. Improving gonad color and somatic index in the European sea urchin Paracentrotus lividus. Aquaculture 2005, 245, 101–109. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Pavlidis, M.; Jimeno, C.D.; Vardanis, G.; Divanach, P. The effect of different carotenoid sources on skin coloration of red porgy (Pagrus pagrus). Aquac. Res. 2005, 36, 1517–1525. [Google Scholar] [CrossRef]
- Kop, A.; Durmaz, Y. The effect of synthetic and natural pigments on the colour of the cichlids (Cichlasoma severum sp., Heckel 1840). Aquac. Int. 2008, 16, 117–122. [Google Scholar] [CrossRef]
- Sales, J.; Janssens, P.X. Nutrient requirements of ornamental fish. Aquat. Living Resour. 2003, 16, 533–540. [Google Scholar] [CrossRef]
- Vadas, R.L. Preferential feeding: An optimization strategy in sea urchins. Ecol. Monogr. 1977, 47, 337–371. [Google Scholar] [CrossRef]
- Plank, L.R.; Lawrence, J.M.; Lawrence, A.L.; Olvera, R.M. The effect of dietary carotenoids on gonad production and carotenoid profiles in the sea urchin Lytechinus variegatus. J. World Aquac. Soc. 2002, 33, 127–137. [Google Scholar] [CrossRef]
- Peng, J.; Xiang, W.Z.; Tang, Q.M.; Sun, N.; Chen, F.; Yuan, J.P. Comparative analysis of astaxanthin and its esters in the mutant E1 of Haematococcus pluvialis and other green algae by HPLC with a C30 column. Sci. China Ser. Life Sci. 2008, 51, 1108–1115. [Google Scholar] [CrossRef]
- Lau, D.C.C.; Lau, S.C.K.; Qian, P.Y.; Qiu, J.W. Morphological plasticity and resource allocation in response to food limitation and hyposalinity in a sea urchin. J. Shellfish Res. 2009, 28, 383–388. [Google Scholar] [CrossRef]
- Shpigel, M.; Schlosser, S.C.; Ben-Amotz, A.; Lawrence, A.L.; Lawrence, J.M. Effects of dietary carotenoid on the gut and the gonad of the sea urchin Paracentrotus lividus. Aquaculture 2006, 261, 1269–1280. [Google Scholar] [CrossRef]
- Montero-Torreiro, M.F.; Garcia-Martinez, P. Seasonal changes in the biochemical composition of body components of the sea urchin, Paracentrotus lividus, in Lorbe (Galicia, north-western Spain). J. Mar. Biol. Assoc. UK 2003, 83, 575–581. [Google Scholar] [CrossRef]
- Haug, E.; Guillou, M.; Connan, S.; Goulard, F.; Diouris, M. HPLC analysis of algal pigments to define diet of sea urchins. J. Mar. Biol. Assoc. UK 2003, 83, 571–573. [Google Scholar] [CrossRef]
- Davies, S.J. Physiological Assessment of Natural Pigmenting Carotenoid Sources for Salmonid Fish: Current Research and Future Perspectives. In Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech’s 21st Annual Symposium, Lexington, KY, USA, 22–25 May 2005; Lyons, T.P., Jacques, K.A., Eds.; Nottingham University Press: Nottingham, UK, 2005; pp. 295–306. [Google Scholar]
- Buttle, L.; Crampton, V.; Williams, P. The effect of feed pigment type on flesh pigment deposition and colour in farmed Atlantic salmon. Salmo Salar Aquac. Res. 2001, 32, 103–111. [Google Scholar] [CrossRef]
- Tshushima, M.; Kawakami, T.; Matsuno, T. Metabolism of carotenoids in sea-urchin Pseudocentrotus depressus. Comp. Biochem. Phys. 1993, 106, 737–741. [Google Scholar]
- Tang, G.; Dolnikowski, G.G.; Blanco, M.C. Serum carotenoids and retinoids in ferrets fed canthaxanthin. J. Nutr. Biochem. 1993, 4, 58–63. [Google Scholar] [CrossRef]
- Suckling, C.C.; Symonds, R.C.; Kelly, M.S.; Young, A.J. The effect of artificial diets on gonad colour and biomass in the edible sea urchin Psammechinus miliaris. Aquaculture 2011, 318, 335–342. [Google Scholar] [CrossRef]
- Kawakami, T.; Tsushima, M.; Katabami, Y.; Mine, M.; Ishida, A.; Matsuno, T. Effect of β,β-carotene, β-echinenone, astaxanthin, fucoxanthin, vitamin A and vitamin E on the biological defense of the sea urchin Pseudocentrotus depressus. J. Exp. Mar. Biol. Ecol. 1998, 226, 165–174. [Google Scholar] [CrossRef]
- Symonds, R.C.; Kelly, M.S.; Caris-Veyrat, C.; Young, A.J. Carotenoids in the sea urchin Paracentrotus lividus: Occurrence of 9′-cis-echinenone as the dominant carotenoid in gonad colour determination. Comp. Biochem. Phys. 2007, 148, 432–444. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng, J.; Yuan, J.-P.; Wang, J.-H. Effect of Diets Supplemented with Different Sources of Astaxanthin on the Gonad of the Sea Urchin Anthocidaris crassispina. Nutrients 2012, 4, 922-934. https://doi.org/10.3390/nu4080922
Peng J, Yuan J-P, Wang J-H. Effect of Diets Supplemented with Different Sources of Astaxanthin on the Gonad of the Sea Urchin Anthocidaris crassispina. Nutrients. 2012; 4(8):922-934. https://doi.org/10.3390/nu4080922
Chicago/Turabian StylePeng, Juan, Jian-Ping Yuan, and Jiang-Hai Wang. 2012. "Effect of Diets Supplemented with Different Sources of Astaxanthin on the Gonad of the Sea Urchin Anthocidaris crassispina" Nutrients 4, no. 8: 922-934. https://doi.org/10.3390/nu4080922
APA StylePeng, J., Yuan, J.-P., & Wang, J.-H. (2012). Effect of Diets Supplemented with Different Sources of Astaxanthin on the Gonad of the Sea Urchin Anthocidaris crassispina. Nutrients, 4(8), 922-934. https://doi.org/10.3390/nu4080922





