Long-Chain Polyunsaturated Fatty Acids in Inborn Errors of Metabolism
Abstract
:Abbreviations
| AA | arachidonic acid |
| ALA | α-linolenic acid |
| DHA | docosahexaenoic acid |
| E | erythrocyte |
| EPA | eicosapentaenoic acid |
| EPC | erythrocyte phosphatidylcholine |
| EPEA | erythrocyte phosphatidylethanolamine |
| EPL | erythrocyte phospholipid |
| HPA | hyperphenylalaninemi |
| LA | linoleic acid |
| MMA | methylmalonic academia |
| MSUD | maple syrup urine disease |
| P | plasma |
| PA | propionic acidemia |
| PCE | plasma cholesteryl ester |
| PKU | phenylketonuria |
| PPL | plasma phospholipid |
| PTG | plasma triacylglycerol |
1. Introduction
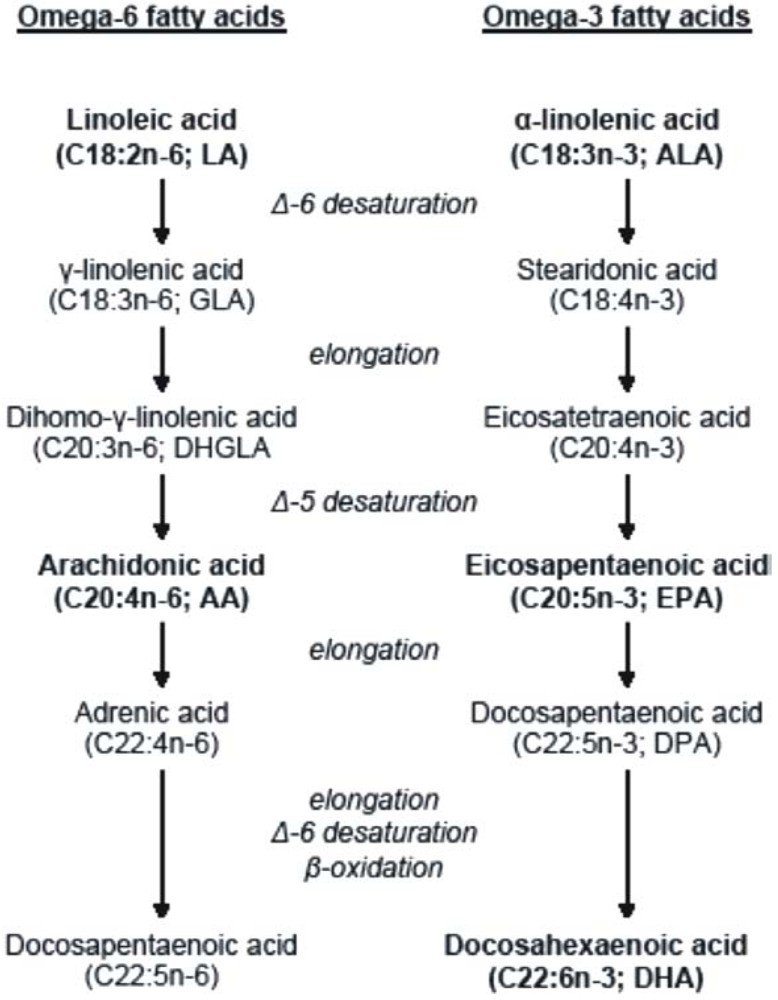
2. Long-Chain Polyunsaturated Fatty Acids
3. Literature Search
4. Long-Chain Polyunsaturated Fatty Acids in Inborn Errors of Metabolism
4.1. Observational studies
| Study | Number of participants, age | Biomarker | LA | AA | ALA | EPA | DHA |
|---|---|---|---|---|---|---|---|
| Galli et al., 1991 [24] | PKU(15)–Control(12) 3–12 yr | P | — | ↓ | n.d. | ↓ | ↓ |
| PPL | — | ↓ | n.d. | — | ↓ | ||
| PCE | — | — | n.d. | n.d. | n.d. | ||
| E | — | — | n.d. | — | — | ||
| Sanjurjo et al., 1994 [28] | PKU(40)–Control(50) 2 mo–20 yr | P | ↑ | ↓ | — | — | ↓ |
| EPL | — | ↑ | ↓ | ↓ | ↓ | ||
| Sanjurjo et al., 1997 [26] | IEM*(13)–Control(50) 1–17 yr | P | ↑ | ↓ | ↓ | ↓ | ↓ |
| EPL | ↑ | — | ↓ | — | ↓ | ||
| Decsi et al., 1997 [19] | PA(5)–Control(18) 3.5–9.5 yr | PPL | — | — | — | — | — |
| PTG | — | — | — | — | — | ||
| PCE | — | — | — | — | — | ||
| Pöge et al., 1998 [20] | PKU(8)–Control(12) 1 PKU(9)–Control(8) 2; 1: 1–6 yr; 2: 11–18 yr | PPL | — | — | — | — | — |
| — | — | — | — | — | |||
| PCE | — | — | — | — | ↓ | ||
| — | — | — | — | — | |||
| EPC | — | — | — | — | ↓ | ||
| — | — | — | — | — | |||
| EPEA | — | — | — | ↓ | ↓ | ||
| — | — | — | — | — | |||
| van Gool et al., 2000 [27] | PKU(9)–Control(18) 6 mo–25 yr | PPL | — | — | ↓ | ↓ | ↓ |
| EPL | ↑ | ↓ | ↓ | ↓ | ↓ | ||
| Acosta et al., 2001 [21] | PKU(13)–Control(13) a PKU(7)–Control(6) b PKU(8)–Control(7) c 1–13 yr | P | — | — | ↑ | — | — |
| — | — | — | ↓ | — | |||
| — | — | — | — | — | |||
| E | ↑ | — | — | — | — | ||
| — | — | — | — | — | |||
| — | — | — | — | — | |||
| Moseley et al., 2002 [25] | PKU(27)–Control(120) 7–39 yr | P | — | ↓ | ↑ | ↓ | ↓ |
| E | — | — | — | ↓ | ↓ | ||
| Vlaardingerbroek et al., 2006 [22] | IEM**(33)–Control(38) 1–18 yr | PPL | ↑ | — | ↑ | ↓ | ↓ |
| EPL | — | — | — | ↓ | ↓ | ||
| Mazer et al., 2010 [23] | MSUD(6)–Control(12) 12–30 yr | P | — | — | ↑ | — | ↓ |
| E | — | — | ↑ | — | ↓ |
4.2. Randomized controlled trials
| Study | Number of participants; age | Short description of intervention | Biomarker | DHA * (treatment vs. control group) | Clinical outcomes |
|---|---|---|---|---|---|
| [29] | 42 infants with PKU (21 in treatment and 21 in control group); 8–39 days | Supplemented formula (0.7 g AA and 0.3 g DHA/100 g fatty acids) for 1 yr | EPL | 3.60 (1.06) vs. 1.40 (0.44) a↑ | — P1 and P100 latency; — mental and physical development (Bayley Test); no adverse reactions |
| [30] | 21 infants with PKU (10 in treatment and 11 in control group); <4 wk | Supplemented formula (0.46 g AA and 0.27 g DHA/100 g fatty acids) for 1 yr | PPL | 3.08 (0.10) vs. 1.52 (0.19) a↑ | no adverse reactions |
| [31] | 21 children with PKU (10 in treatment and 11 in control group); 5–10 yr | 2.5–4 g fish oil (18 g EPA, 4 g DPA and 12 g DHA/100 g fatty acid) daily for 6 mo | P | 2.94 (0.88) vs. 0.73 (0.08) a↑ | ↓ plasma triacylglycerol; no adverse reactions |
| [16,32,33] | 20 children with HPA (10 in treatment and 10 in control group); 10 ± 7 yr | 1 capsule (37 mg AA, 27.5 mg EPA, 20 mg DPA and 40 mg DHA/0.5 g capsule) per 4 kg body weight for 1 yr | P | 2.3 (1.1) vs. 1.1 (0.3) a↑ | ↓ P100 wave latency; — plasma triacylglycerol; no adverse reactions |
| PPL | 3.1 (1.6) vs. 1.6 (0.4) a↑ | ||||
| PTG | 0.6 (0.5) vs. 0.3 (0.3) a— | ||||
| PCE | 0.5 (0.2) vs. 0.2 (0.1) a↑ | ||||
| E | 2.8 (1.5) vs. 1.5 (0.5) a— | ||||
| EPC | 0.9 (0.3) vs. 0.4 (0.2) a↑ | ||||
| EPEA | 3.7 (1.7) vs. 1.3 (0.9) a↑ | ||||
| [34] | 44 children with PKU (24 in treatment and 20 in control group); 1–10 yr | EFA supplemented protein substitute (17.2 g LA and 4.5 g ALA/100 g fatty acid) for 20 wk | EPL | 2.07 (0.8) vs. 1.64 (0.4) a↑ | no adverse reactions |
| [35] | 4 children with MMA; 9–16 yr; crossover design | 25 mg/kg DHA daily for 3 mo | P | 5.14 (2.72–7.94) vs. 1.89 (1.12–2.31) b↑ | ↓ plasma triacylglycerol; no adverse reactions |
5. Conclusions
References
- Lanpher, B.; Brunetti-Pierri, N.; Lee, B. Inborn errors of metabolism: The flux from Mendelian to complex diseases. Nat. Rev. Genet. 2006, 7, 449–460. [Google Scholar]
- Rao, A.N.; Kavitha, J.; Koch, M.; Suresh, K.V. Inborn errors of metabolism: Review and data from a tertiary care center. Indian J. Clin. Biochem. 2009, 24, 215–222. [Google Scholar]
- Levy, P.A. Inborn errors of metabolism: Part 1: Overview. Pediatr. Rev. 2009, 30, 131–137. [Google Scholar]
- Raghuveer, T.S.; Garg, U.; Graf, W.D. Inborn errors of metabolism in infancy and early childhood: An update. Am. Fam. Physician. 2006, 73, 1981–1990. [Google Scholar]
- Burton, B.K. Inborn errors of metabolism in infancy: A guide to diagnosis. Pediatrics 1998, 102, E69:1–E69:9. [Google Scholar]
- Schwartz, I.V.; Souza, C.F.; Giugliani, R. Treatment of inborn errors of metabolism. J. Pediatr. (Rio J.) 2008, 84, S8–S19. [Google Scholar]
- Kabra, M. Dietary management of inborn errors of metabolism. Indian J. Pediatr. 2002, 69, 421–426. [Google Scholar]
- Przyrembel, H.; Bremer, H.J. Nutrition, physical growth, and bone density in treated phenylketonuria. Eur. J. Pediatr. 2000, 159, S129–S135. [Google Scholar]
- Feillet, F.; Agostoni, C. Nutritional issues in treating phenylketonuria. J. Inherit. Metab. Dis. 2010. [Google Scholar]
- Das, U.N. Essential Fatty acids—a review. Curr. Pharm. Biotechnol. 2006, 7, 467–482. [Google Scholar]
- Szabó, É.; Soltész, Gy.; Decsi, T. Long-chain polyunsaturated fatty acid supply in diabetes mellitus. In Handbook of Type 1 Diabetes Mellitus: Etiology, Diagnosis, and Treatment, 1st; Aucoin, L., Prideux, T., Eds.; Nova Science Publishers: New York, NY, USA, 2010; pp. 265–295. [Google Scholar]
- Infante, J.P.; Huszagh, V.A. On the molecular etiology of decreased arachidonic (20:4n-6), docosapentaenoic (22:5n-6) and docosahexaenoic (22:6n-3) acids in Zellweger syndrome and other peroxisomal disorders. Mol. Cell. Biochem. 1997, 168, 101–115. [Google Scholar]
- Davis, B.C.; Kris-Etherton, P.M. Achieving optimal essential fatty acid status in vegetarians: Current knowledge and practical implications. Am. J. Clin. Nutr. 2003, 78, 640S–646S. [Google Scholar]
- Koletzko, B.; Beblo, S.; Demmelmair, H.; Müller-Felber, W.; Hanebutt, F.L. Does dietary DHA improve neural function in children? Observations in phenylketonuria. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 159–164. [Google Scholar]
- Sanders, T.A. DHA status of vegetarians. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 137–141. [Google Scholar]
- Agostoni, C.; Massetto, N.; Biasucci, G.; Rottoli, A.; Bonvissuto, M.; Bruzzese, M.G.; Giovannini, M.; Riva, E. Effects of long-chain polyunsaturated fatty acid supplementation on fatty acid status and visual function in treated children with hyperphenylalaninemia. J. Pediatr. 2000, 137, 504–509. [Google Scholar]
- Agostoni, C.; Verduci, E.; Fiori, L.; Riva, E.; Giovannini, M. Breastfeeding rates among hyperphenylalaninemic infants. Acta Paediatr. 2000, 89, 366–367. [Google Scholar]
- MacDonald, A.; Depondt, E.; Evans, S.; Daly, A.; Hendriksz, C.; Chakrapani, A.A.; Saudubray, J.M. Breast feeding in IMD. J. Inherit. Metab. Dis. 2006, 29, 299–303. [Google Scholar]
- Decsi, T.; Sperl, W.; Koletzko, B. Essential fatty acids in clinically stable children with propionic acidaemia. J. Inherit. Metab. Dis. 1997, 20, 778–782. [Google Scholar]
- Pöge, A.P.; Bäumann, K.; Müller, E.; Leichsenring, M.; Schmidt, H.; Bremer, H.J. Long-chain polyunsaturated fatty acids in plasma and erythrocyte membrane lipids of children with phenylketonuria after controlled linoleic acid intake. J. Inherit. Metab. Dis. 1998, 21, 373–381. [Google Scholar]
- Acosta, P.B.; Yannicelli, S.; Singh, R.; Eisas, L.J., II; Kennedy, M.J.; Bernstein, L.; Rohr, F.; Trahms, C.; Koch, R.; Breck, J. Intake and blood levels of fatty acids in treated patients with phenylketonuria. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Vlaardingerbroek, H.; Hornstra, G.; de Koning, T.J.; Smeitink, J.A.; Bakker, H.D.; de Klerk, H.B.; Rubio-Gozalbo, M.E. Essential polyunsaturated fatty acids in plasma and erythrocytes of children with inborn errors of amino acid metabolism. Mol. Genet. Metab. 2006, 88, 159–165. [Google Scholar]
- Mazer, L.M.; Yi, S.H.; Singh, R.H. Docosahexaenoic acid status in females of reproductive age with maple syrup urine disease. J. Inherit. Metab. Dis. 2010, 33, 121–127. [Google Scholar]
- Galli, C.; Agostoni, C.; Mosconi, C.; Riva, E.; Salari, P.C.; Giovannini, M. Reduced plasma C-20 and C-22 polyunsaturated fatty acids in children with phenylketonuria during dietary intervention. J. Pediatr. 1991, 119, 562–567. [Google Scholar]
- Moseley, K.; Koch, R.; Moser, A.B. Lipid status and long-chain polyunsaturated fatty acid concentrations in adults and adolescents with phenylketonuria on phenylalanine-restricted diet. J. Inherit. Metab. Dis. 2002, 25, 56–64. [Google Scholar]
- Sanjurjo, P.; Ruiz, J.I.; Montejo, M. Inborn errors of metabolism with a protein-restricted diet: Effect on polyunsaturated fatty acids. J. Inherit. Metab. Dis. 1997, 20, 783–789. [Google Scholar]
- van Gool, C.J.; van Houwelingen, A.C.; Hornstra, G. The essential fatty acid status in phenylketonuria patients under treatment. J. Nutr. Biochem. 2000, 11, 543–547. [Google Scholar]
- Sanjurjo, P.; Perteagudo, L.; Rodríguez Soriano, J.; Vilaseca, A.; Campistol, J. Polyunsaturated fatty acid status in patients with phenylketonuria. J. Inherit. Metab. Dis. 1994, 17, 704–709. [Google Scholar]
- Agostoni, C.; Harvie, A.; McCulloch, D.L.; Demellweek, C.; Cockburn, F.; Giovannini, M.; Murray, G.; Harkness, R.A.; Riva, E. A randomized trial of long-chain polyunsaturated fatty acid supplementation in infants with phenylketonuria. Dev. Med. Child. Neurol. 2006, 48, 207–212. [Google Scholar]
- Koletzko, B.; Sauerwald, T.; Demmelmair, H.; Herzog, M.; von Schenck, U.; Böhles, H.; Wendel, U.; Seidel, J. Dietary long-chain polyunsaturated fatty acid supplementation in infants with phenylketonuria: A randomized controlled trial. J. Inherit. Metab. Dis. 2007, 30, 326–332. [Google Scholar]
- Agostoni, C.; Riva, E.; Biasucci, G.; Luotti, D.; Bruzzese, M.G.; Marangoni, F.; Giovannini, M. The effects of n-3 and n-6 polyunsaturated fatty acids on plasma lipids and fatty acids of treated phenylketonuric children. Prostaglandins Leukot. Essent. Fatty Acids 1995, 53, 401–404. [Google Scholar]
- Agostoni, C.; Scaglioni, S.; Bonvissuto, M.; Bruzzese, M.G.; Giovannini, M.; Riva, E. Biochemical effects of supplemented long-chain polyunsaturated fatty acids in hyperphenylalaninemia. Prostaglandins Leukot. Essent. Fatty Acids 2001, 64, 111–115. [Google Scholar]
- Agostoni, C.; Verduci, E.; Massetto, N.; Fiori, L.; Radaelli, G.; Riva, E.; Giovannini, M. Long term effects of long chain polyunsaturated fats in hyperphenylalaninemic children. Arch. Dis. Child. 2003, 88, 582–583. [Google Scholar]
- Cleary, M.A.; Feillet, F.; White, F.J.; Vidailhet, M.; Macdonald, A.; Grimsley, A.; Maurin, N.; de Baulny, H.O.; Rutherford, P.J. Randomised controlled trial of essential fatty acid supplementation in phenylketonuria. Eur. J. Clin. Nutr. 2006, 60, 915–920. [Google Scholar]
- Aldámiz-Echevarría, L.; Sanjurjo, P.; Elorz, J.; Prieto, J.A.; Pérez, C.; Andrade, F.; Rodríguez-Soriano, J. Effect of docosahexaenoic acid administration on plasma lipid profile and metabolic parameters of children with methylmalonic acidaemia. J. Inherit. Metab. Dis. 2006, 29, 58–63. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fekete, K.; Decsi, T. Long-Chain Polyunsaturated Fatty Acids in Inborn Errors of Metabolism. Nutrients 2010, 2, 965-974. https://doi.org/10.3390/nu2090965
Fekete K, Decsi T. Long-Chain Polyunsaturated Fatty Acids in Inborn Errors of Metabolism. Nutrients. 2010; 2(9):965-974. https://doi.org/10.3390/nu2090965
Chicago/Turabian StyleFekete, Katalin, and Tamás Decsi. 2010. "Long-Chain Polyunsaturated Fatty Acids in Inborn Errors of Metabolism" Nutrients 2, no. 9: 965-974. https://doi.org/10.3390/nu2090965
APA StyleFekete, K., & Decsi, T. (2010). Long-Chain Polyunsaturated Fatty Acids in Inborn Errors of Metabolism. Nutrients, 2(9), 965-974. https://doi.org/10.3390/nu2090965




