Isolated White Lupin Proteins Beneficially Modulate the Intestinal Microbiota Composition in Rats
Highlights
- White lupin protein isolate beneficially modulated the intestinal microbiota composition in rats.
- The beneficial modulation of intestinal microbiota generally associated with legume-based diets is likely to be due, at least in part, to their constituent protein components.
- Lactalbumin induced a generally healthier microbiota composition than casein.
- Casein modulated the intestinal microbiota to a composition compatible with improved bowel movement frequency and lipid metabolism.
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets
2.2. Animals and Treatments
2.3. RT-qPCR Microbiota Composition Analysis
2.4. High-Throughput Analysis of Microbial Community
2.5. Analyses of Predicted Microbial Functions
2.6. Statistical Analysis
3. Results
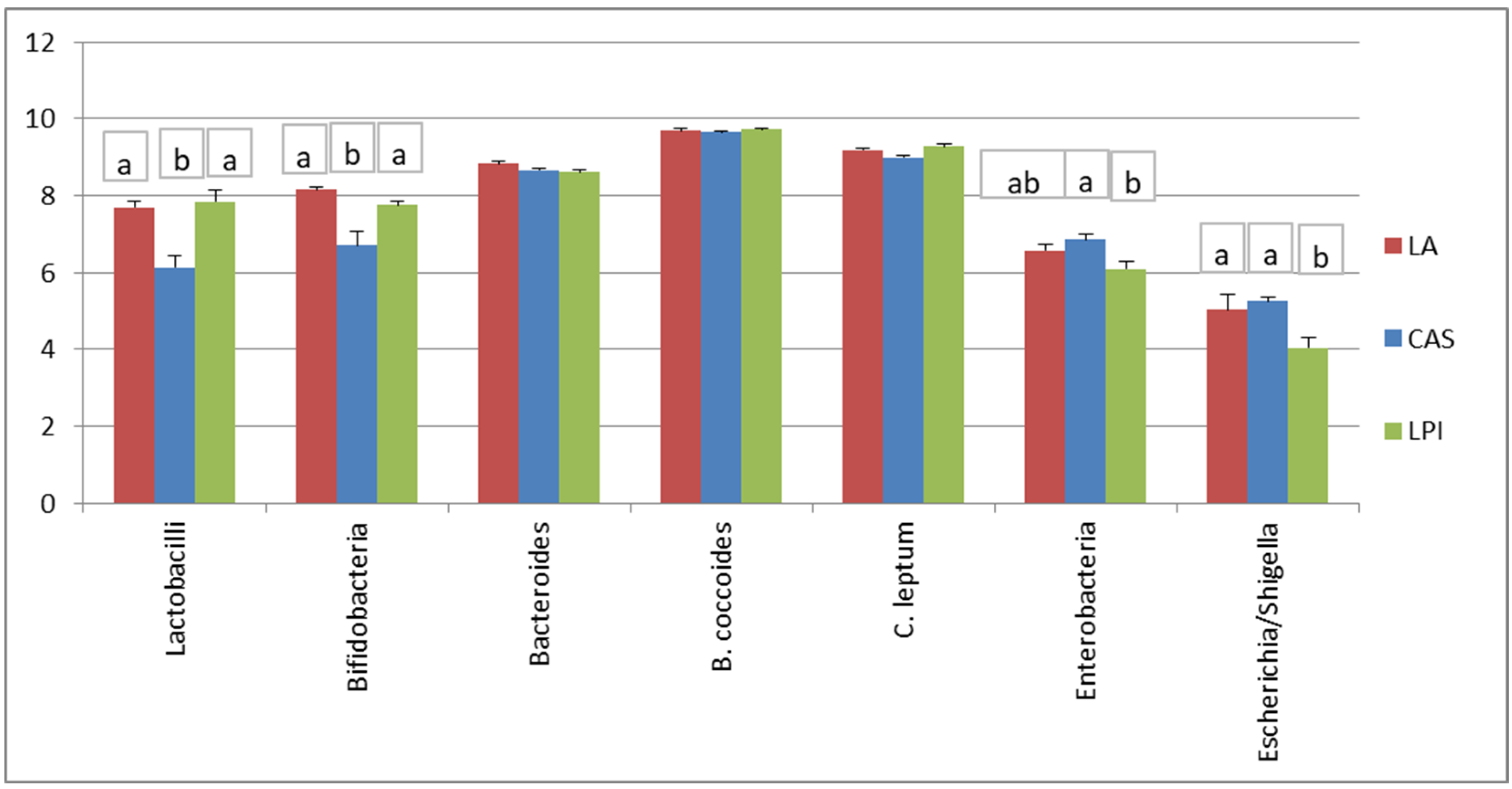
3.1. RT-qPCR Microbiota Composition
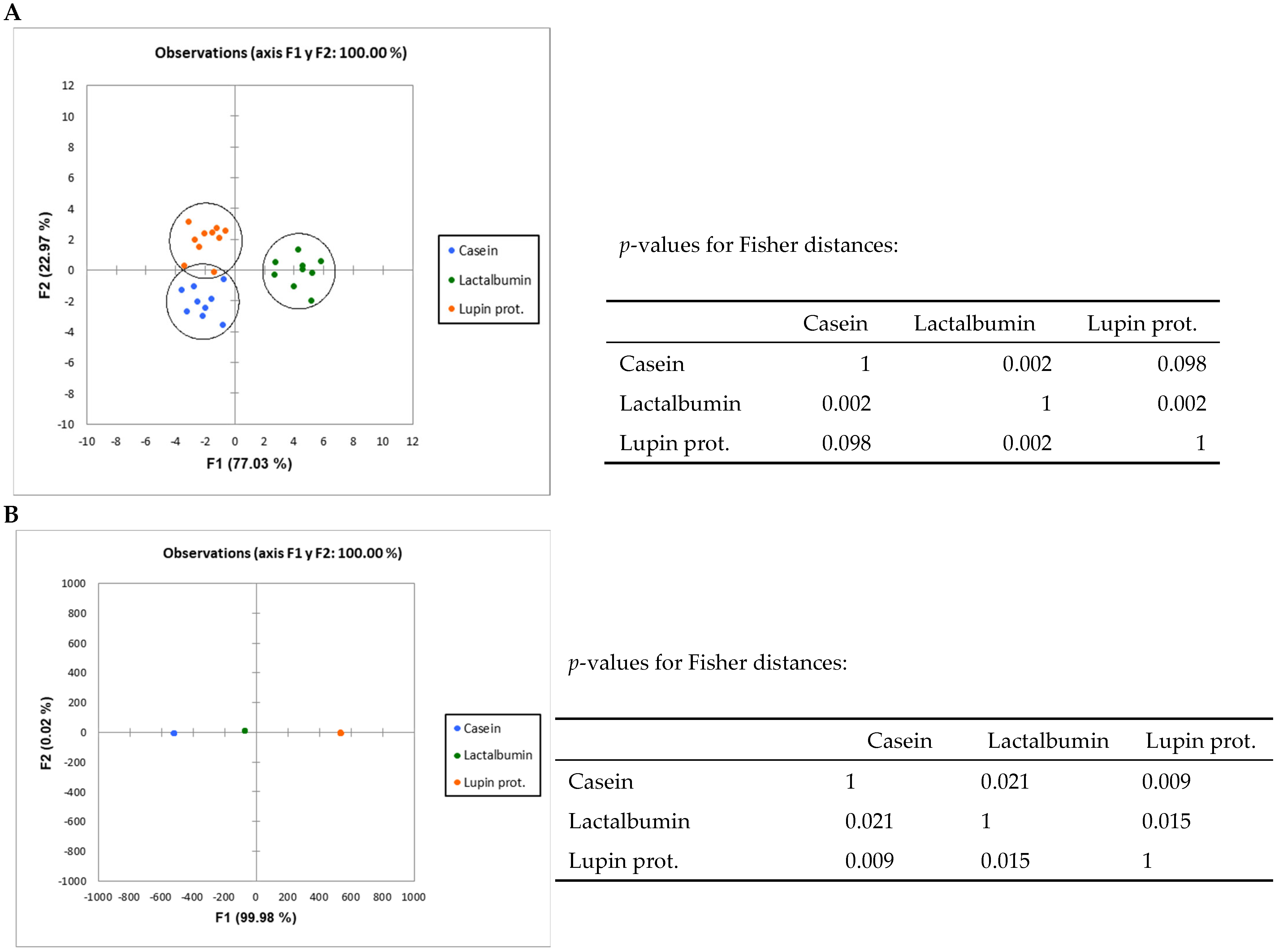
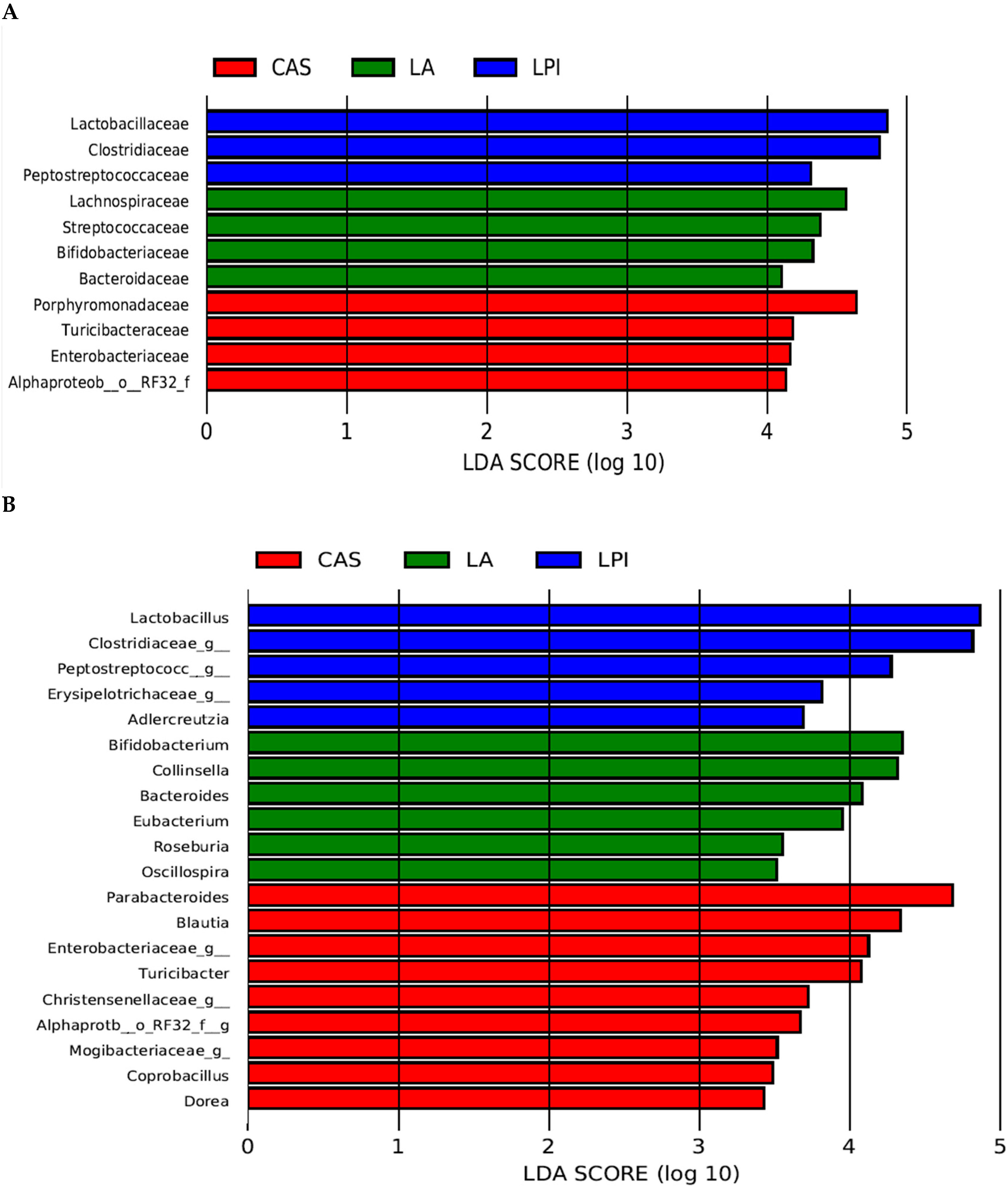
3.2. Results on High-Throughput Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Xu, Y.; Xiong, J.; Wang, X.; He, F.; Cheng, G. Dietary protein sources, gut microbiome, and puberty timing in children: Findings from a cohort study. Signal Transduct. Target. Ther. 2024, 9, 167. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Shen, A.; Zhang, T.; Jiang, L.; El-Seedi, H.; Zhang, G.; Sui, X. Legumes as an alternative protein source in plant-based foods: Applications, challenges, and strategies. Curr. Res. Food Sci. 2024, 9, 100876. [Google Scholar] [CrossRef]
- Lisciani, S.; Marconi, S.; Le Donne, C.; Camilli, E.; Aguzzi, A.; Gabrielli, P.; Gambelli, L.; Kunert, K.; Marais, D.; Vorster, B.J.; et al. Legumes and common beans in sustainable diets: Nutritional quality, environmental benefits, spread and use in food preparations. Front. Nutr. 2024, 11, 1385232. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Ramos, F.; Sanches Silva, A. Lupin (Lupinus albus L.) Seeds: Balancing the good and the bad and addressing future challenges. Molecules 2022, 27, 8557. [Google Scholar] [CrossRef] [PubMed]
- Prusinski, J. White Lupin (Lupinus albus L.)—Nutritional and health values in human nutrition—A Review. Czech J. Food Sci. 2017, 35, 95–105. [Google Scholar] [CrossRef]
- Friedman, M. Nutritional value of proteins from different food sources. A review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Rubio, L.A.; Clemente, A. In vivo (rat) and In vitro (Caco-2 cells) absorption of amino acids from legume as compared to animal proteins. Arch. Anim. Nutr. 2009, 63, 413–426. [Google Scholar] [CrossRef]
- Caligari, S.; Chiesa, G.; Camisassi, D.; Johnson, S.K.; Gilio, D.; Marchesi, M.; Parolini, C.; Rubio, L.A.; Sirtori, C.R. Lupin (Lupinus albus) protein isolate has adequate nutritional value and reduces large intestinal weight in rats after restricted and ad libitum feeding. Ann. Nutr. Metab. 2006, 50, 528–537. [Google Scholar] [CrossRef]
- Arpón, A.; Riezu-Boj, J.I.; Milagro, F.I.; Marti, A.; Razquin, C.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Casas, R.; Fitó, M.; et al. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J. Physiol. Biochem. 2016, 73, 445–455. [Google Scholar] [CrossRef]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Diether, N.; Willing, B. Microbial fermentation of dietary protein: An important factor in diet–microbe–host interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef]
- Hector, A.J.; Phillips, S.M. Protein recommendations for weight loss in elite athletes: A focus on body composition and performance. Int. J. Sport Nutr. Exerc. Metab. 2023, 28, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.-D.A. Effect of dietary protein and processing on gut microbiota—A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef]
- Salonen, A.; de Vos, W.M. Impact of diet on human intestinal microbiota and health. Annu. Rev. Food Sci. Technol. 2014, 5, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 34, 289–306. [Google Scholar] [CrossRef]
- Rubio, L.A. Dietary Milk or isolated legume proteins modulate intestinal microbiota composition in rats. Nutrients 2024, 16, 149. [Google Scholar] [CrossRef]
- D’Agostina, A.; Antonioni, C.; Resta, D.; Arnoldi, A.; Bez, J.; Knauf, U.; Wäsche, A. Optimization of a pilot-scale process for producing lupin protein isolates with valuable technological properties and minimum thermal damage. J. Agric. Food Chem. 2006, 54, 92–98. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Laboratory Animals; National Academy Press: Washington, DC, USA, 1995. [Google Scholar]
- Cohen, S.A.; Meys, M.; Tarwin, T.L. The Pico-Tag Method: A Manual of Advanced Techniques for Amino Acid Analysis; Millipore Corporation: Bedford, MA, USA, 1989. [Google Scholar]
- van Barneveld, R.J. Understanding the nutritional chemistry of lupin (Lupinus spp.) seed to improve livestock production efficiency. Nutr. Res. Rev. 1999, 12, 203–230. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Jesse, Z.; Gregory, J.; Daniel, M.D.; Dan, K.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814. [Google Scholar] [CrossRef] [PubMed]
- De-Santis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 7, 5069–5072. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Lin, H.; Das Peddada, S. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Arnoldi, A. The healthy-profood project: Overview and main inputs. Grain Legumes 2005, 43, 14. [Google Scholar]
- Scarafoni, A.; Magni, C.; Duranti, M. Molecular nutraceutics as a mean to investigate the positive effects of legume seed proteins on human health. Trends Food Sci. Technol. 2007, 18, 454–463. [Google Scholar]
- Parolini, C.; Rigamonti, E.; Marchesi, M.; Busnelli, M.; Cinquanta, P.; Manzini, S.; Sirtori, C.R.; Chiesa, G. Cholesterol-Lowering effect of dietary Lupinus angustifolius proteins in adult rats through regulation of genes involved in cholesterol homeostasis. Food Chem. 2012, 132, 1475. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.A.; Grant, G.; Spencer, R.; Pusztai, A. The effects of feeding lupin (Lupinus angustifolius) seed meal or its insoluble fraction on the intestinal microflora population in the rat. Microb. Ecol. Health Dis. 1995, 8, 101–105. [Google Scholar]
- Rubio, L.A.; Peinado, M.J. Replacement of soybean meal with lupin or chickpea seed meal in diets for fattening Iberian pigs promotes a healthier ileal microbiota composition. Adv. Microbiol. 2014, 4, 498–503. [Google Scholar] [CrossRef]
- Rist, V.T.S.; Weiss, E.; Sauer, N.; Mosenthin, R.; Eklund, M. Effect of dietary protein supply originating from soybean meal or casein on the intestinal microbiota of piglets. Anaerobe 2014, 25, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Davin-Regli, A.; Lavigne, J.P.; Pages, J.M. Enterobacter spp.: Update on Taxonomy, Clinical Aspects, and Emerging Antimicrobial Resistance. Clin. Microb. Rev. 2019, 32, e00002-19. [Google Scholar] [CrossRef]
- Kim, E.; Kim, D.-B.; Park, J.-Y. Changes of mouse gut microbiota diversity and composition by modulating dietary protein and carbohydrate contents: A pilot study. Prev. Nutr. Food Sci. 2016, 21, 57–61. [Google Scholar] [CrossRef]
- Zakostelska, Z.; Kverka, M.; Klimesova, K.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Hornova, M.; Srutkova, D.; Hudcovic, T.; Ridl, J.; et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS ONE 2011, 6, e27961. [Google Scholar] [CrossRef]
- Simon, E.; Calinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, prebiotics, and synbiotics: Implications and beneficial effects against irritable bowel syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T Cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef]
- Fujio-Vejar, S.; Vasquez, Y.; Morales, P.; Magne, F.; Vera-Wolf, P.; Ugalde, J.A.; Navarrete, P.; Gotteland, M. The gut microbiota of healthy chilean subjects reveals a high abundance of the phylum Verrucomicrobia. Front. Microbiol. 2017, 8, 1221. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, M.; Manzini, S.; Chiesa, G. The Gut Microbiota Affects Host Pathophysiology as an Endocrine Organ: A Focus on Cardiovascular Disease. Nutrients 2019, 12, 79. [Google Scholar] [CrossRef]
- Oki, K.; Toyama, M.; Banno, T.; Chonan, O.; Benno, Y.; Watanabe, K. Comprehensive analysis of the faecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol. 2016, 16, 284. [Google Scholar] [CrossRef]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.M.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef] [PubMed]


| Amino Acids | LA 1 | CAS | LPI |
|---|---|---|---|
| Histidine | 14.1 | 27.5 | 17.9 |
| Isoleucine | 40.2 | 40.9 | 39.1 |
| Leucine | 65.3 | 74.9 | 60.0 |
| Lysine | 80.1 | 97.9 | 47.5 |
| Methionine + Cysteine | 55.5 | 39.2 | 15.3 |
| Phenylalanine + Tyrosine | 46.3 | 95.8 | 83.4 |
| Threonine | 50.0 | 39.6 | 30.0 |
| Tryptophan 2 | 16.7 | 10.0 | 5.0 |
| Valine | 37.4 | 53.3 | 30.2 |
| Diet | LA 1 | CAS | LPI |
|---|---|---|---|
| Lactalbumin | 138 | - | - |
| Casein | - 2 | 118 | - |
| Lupin proteins isolate | - | - | 128 |
| EAA | |||
| Lysine | - | - | 5.0 |
| Methionine | - | 1.9 | 4.6 |
| Threonine | - | - | 0.3 |
| Tryptophan | - | - | 1.0 |
| Valine | - | - | 1.1 |
| Taxonomy | Diet 1 | ||
|---|---|---|---|
| LA | CAS | LPI | |
| Family | |||
| LA | 0 | 0.023 | 0.001 |
| CAS | 0 | 0.001 | |
| LPI | 0 | ||
| Genus | |||
| LA | 0 | 0.021 | <0.001 |
| CAS | 0 | <0.001 | |
| LPI | 0 | ||
| Species | |||
| LA | 0 | 0.021 | <0.001 |
| CAS | 0 | <0.001 | |
| LPI | 0 | ||
| Taxonomy | Diet 1 | |||
|---|---|---|---|---|
| LA 2 | CAS | LPI | p Values | |
| Phylum | ||||
| Bacteroidetes | 4900 | 3830 | 2755 | 0.231 |
| Firmicutes | 14,091 | 9824 | 17,882 | 0.514 |
| Proteobacteria | 1025 ab | 1201 a | 410 b | 0.085 |
| Actinobacteria | 2406 a | 1229 ab | 1007 b | 0.085 |
| Verrucomicrobia | 1081 | 850 | 892 | 0.838 |
| Tenericutes | 29 | 49 | 136 | 0.198 |
| F/B | 3 a | 3 a | 5 b | 0.006 |
| Genus | ||||
| Allobaculum | 491 | 502 | 458 | 0.947 |
| Clostridiales;f__;g__ | 124 ab | 103 b | 187 a | 0.072 |
| Lachnospiraceae;g__ | 178 | 142 | 132 | 0.175 |
| Ruminococcaceae;g__ | 138 | 163 | 186 | 0.273 |
| Bacteroidales;f__S24-7;g__ | 331 | 146 | 258 | 0.119 |
| Ruminococcus | 165 | 150 | 195 | 0.533 |
| Rikenellaceae;g__ | 106 ab | 154 a | 65 b | 0.049 |
| Parabacteroides | 321 b | 615 a | 275 b | 0.005 |
| Collinsella | 239 a | 4 b | 1 b | <0.0001 |
| Alphaproteobacteria;o__RF32;f__;g__ | 26 a | 36 a | 2 b | <0.0001 |
| Bifidobacterium | 315 a | 245 ab | 182 b | 0.109 |
| Erysipelotrichaceae;g__ | 13 b | 5 b | 42 a | <0.0001 |
| Bacteroides | 137 a | 128 a | 23 b | 0.004 |
| Peptostreptococcaceae;g__ | 73 b | 192 a | 205 a | 0.002 |
| Lactobacillus | 447 a | 107 b | 652 a | 0.001 |
| Akkermansia | 214 | 270 | 258 | 0.796 |
| Clostridiaceae;g__ | 122 c | 372 b | 576 a | <0.0001 |
| Blautia | 196 a | 198 a | 37 b | 0.002 |
| Turicibacter | 122 a | 136 a | 47 a | 0.097 |
| Sutterella | 86 ab | 94 a | 44 b | 0.044 |
| [Eubacterium] | 78 a | 39 ab | 7 b | 0.054 |
| Enterobacteriaceae;g__ | 62 b | 122 a | 10 c | <0.0001 |
| Species | ||||
| Allobaculum;s__ | 491 | 502 | 458 | 0.947 |
| Clostridiales;f__;g__;s__ | 124 ab | 103 b | 187 a | 0.072 |
| Lachnospiraceae;g__;s__ | 178 | 142 | 132 | 0.175 |
| Bacteroidales;f__S24-7;g__;s__ | 331 | 146 | 258 | 0.119 |
| Ruminococcaceae;g__;s__ | 138 | 163 | 186 | 0.273 |
| Ruminococcus;s__ | 54 b | 133 ab | 138 a | 0.072 |
| Ruminococcus;s__bromii | 117 | 59 | 38 | 0.141 |
| Rikenellaceae;g__;s__ | 106 ab | 154 a | 65 b | 0.049 |
| Parabacteroides;s__ | 350 b | 615 a | 275 b | 0.006 |
| Collinsella;s__aerofaciens | 114 a | 0.2 b | 0.3 b | 0.030 |
| Alphaproteobacteria;o__RF32;f__;g__;s__ | 29 a | 32 a | 2 b | 0.001 |
| Bifidobacterium;s__ | 180 | 99 | 151 | 0.485 |
| Bifidobacterium;s__animalis | 196 a | 85 ab | 52 b | 0.064 |
| Erysipelotrichaceae;g__;s__ | 13 b | 5 b | 49 a | <0.0001 |
| Bacteroides;s__ | 137 a | 128 a | 23 b | 0.004 |
| Peptostreptococcaceae;g__;s__ | 73 b | 192 a | 205 a | 0.002 |
| Lactobacillus;s__ | 381 b | 106 c | 627 a | <0.0001 |
| Lactobacillus;s__reuteri | 66 a | 1 b | 101 a | 0.001 |
| Akkermansia;s__muciniphila | 192 | 270 | 235 | 0.655 |
| Clostridiaceae;g__;s__ | 122 c | 372 b | 576 a | <0.0001 |
| Blautia;s__producta | 110 a | 86 a | 32 b | 0.015 |
| Blautia;s__ | 111 a | 112 a | 5.200 b | 0.001 |
| Turicibacter;s__ | 109 ab | 152 a | 52 b | 0.100 |
| Sutterella;s__ | 86 ab | 94 a | 49 b | 0.086 |
| [Eubacterium];s__dolichum | 78 a | 39 ab | 7 b | 0.054 |
| Enterobacteriaceae;g__;s__ | 48 b | 122 a | 16 b | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, L.A.; Chiesa, G. Isolated White Lupin Proteins Beneficially Modulate the Intestinal Microbiota Composition in Rats. Nutrients 2025, 17, 551. https://doi.org/10.3390/nu17030551
Rubio LA, Chiesa G. Isolated White Lupin Proteins Beneficially Modulate the Intestinal Microbiota Composition in Rats. Nutrients. 2025; 17(3):551. https://doi.org/10.3390/nu17030551
Chicago/Turabian StyleRubio, Luis A., and Giulia Chiesa. 2025. "Isolated White Lupin Proteins Beneficially Modulate the Intestinal Microbiota Composition in Rats" Nutrients 17, no. 3: 551. https://doi.org/10.3390/nu17030551
APA StyleRubio, L. A., & Chiesa, G. (2025). Isolated White Lupin Proteins Beneficially Modulate the Intestinal Microbiota Composition in Rats. Nutrients, 17(3), 551. https://doi.org/10.3390/nu17030551








