Unlocking Opportunities and Overcoming Challenges in Genetically Engineered Biofortification
Abstract
1. Introduction
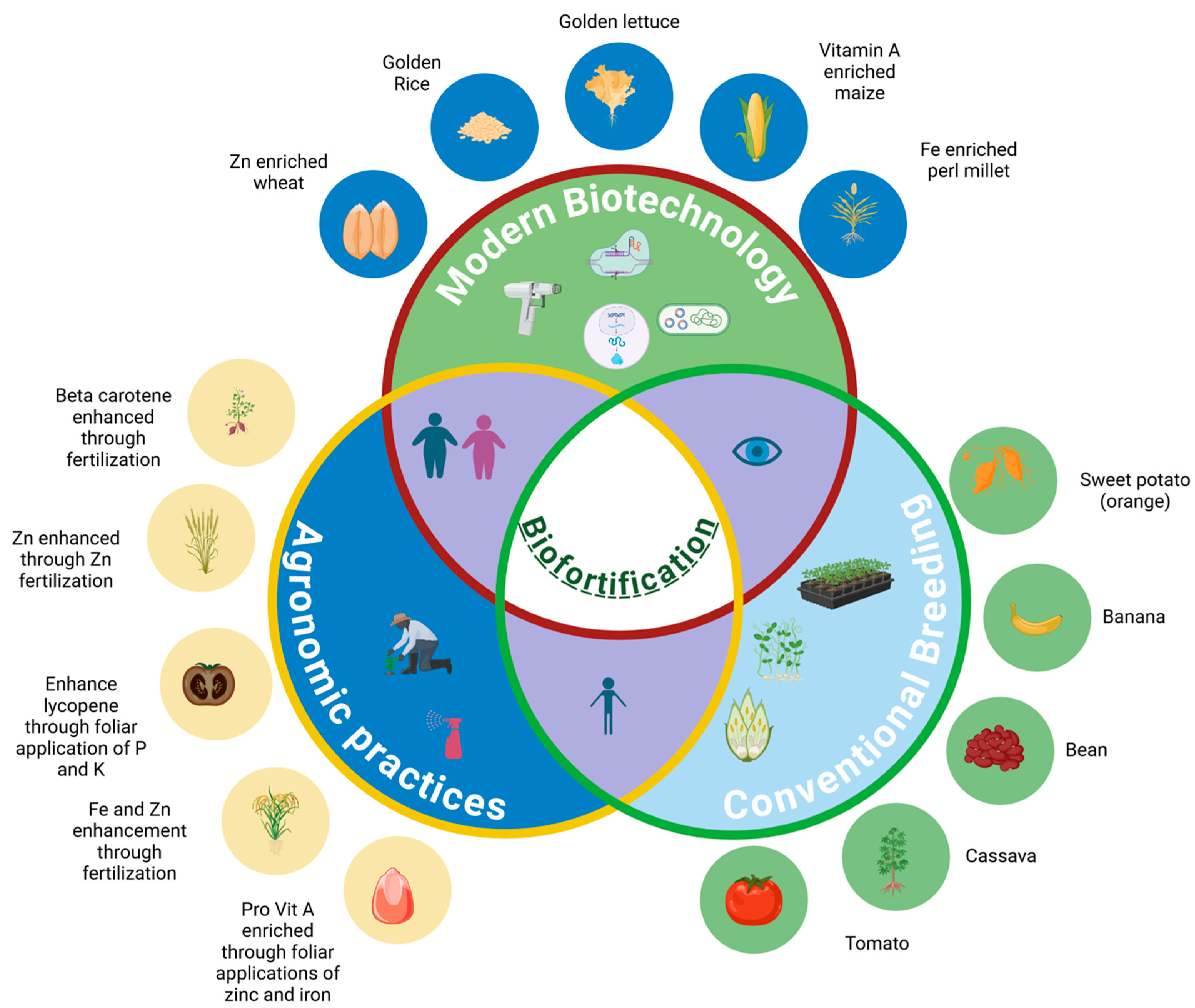
2. Common Crop Biofortification Schemes
3. Plant Genetic Engineering as a Strategy for Biofortification
4. Examples of Biofortified and Nutritionally Enhanced Crops
4.1. Nutritionally Enhanced Tomatoes
4.2. β-Carotene Biofortification
4.3. Omega 3 Biofortifiaction
4.4. Potato
4.5. Iron-Enhanced Crops
4.6. Bean
4.7. Cassava
4.8. Rice
4.9. Wheat
5. Molecular Farming for Animal Proteins Using Plant Platforms
6. Biochemical Studies on Nutrient Biofortification in Crops
7. Opportunities and Challenges of Genetically Engineered Biofortified Crops
7.1. Opportunities
7.2. Challenges
8. Fate of Biologically Active Compounds in Biofortified Crops
9. A Regulatory Milestone in Promoting Biofortification Research
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- UNICEF. The State of the World’s Children 2020: Nutrition, for Every Child; UNICEF: Pyrmont, NSW, Australia, 2020. [Google Scholar]
- Lowe, N.; Lowe, N.; Qualter, P.; Sinclair, J.; Gupta, S.; Zaman, M. School Feeding to Improve Cognitive Performance in Disadvantaged Children: A 3-Arm Parallel Controlled Trial in Northwest Pakistan. Nutrients 2023, 15, 1768. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). The State of Food Security and Nutrition in the World 2019; FAO: Roma, Italy, 2019. [Google Scholar]
- Jack, A. Nutrition Under Siege. One Peaceful World; Kushi Institute newsletter: Becket, MA, USA, 1998; pp. 1–8. [Google Scholar]
- Bhardwaj, R.L.; Parashar, A.; Parewa, H.P.; Vyas, L. An alarming decline in the nutritional quality of foods: The biggest challenge for future generations’ health. Foods 2024, 13, 877. [Google Scholar] [CrossRef]
- Pfeiffer, W.H.; McClafferty, B. Biofortification: Breeding micronutrient-dense crops. In Breeding Major Food Staples; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Datta, K.; Datta, S.K. Indica rice (Oryza sativa, BR29 and IR-64). In Methods in Molecular Biology: Agrobacterium Protocols; Wang, K., Ed.; Humana Press: Totowa, NJ, USA, 2006; Volume 344, pp. 201–211. [Google Scholar]
- Ierna, A.; Pellegrino, A.; Mauro, R.P.; Leonardi, C. Micronutrient Foliar Fertilization for the Biofortification of Raw and Minimally Processed Early Potatoes. Agronomy 2020, 10, 1744. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, C.; Shen, Z.; Yan, C. Crop Quality Improvement Through Genome Editing Strategy. Front. Genome Ed. 2022, 3, 819687. [Google Scholar] [CrossRef]
- Kumar, D.; Yadav, A.; Ahmad, R.; Dwivedi, U.N.; Yadav, K. CRISPR-Based Genome Editing for Nutrient Enrichment in Crops: A Promising Approach Toward Global Food Security. Front. Genet. 2022, 13, 932859. [Google Scholar] [CrossRef] [PubMed]
- Buchholzer, M.; Frommer, W.B. An increasing number of countries regulate genome editing in crops. New Phytol. 2022, 237, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Financial Times. Proposed EU Ban On Gene-Edited Crop Patents Prompts Dispute. 2024. Available online: https://www.ft.com/content/5e3cc871-1377-4c65-88e5-e9096fb4db1f (accessed on 13 June 2024).
- Li, J.; Scarano, A.; Gonzalez, N.M.; D’orso, F.; Yue, Y.; Nemeth, K.; Saalbach, G.; Hill, L.; Martins, C.d.O.; Moran, R.; et al. Biofortified tomatoes provide a new route to vitamin D sufficiency. Nat. Plants 2022, 8, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Karlson, D.; Mojica, J.P.; Poorten, T.J.; Lawit, S.J.; Jali, S.; Chauhan, R.D.; Pham, G.M.; Marri, P.; Guffy, S.L.; Fear, J.M.; et al. Targeted Mutagenesis of the Multicopy Myrosinase Gene Family in Allotetraploid Brassica juncea Reduces Pun-gency in Fresh Leaves across Environments. Plants 2022, 11, 2494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.M.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Gonzali, S.; Perata, P. Anthocyanins from Purple Tomatoes as Novel Antioxidants to Promote Human Health. Antioxidants 2020, 9, 1017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ezura, K.; Nakamura, A.; Mitsuda, N. Genome-wide characterization of the TALE homeodomain family and the KNOX-BLH interaction network in tomato. Plant Mol. Biol. 2022, 109, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Gerondopoulos, A.; Linford, A.; Rigden, D.J.; Barr, F.A. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J. Cell Biol. 2010, 191, 367–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takayama, H.; Landes, E.; Truby, L.; Fujita, K.; Kirtane, A.J.; Mongero, L.; Yuzefpolskaya, M.; Colombo, P.C.; Jorde, U.P.; Kurlansky, P.A.; et al. Feasibility of smaller arterial cannulas in venoarterial extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2015, 149, 1428–1433. [Google Scholar] [CrossRef]
- Takayama, M.; Matsukura, C.; Ariizumi, T.; Ezura, H. Activating glutamate decarboxylase activity by removing the autoin-hib-itory domain leads to hyper γ-aminobutyric acid (GABA) accumulation in tomato fruit. Plant Cell Rep. 2017, 36, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Al-Babili, S.; Hoa, T.T.; Schaub, P. Exploring the potential of the bacterial carotene desaturase CrtI to increase the beta-carotene content in Golden Rice. J. Exp. Bot. 2006, 57, 1007–1014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ye, X.; Al-Babili, S.; Kloti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the provitamin A (β-carotene) biosynthetic pathway into rice endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef]
- Tang, G.; Qin, J.; Dolnikowski, G.G.; Russell, R.M.; Grusak, M.A. Golden Rice is an effective source of vitamin A. Am. J. Clin. Nutr. 2009, 89, 1776–1783. [Google Scholar] [CrossRef]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Perez-Colao, P.; Reig-Lopez, D.; Di, X.; Llorente, B.; Rodriguez-Concepcion, M. Boosting pro-vitamin A content and bi-oaccessibility in leaves by combining engineered biosynthesis and storage pathways with high-light treatments. Plant J. 2024, 119, 2951–2966. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Alok, A.; Shivani Kumar, P.; Kaur, N.; Awasthi, P.; Chaturvedi, S.; Pandey, P.; Pandey, A.; Pandey, A.K.; Tiwari, S. CRISPR/Cas9 directed editing of lycopene epsilon-cyclase modulates metabolic flux for β-carotene biosynthesis in banana fruit. Metab. Eng. 2020, 59, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Calerón, M.; Sayanova, O.; Napier, J.A. An alternative to fish oils: Metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog. Lipid Res. 2010, 49, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lopez, N.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Successful high-level accumulation of fish oil omega-3 long-chain poly-unsaturated fatty acids in a transgenic oilseed crop. Plant J. 2014, 77, 198–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belide, S.; Shrestha, P.; Kennedy, Y.; Leonforte, A.; Devine, M.D.; Petrie, J.R.; Singh, S.P.; Zhou, X.R. Engineering docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA) in Brassica juncea. Plant Biotechnol. J. 2022, 20, 19–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- University of Minnesota (n.d.). The importance of Technology Transfer|Better World. Available online: https://autm.net/about-tech-transfer/better-world-project/bwp-stories/talen (accessed on 13 June 2024).
- Ye, J.; Shakya, R.; Shrestha, P.; Rommens, C.M. Tuber-specific silencing of the acid invertase gene substantially lowers the acry-la-mide-forming potential of potato. J. Agric. Food Chem. 2010, 58, 12162–12167. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Martínez-Prada, M.; Curtin, S.J.; Gutiérrez-González, J.J. Potato improvement through genetic engineering. GM Crops Food. 2021, 12, 479–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chincinska, I.A.; Miklaszewska, M.; Sołtys-Kalina, D. Recent advances and challenges in potato improvement using CRISPR/Cas genome editing. Planta. 2022, 257, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huertas, R.; Karpinska, B.; Ngala, S.; Mkandawire, B.; Maling’A, J.; Wajenkeche, E.; Kimani, P.M.; Boesch, C.; Stewart, D.; Hancock, R.D.; et al. Biofortification of common bean (Phaseolus vulgaris L.) with iron and zinc: Achievements and challenges. Food Energy Secur. 2022, 12, e406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narayanan, N.; Beyene, G.; Chauhan, R.D.; Gaitán-Solís, E.; Gehan, J.; Butts, P.; Siritunga, D.; Okwuonu, I.; Woll, A.; Jiménez-Aguilar, D.M.; et al. Biofortification of field-grown cassava by engineering expression of an iron transporter and ferritin. Nat. Biotechnol. 2019, 37, 144–151, Erratum in: Nat. Biotechnol. 2019, 37, 323. https://doi.org/10.1038/s41587-019-0066-6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wairich, A.; Ricachenevsky, F.K.; Lee, S. A tale of two metals: Biofortification of rice grains with iron and zinc. Front. Plant Sci. 2022, 13, 944624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, B.B.; Mishra, S.K.; Banoth, S.K.; Baliyan, S.; Chauhan, H. Iron and zinc biofortification of rice by synergistic expression of OsNAS2 gene with monocot (Pennisetum glaucum) and dicot (Phaseolus vulgaris) ferritins. Plant Physiol. Biochem. 2023, 205, 108195. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Gruissem, W.; Bhullar, N.K. Novel rice iron biofortification approaches using expression of ZmYS1 and OsTOM1 controlled by tissue-specific promoters. J. Exp. Bot. 2022, 73, 5440–5459. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Li, X.; Li, R.; Huang, J.; Fan, J.; Zhang, J.; Wang, Y.; Zhang, C. Overexpression of Nicotianamine Synthase (AtNAS1) Increases Iron Accumulation in the Tuber of Potato. Plants 2022, 11, 2741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beasley, J.T.; Bonneau, J.P.; Moreno-Moyano, L.T.; Callahan, D.L.; Howell, K.S.; Tako, E.; Taylor, J.; Glahn, R.P.; Appels, R.; Johnson, A.A.T. Multi-year field evaluation of nicotianamine biofortified bread wheat. Plant J. 2022, 109, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liao, S.; Li, M.; Wei, J.; Zhu, B.; Gu, L.; Li, L.; Du, X. TmNAS3 from Triticum monococum directly regulated by TmbHLH47 increases Fe content of wheat grain. Gene 2022, 811, 146096. [Google Scholar] [CrossRef] [PubMed]
- Aluru, M.; Xu, Y.; Guo, R.; Wang, Z.; Li, S.; White, W.; Wang, K.; Rodermel, S. Generation of Transgenic Maize with Enhanced Provitamin A Content. J. Exp. Bot. 2008, 59, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Boonyaves, K.; Wu, T.Y.; Gruissem, W.; Bhullar, N.K. Enhanced Grain Iron Levels in Rice Expressing an Iron-Regulated Metal Transporter, Nicotianamine Synthase, and Ferritin Gene Cassette. Front. Plant Sci. 2017, 8, 130. [Google Scholar] [CrossRef]
- Singh, S.P.; Keller, B.; Gruissem, W.; Bhullar, N.K. Rice Nicotianamine Synthase 2 Expression Improves Dietary Iron and Zinc Levels in Wheat. Theor. Appl. Genet. 2017, 130, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Konda, A.R.; Nazarenus, T.J.; Nguyen, H.; Yang, J.; Gelli, M.; Swenson, S.; Cahoon, E.B. Metabolic Engineering of Soybean Seeds for Enhanced Vitamin E Tocochromanol Content and Effects on Oil Antioxidant Properties in Polyunsaturated Fatty Acid-Rich Germplasm. Metab. Eng. 2020, 57, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Lee, C.J.; Park, S.U.; Lim, Y.H.; Park, W.S.; Kim, H.J.; Ahn, M.J.; Kwak, S.S.; Kim, H.S. Overexpression of the Golden SNP-Carrying Orange Gene Enhances Carotenoid Accumulation and Heat Stress Tolerance in Sweetpotato Plants. Antioxidants 2021, 10, 51. [Google Scholar] [CrossRef]
- Good Food Institute (GFI) (n.d.). Plant Molecular Farming: Key Facts. Available online: https://gfi.org/resource/plant-molecular-farming-facts/ (accessed on 13 June 2024).
- Ivanovich, C.C.; Sun, T.; Gordon, D.R.; Ocko, I.B. Future warming from global food consumption. Nat. Clim. Change 2023, 13, 297–302. [Google Scholar] [CrossRef]
- Schillberg, S.; Finnern, R. Plant molecular farming for the production of valuable proteins—Critical evaluation of achievements and future challenges. J. Plant Physiol. 2021, 258–259, 153359. [Google Scholar] [CrossRef] [PubMed]
- Watson, E. Proudly Genetically Modified: Moolec Molecular Farming co. Gears up to Launch Meat Proteins from GM Crops. Food Navigator-USA. 2022. Available online: https://www.foodnavigator-usa.com/Article/2022/04/25/Proudly-genetically-modified-Moolec-molecular-farming-co-gears-up-to-launch-meat-proteins-from-GM-crops (accessed on 25 April 2022).
- D’Amelia, V.; Staiti, A.; D’Orso, F.; Maisto, M.; Piccolo, V.; Aversano, R.; Carputo, D. Targeted mutagenesis of StISAC stabilizes the production of anthocyanins in potato cell culture. Plant Direct 2022, 6, e433. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Wu, L.; Wang, M.; Yang, W.; Wang, X.; Ma, W.; Sun, W.; Chen, S.; Xiang, L.; Shi, Y. CRISPR/Cas9-Mediated Targeted Mutagenesis of FtMYB45 Promotes Flavonoid Biosynthesis in Tartary Buckwheat (Fagopyrum tataricum). Front. Plant Sci. 2022, 13, 879390. [Google Scholar] [CrossRef]
- Xu, H.; Wang, N.; Liu, J.; Qu, C.; Wang, Y.; Jiang, S.; Lu, N.; Wang, D.; Zhang, Z.; Chen, X. The molecular mechanism underlying anthocyanin metabolism in apple using the MdMYB16 and MdbHLH33 genes. Plant Mol. Biol. 2017, 94, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Li, C.; Ma, X.; Luo, K. PtrMYB57 contributes to the negative regulation of anthocyanin and proanthocyanidin biosynthesis in poplar. Plant Cell Rep. 2017, 36, 1263–1276. [Google Scholar] [CrossRef]
- Sun, C.; Deng, L.; Du, M.; Zhao, J.; Chen, Q.; Huang, T.; Jiang, H.; Li, C.-B.; Li, C. A transcriptional network promotes anthocyanin biosynthesis in tomato flesh. Mol. Plant 2020, 13, 42–58. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H.; et al. Development of “Purple Endosperm Rice” by engineering anthocyanin biosynthesis in the endosperm with a high-efficiency transgene stacking system. Mol. Plant 2017, 10, 918–929. [Google Scholar] [CrossRef]
- Liu, X.Q.; Li, S.Z.; Yang, W.Z.; Mu, B.N.; Jiao, Y.; Zhou, X.J.; Zhang, C.Y.; Fan, Y.L.; Chen, R.M. Synthesis of Seed-Specific Bidirectional Promoters for Metabolic Engineering of Anthocyanin-Rich Maize. Plant Cell Physiol. 2018, 59, 1942–1955. [Google Scholar] [CrossRef]
- Tu, M.X.; Fang, J.H.; Zhao, R.K.; Liu, X.Y.; Yin, W.C.; Wang, Y.; Wang, X.H.; Wang, X.P.; Fang, Y.L. CRISPR/Cas9-mediated mutagenesis of VvbZIP36 promotes anthocyanin accumulation in grapevine (Vitis vinifera). Hortic. Res. 2022, 9, uhac022. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Matsukura, C.; Takayama, M.; Asamizu, E.; Ezura, H. Suppression of g-aminobutyric acid (GABA) transaminases induces prominent GABA accumulation, dwarfism and infertility in the tomato (Solanum lycopersicum L.). Plant Cell Physiol. 2013, 54, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, R.; Li, X.; Fu, D.; Zhu, B.; Tian, H.; Luo, Y.; Zhu, H. Multiplexed CRISPR/Cas9-mediated metabolic engineering of g-aminobutyric acid levels in Solanum lycopersicum. Plant Biotechnol. J. 2018, 16, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, D.H.; Kim, M.-S.; Jung, Y.J.; Kang, K.K. Physicochemical Properties and Antioxidant Activity of CRISPR/Cas9-Edited Tomato SGR1 Knockout (KO) Line. Int. J. Mol. Sci. 2024, 25, 5111. [Google Scholar] [CrossRef] [PubMed]
- Akama, K.; Akter, N.; Endo, H.; Kanesaki, M.; Endo, M.; Toki, S. An In Vivo Targeted Deletion of the Calmodulin-Binding Domain from Rice Glutamate Decarboxylase 3 (OsGAD3) Increases γ-Aminobutyric Acid Content in Grains. Rice 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 2017, 7, 7057. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, M.; Zhang, M.; Yang, M.; Dai, S.; Meng, Q.; Lv, W.; Zhuang, K. ETHYLENE-INSENSITIVE 3-LIKE 2 regulates β-carotene and ascorbic acid accumulation in tomatoes during ripening. Plant Physiol. 2023, 192, 2067–2080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tripathi, L.; Ntui, V.O.; Tripathi, J.N. Application of CRISPR/Cas-based gene-editing for developing better banana. Front. Bioeng. Biotechnol. 2024, 12, 1395772. [Google Scholar] [CrossRef]
- Do, P.T.; Nguyen, C.X.; Bui, H.T.; Tran, L.T.N.; Stacey, G.; Gillman, J.D.; Zhang, Z.J.; Stacey, M.G. Demonstration of highly efficient dual gRNA CRISPR/Cas9 editing of the homeologous GmFAD2-1A and GmFAD2-1B genes to yield a high oleic, low linoleic and α-linolenic acid phenotype in soybean. BMC Plant Biol. 2019, 19, 311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jarvis, B.A.; Romsdahl, T.B.; McGinn, M.G.; Nazarenus, T.J.; Cahoon, E.B.; Chapman, K.D.; Sedbrook, J.C. CRISPR/Cas9-Induced fad2 and rod1 Mutations Stacked With fae1 Confer High Oleic Acid Seed Oil in Pennycress (Thlaspi arvense L.). Front. Plant Sci. 2021, 12, 652319. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, Y.; Dueñas, C., Jr.; Arcillas, E.; Macalalad-Cabral, R.J.; Kohli, A.; Reinke, R.; Slamet-Loedin, I.H. CRISPR-mediated promoter editing of a cis-regulatory element of OsNAS2 increases Zn uptake/translocation and plant yield in rice. Front. Genome Ed. 2024, 5, 1308228. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yamaji, N.; Ma, J.F. Role of a vacuolar iron transporter OsVIT2 in the distribution of iron to rice grains. New Phytol. 2021, 230, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Saleem, B.; Rehman, N.; Zafar, S.A.; Naeem, M.K.; Khan, M.R. CRISPR/Cas9 mediated disruption of Inositol Pentakisphosphate 2-Kinase 1 (TaIPK1) reduces phytic acid and improves iron and zinc accumulation in wheat grains. J. Adv. Res. 2021, 37, 33–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horton, S.; Alderman, H.; Rivera, J.A. Copenhagen Consensus 2008 Challenge Paper Malnutrition and Hunger. 2008. Available online: https://copenhagenconsensus.com/sites/default/files/CP_Malnutrition_and_Hunger_-_Horton.pdf (accessed on 9 April 2012).
- Bouis, H.; Birol, E.; Boy, E.; Gannon, B.M.; Haas, J.D.; Low, J.; Mehta, S.; Michaux, K.; Mudyahoto, B.; Pfeiffer, W.; et al. Food Biofortificatio: Reaping the Benefits of Science to Overcome Hidden Hunger; CAST: Ames, IA, USA, 2020. [Google Scholar]
- Vaiknoras, K.; Larochelle, C. The impact of iron-biofortified bean adoption on bean productivity, consumption, purchases and sales. World Dev. 2020, 139, 105260. [Google Scholar] [CrossRef]
- McNulty, M.J.; Gleba, Y.; Tusé, D.; Hahn-Löbmann, S.; Giritch, A.; Nandi, S.; McDonald, K.A. Techno-economic analysis of a plant-based platform for manufacturing antimicrobial proteins for food safety. Biotechnol. Prog. 2019, 36, e2896. [Google Scholar] [CrossRef] [PubMed]
- Sinke, P.; Swartz, E.; Sanctorum, H.; Van Der Giesen, C.; Odegard, I. Ex-ante life cycle assessment of commercial-scale cultivated meat production in 2030. Int. J. Life Cycle Assess. 2023, 28, 234–254. [Google Scholar] [CrossRef]
- Hefferon, K. Prospects and challenges associated with GM biofortified crops. In Genetic Engineering and Genome Editing for Zinc Biofortification of Rice; Swamy, B.P.M., Macovei, A., Trijatmiko, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 153–165. [Google Scholar] [CrossRef]
- De Steur, H.; Blancquaert, D.; Strobbe, S.; Lambert, W.; Gellynck, X.; Van Der Straeten, D. Status and market po-ten-tial of transgenic biofortified crops. Nat. Biotechnol. 2015, 33, 25–29. [Google Scholar] [CrossRef]
- Mmbando, G.S. The Adoption of Genetically Modified Crops in Africa: The Public’s Current Perception, the Regu-la-tory Obstacles, and Ethical Challenges. GM Crops Food 2024, 15, 1–15. [Google Scholar]
- Talsma, E.F.; Melse-Boonstra, A.; Brouwer, I.D. Acceptance and adoption of biofortified crops in low-and mid-dle-income countries: A systematic review. Nutr. Rev. 2017, 75, 798–829. [Google Scholar] [CrossRef] [PubMed]
- Muzhingi, T.; Langyintuo, A.S.; Malaba, L.C.; Banziger, M. Consumer acceptability of yellow maize products in Zimbabwe. Food Policy 2007, 33, 352–361. [Google Scholar] [CrossRef]
- De Groote, H.; Kimenju, S.C. Consumer preferences for maize products in urban Kenya. Food Nutr. Bull. 2012, 33, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Vaiknoras, K.; Larochelle, C.; Birol, E.; Asare-Marfo, D.; Herrington, C. Promoting rapid and sustained adoption of biofortified crops: What we learned from iron-biofortified bean delivery approaches in Rwanda. Food Policy 2019, 83, 271–284. [Google Scholar] [CrossRef]
- Birol, E.; Bouis, H.E. Role of socio-economic research in developing, delivering and scaling new crop varieties: The case of staple crop biofortification. Front. Plant Sci. 2023, 14, 1099496. [Google Scholar] [CrossRef]
- Katuuramu, D.N.; Wiesinger, J.A.; Luyima, G.B.; Nkalubo, S.T.; Glahn, R.P.; Cichy, K.A. Investigation of genotype by environment interactions for seed zinc and iron concentration and iron bioavailability in common bean. Front. Plant Sci. 2021, 12, 670965. [Google Scholar] [CrossRef] [PubMed]
- Avnee; Sood, S.; Chaudhary, D.R.; Jhorar, P.; Rana, R.S. Biofortification: An approach to eradicate micronutrient deficiency. Front. Nutr. 2023, 10, 1233070. [Google Scholar] [CrossRef] [PubMed]
- Van Der Straeten, D.; Bhullar, N.K.; De Steur, H.; Gruissem, W.; MacKenzie, D.; Pfeiffer, W.; Qaim, M.; Slamet-Loedin, I.; Strobbe, S.; Tohme, J.; et al. Multiplying the efficiency and impact of biofortification through metabolic engineering. Nat. Commun. 2020, 11, 5203. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2008, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.A.; Maqbool, A. Transgenic Crops for Biofortification. Front. Sustain. Food Syst. 2020, 4, 571402. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil. 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Ishii, T.; Araki, M. A future scenario of the global regulatory landscape regarding genome-edited crops. GM Crops Food 2017, 8, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Shohael, A.M.; Hefferon, K.L. Agricultural biotechnology in Bangladesh: The way forward. In Agricultural Bioeconomy; Academic Press: Cambridge, MA, USA, 2023; pp. 143–166. [Google Scholar]
- Ahmed, S.; Shohael, A.M.; Ahamed, T.; Ahmed, R.; Ahmed, S.; Hassan, H.M.S. Understanding public perspectives on genetically engineered Brinjal and the adoption of modern biotechnology in Bangladesh. Front. Bioeng. Biotechnol. 2024, 12, 1471201. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shohael, A.M.; Kelly, J.; Venkataraman, S.; Hefferon, K. Unlocking Opportunities and Overcoming Challenges in Genetically Engineered Biofortification. Nutrients 2025, 17, 518. https://doi.org/10.3390/nu17030518
Shohael AM, Kelly J, Venkataraman S, Hefferon K. Unlocking Opportunities and Overcoming Challenges in Genetically Engineered Biofortification. Nutrients. 2025; 17(3):518. https://doi.org/10.3390/nu17030518
Chicago/Turabian StyleShohael, Abdullah Mohammad, Jojo Kelly, Srividhya Venkataraman, and Kathleen Hefferon. 2025. "Unlocking Opportunities and Overcoming Challenges in Genetically Engineered Biofortification" Nutrients 17, no. 3: 518. https://doi.org/10.3390/nu17030518
APA StyleShohael, A. M., Kelly, J., Venkataraman, S., & Hefferon, K. (2025). Unlocking Opportunities and Overcoming Challenges in Genetically Engineered Biofortification. Nutrients, 17(3), 518. https://doi.org/10.3390/nu17030518








