Short- and Long-Term Nutritional Status in Children and Adolescents with Celiac Disease Following a Gluten-Free Diet: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Outcome Measures
2.5. Risk of Bias
3. Results
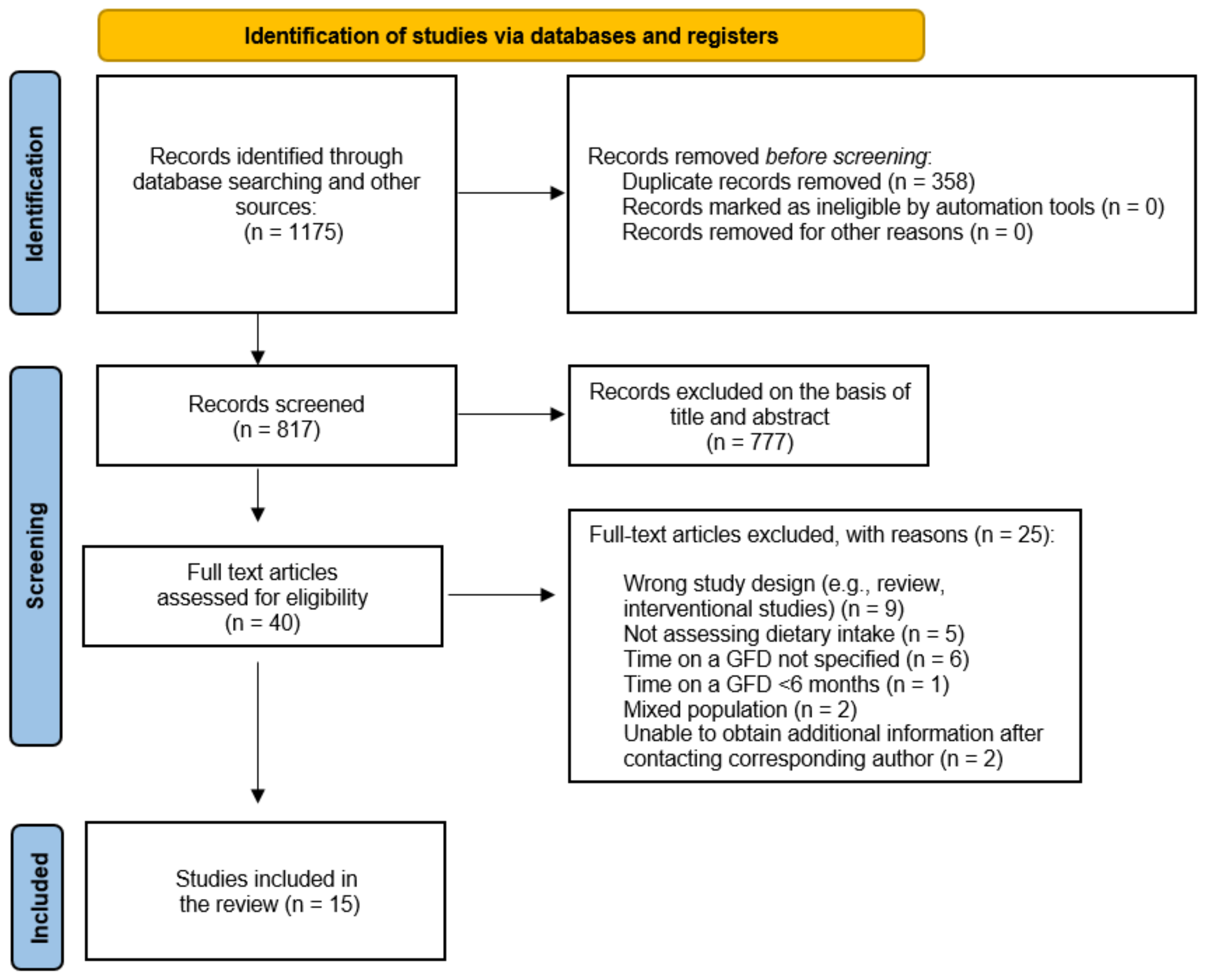
3.1. Study Selection and Quality Assessment
3.2. Study Characteristics
3.3. Associations of GFD with Energy and Macronutrient Intake
- Short-term associations
- Long-term associations
3.4. Associations of GFD with Micronutrient Intake
3.4.1. Fat-Soluble Vitamins
| Study | Duration of GFD | Results | |||
|---|---|---|---|---|---|
| Vitamin A | Vitamin D | Vitamin E | Vitamin K | ||
| Allowaymi et al. [33] | Short term ≥6 months | ↓ (compared to DRI) G: 247.09 μg (p = 0.000) B: 326.16 μg (p = 0.004) DRI: 600 μg | ↓ (compared to DRI) G: 2.50 mg (p = 0.000) B: 3.26 mg (p = 0.000) DRI: 15 mg | ↓ (compared to DRI) G: 1.59 mg (p = 0.000) B: 1.45 mg (p = 0.000) DRI: 11 mg | ↓ for boys (compared to DRI) NS for girls G: 62.25 μg (p = 0.865) B: 34.71 μg (p = 0.021) DRI: 60 μg |
| Zuccotti et al. [46] | Short term ≥6 months | - | ↓ (p < 0.001) (compared to HCs) CD: 0.8 ± 0.6 μg HC: 3.1 ± 2.8 μg | - | - |
| Ballestero- Fernández et al., 2019 [22] | Long term ≥1 y | NS | NS | NS | NS |
| Balamtekin et al., 2015 [34] | Long term 4.0 ± 3.3 y | NS | - | ↑ (p = 0.004) (compared to HCs) CD: 19.7 ± 6.5 mgHC: 14.3 ± 6.5 mg | - |
| Larretxi et al., 2018 [39] | Long term 6.43 ± 4.18 y | ↓ (p < 0.001) (compared to GCD) GF: 555 ± 224 μg GC: 602 ± 231 μg | ↓ (p < 0.001) (compared to GCD) GF: 3.3 ± 3.5 μgGC: 3.8 ± 3.3 μg | ↑ (p < 0.001) (compared to GCD) GF: 10.4 ± 3.9 mgGC: 9.6 ± 3.4 mg | - |
| Sila et al., 2020 [44] | Long term 34.1 ± 25.4 y | - | ↑ (p < 0.001) (compared to HCs) CD: 2.2 ± 2.4 μgHC: 1.1 ± 1.7 μg | - | - |
| Szaflarska-Popławska et al., 2022 [45] | Long term 5.02 ± 3.87 y | - | NS | - | - |
| Kozioł-Kozakowska et al., 2021 [38] | Diagnosis (T0) Short term 6 months (T1) Long term 12 months (T2) | NS | ↓Before diagnosis ↑ (p < 0.01) After 6mo and 12 months T0: 1.72 ± 0.92 μg 11.47 ± 6.19% RDA T1: 2.15 ± 1.74 μg 14.30 ± 11.58% RDA T2: 3.59 ± 8.09 μg 23.90 ± 53.91% RDA | ↓ Before diagnosis 6.15 ± 2.98 μg 87.01 ± 41.68% RDA NS after 6mo and 12 months | ↓ Before diagnosis 4.88 ± 7.59 μg 8.43 ± 13.50% RDA NS after 6mo and 12 months |
| Mager et al., 2011 [41] | Diagnosis (T0) Long term 12 months (T2) | NS | ↓ (p < 0.05) (compared to time of diagnosis) T0:4.7 ± 4.3 μg 31.1 ± 28.8% RDA T2: 3.2 ± 2.3 μg 21.1 ± 15.1% RDA | - | NS after 12 months (compared to time of diagnosis) |
3.4.2. Water Soluble Vitamins
3.4.3. Essential Minerals
| Study | Duration of GFD | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Vitamin B1 | Vitamin B2 | Vitamin B3 | Vitamin B6 | Vitamin B12 | Folate | Vitamin C | ||
| Allowaymi et al., 2022 [33] | Short term ≥6 months | ↓ (compared to DRI) G: 0.53 mg (p =0.000) B: 0.69 mg (p =0.022) DRI:0.9 mg | ↓ for girls (compared to DRI) NS for boys G: 0.72 mg (p = 0.004) B: 0.94 mg (p = 0.683) DRI: 0.9 mg | ↓ for girls (compared to DRI) NS for boys G: 7.97 mg (p = 0.000) B: 9.62 mg (p = 0.062) DRI: 12 mg | ↓ (compared to DRI) G: 0.54 mg (p = 0.000) B: 0.66 mg (p = 0.000) DRI: 1.0 mg | NS for boys | ↓ (compared to DRI) G:146.52 μg (p = 0.000) B:142.09 μg (p = 0.000) DRI: 300 μg | ↓ for girls (compared to DRI) NS for boys G: 32.24 mg (p = 0.003) B: 36.18 mg (p = 0.104) DRI: 45 mg |
| Zuccotti et al., 2012 [46] | Short term ≥6 months | - | - | - | NS | NS | NS | - |
| Nestares et al., 2020 [42] | Short term >6 months | - | - | - | - | NS | NS | - |
| Ballestero- Fernández et al., 2019 [22] | Long term ≥1 y | ↓ (p < 0.05) (compared to HC) CD: 110.0% of recommendations HC: 133.3% of recommendations | ↓ (p < 0.05) (compared to HC) CD: 107.1% of recommendations HC: 121.4% of recommendations | ↓ (p < 0.05) (compared to HC) CD: 177.4% of recommendations HC: 231.4% of recommendations | ↓ (p < 0.05) (compared to HC) CD: 118.5% of recommendations HC: 141.9% of recommendations | NS | ↓ (p < 0.05) (compared to HC) CD: 67.45% of recommendations HC: 82.0% of recommendations | NS |
| Balamtekin et al., 2015 [34] | Long term 4.0 ± 3.3y | ↓ (p < 0.001)(compared to HC) CD: 0.6 ± 0.1 mgHC: 0.8 ± 0.1 mg | NS | - | NS | - | NS | NS |
| Larretxi et al., 2018 [39] | Long term 6.43 ± 4.18 y | ↓ (p < 0.001) (compared to GCD) GF: 1.3 ± 0.4 mgGC: 1.4 ± 0.5 mg | ↓ (p < 0.001) (compared to GCD) GF: 1.6 ± 0.4 mgGC: 1.8 ± 0.5 mg | ↓ (p < 0.001) (compared to GCD) GF: 21.2 ± 5.6 mgGC: 23.7 ± 6.2 mg | ↓ (p < 0.001) (compared to GCD) GF: 1.6 ± 0.4 mgGC: 1.9 ± 0.5 mg | ↓ (p < 0.001) (compared to GCD) GF: 5.9 ± 2.9 μgGC: 6.8 ± 3.7 μg | ↓ (p < 0.001) (compared to GCD) GF: 186 ± 76 μgGC: 233 ± 83 μg | NS |
| Sila et al., 2020 [44] | Long term 34.1 ± 25.4 y | NS | - | - | - | - | - | ↑ (p = 0.004)(compared to HC) CD: 91.9 ± 51.8 mgHC: 74.1 ± 49.8 mg |
| Szaflarska-Popławska et al., 2022 [45] | Long term 5.02 ± 3.87 y | ↓ (p < 0.001) (compared to HC) CD:0.6 ± 0.1 mgHC:0.8 ± 0.1 mg | NS | NS | NS | NS | NS | NS |
| Forchielli et al., 2020 [37] | Diagnosis (T0) Short term 6 months (T1) Long term 12 months (T2) | - | - | - | - | - | ↓ (p < 0.001) (after 12 months) T0: 155.5 ± 66.6 μg T2: 118.4 ± 63.5 μg | - |
| Kozioł-Kozakowska et al., 2021 [36] | Diagnosis (T0) Short term 6 months (T1) Long term 12 months (T2) | NS | NS | NS | NS | NS | ↓ Before diagnosis 160.96 ± 60.03 μg 58.20 ± 24.26% RDA NS after 6 months and 12 months | ↑ (p <0.01) After 6 and 12 months Before diagnosis: 63.92 ± 36.48 mg 123.80 ± 75.58% RDA T1: 81.70 ± 53.98 mg 156.77 ± 102.89% RDA T2: 93.21 ± 55.10 mg 179.73 ± 122.25% RDA |
| Study | Duration of GFD | Results | |||||
|---|---|---|---|---|---|---|---|
| Calcium | Iron | Magnesium | Phosphorus | Sodium | Potassium | ||
| Allowaymi et al., 2022 [33] | Short term ≥6 months | ↓ (compared to DRI) G: 496.58 mg (p < 0.001) B: 531.45 mg (p < 0.001) DRI: 1300 mg | ↓for girls (compared to DRI) No significant difference for boys G: 6.22 mg (p < 0.001) B: 7.68 mg (p = 0.666) DRI: 8 mg | ↓ (compared to DRI) G: 94.64 mg (p < 0.001) B: 97.85 mg (p < 0.001) DRI: 240 mg | ↓ (compared to DRI) G: 458.84 mg (p < 0.001) B: 494.23 mg (p < 0.001) DRI: 1250 mg | NS | ↓ (compared to DRI) G: 1032.87 mg (p < 0.001) B: 1175.18 mg (p < 0.001) DRI: 2500 mg |
| Zuccotti et al., 2012 [46] | Short term ≥6 months | NS | NS | NS | NS | NS | NS |
| Nestares et al., 2020 [42] | Short term >6 months | NS | ↓ (p = 0.006) (compared to HC) CD: 7.61 ± 0.49 mg HC: 10.1 ± 0.58 mg | ↓ (p < 0.001) (compared to HC) CD: 165.6 ± 9.23 mg HC: 206.9 ± 10.9 mg | - | - | - |
| Ballestero- Fernández et al., 2019 [22] | Long term ≥1 y | ↓ (p < 0.05) CD: 64.1% of recommendations HC: 74.7% of recommendations | ↓ (p < 0.05) CD: 76.0% of recommendations HC: 103.1% of recommendations | ↓ (p < 0.05) CD: 74.4% of recommendations HC: 82.8% of recommendations | NS | - | - |
| Balamtekin et al., 2015 [34] | Long term 4.0 ± 3.3y | NS | ↓ (p < 0.001)(compared to HCs) CD: 6.9 ± 2.6 mgHC: 11.2 ± 3.2 mg | ↓ (p = 0.01)(compared to HCs) CD: 200.5 ± 68.3 mgHC: 247.6 ± 65.3 mg | ↓ (p = 0.005)(compared to HCs) CD: 899.6 ± 247.7 mgHC: 1088.8 ± 223.8 mg | ↓ (p < 0.001)(compared to HCs) CD:972.7 ± 363.3 mgHC:1655.5 ± 822.8 mg | NS |
| Forchielli et al., 2015 [36] | Long term 6.2 ± 4.1 y | ↓ (p ≤ 0.001) (compared to LARN) CD:595.4 ± 305 mg LARN:1091.1 ± 295.3 mg | ↓ (p < 0.0001)(compared to LARN) CD:6.9 ± 2.6 mgLARN:11.2 ± 3.2 mg | - | - | ↑ (p = 0.002) (compared to LARN) CD:1529.3 ± 1317.8 mgLARN:1173.6 ± 353.7 mg | - |
| Larretxi et al., 2018 [39] | Long term 6.43 ± 4.18 y | ↑ (p = 0.007) (compared to GCD) GF: 900 ± 217 mgGC: 887 ± 308 mg | ↓ (p < 0.001) (compared to GCD) GF: 12.2 ± 3.3 mgGC: 13.3 ± 3.4 mg | ↓ (p < 0.001) (compared to GCD) GF: 242 ± 64 mgGC: 279 ± 69 mg | NS | ↓ (p < 0.001) (compared to GCD) GF: 1819 ± 529 mgGC: 1932 ± 533 mg | ↑ (p = 0.001) (compared to GCD) GF: 2788 ± 585 mgGC: 2747 ± 555 mg |
| Sila et al., 2020 [44] | Long term 34.1 ± 25.4 y | ↑ (p < 0.001) (compared to HCs) CD: 713.3 ± 261.2 mgHCs: 579.9 ± 242.1 mg | NS | - | - | - | - |
| Szaflarska-Popławska et al., 2022 [45] | Long term 5.02 ± 3.87 y | NS | NS | NS | - | - | - |
| Rujner et al. [43] | Long term 2.7–17.3 y | - | - | NS | - | - | - |
| Mager et al., 2011 [41] | Diagnosis (T0) Long term 12 months (T2) | ↓ (p < 0.05) after 12 months (compared to time of diagnosis) T0: 994 ± 697 mg 88.8 ± 59.9% RDA T2: 839 ± 349 mg 70.8 ± 31.7% RDA | - | NS | NS | ↓ (p < 0.05) (after 12 months) (compared to time of diagnosis) T0: 2752 ± 1396 mg 160.4 ± 53.8% RDA T2: 2104 ± 600 mg 123.8 ± 68.3% RDA | - |
| Kozioł-Kozakowska et al., 2021 [36] | Diagnosis (T0) Short term 6 months (T1) Long-term 12 months (T2) | ↓Before diagnosis 537.13 ± 233.32 mg 50.56 ± 23.60% RDA No significant difference after 6 months and 12 months | ↓Before diagnosis 8.40 ± 3.01 mg 79.29 ± 38.82% RDA No significant difference after 6 months and 12 months | NS | NS | NS | ↑ (p <0.03) After 6 months and 12 months T0: 2055.26 ± 687.85 mg 119.68 ± 6.19% RDA T1: 2901.67 ± 685.63 mg 128.51 ± 56.75% RDA T2: 2335.84 ± 568.94 mg 137.88 ± 60.32% RDA |
3.4.4. Trace Minerals
| Study | Duration of GFD | Results | |||||
|---|---|---|---|---|---|---|---|
| Zinc | Selenium | Iodine | Chlorine | Copper | Chromium | ||
| Allowaymi et al., 2022 [33] | Short term ≥6 months | ↓ (compared to DRI) G: 3.15 mg (p = 0.000) B: 3.37 mg (p = 0.000) DRI: 8 mg | - | - | - | - | - |
| Zuccotti et al., 2012 [46] | Short term ≥6 months | NS | - | - | - | - | - |
| Nestares et al., 2020 [42] | Short term >6 months | NS | ↓ (p < 0.001) (compared to HC) CD: 45.5 ± 3.5 μg HC: 68.9 ± 4.2 μg | NS | - | - | - |
| Ballestero- Fernández et al., 2019 [22] | Long term ≥1 y | NS | ↓ (p < 0.05) CD: 159.9% of recommendations HC: 268.3% of recommendations | NS | NS | - | - |
| Balamtekin et al., 2015 [34] | Long term 4.0 ± 3.3 y | ↓ (p < 0.001)(compared to HC) CD: 5.9 ± 1.8 mgHC: 9.2 ± 2.1 mg | - | - | - | - | - |
| Larretxi et al., 2018 [39] | Long term 6.43 ± 4.18 y | ↑ (p < 0.001) (compared to gluten-containing diet) GF: 15.1 ± 13.3 mgGC: 8.7 ± 2.6 mg | ↓ (p < 0.001) (compared to gluten-containing diet) GF: 36.9 ± 17.6 μgGC: 52.0 ± 20.2 μg | ↓ (p < 0.001) (compared to gluten-containing diet) GF: 63.0 ± 28.6 μgGC: 75.3 ± 32.1 μg | ↓ (p < 0.001) (compared to gluten-containing diet) GF: 697 ± 322 mgGC: 1118 ± 518 mg | ↓ (p < 0.001) (compared to gluten-containing diet) GF: 0.9 ± 0.4 mgGC: 1.2 ± 0.5 mg | - |
| Sila et al., 2020 [44] | Long term 34.1 ± 25.4 y | NS | - | - | - | - | - |
| Szaflarska-Popławska et al., 2022 [45] | Long term 5.02 ± 3.87 y | NS | - | - | - | - | - |
| Kozioł-Kozakowska et al., 2021 [36] | Diagnosis (T0) Short term 6 months (T1) Long term 12 months (T2) | ↑ (p <0.01) After 6mo and 12 months T0: 6.5 ± 0.92 mg 114.08 ± 44.97% RDA T1: 6.86 ± 1.75 mg 119.27 ± 35.84% RDA T2: 7.48 ± 1.57 mg 129.51 ± 39.58% RDA | ↓ Before diagnosis 6.04 ± 7.62 μg 18.86 ± 25.26% RDA NS after 6 months and 12 months | ↓ Before diagnosis ↑ (p <0.01) After 6 months and 12 months T0: 13.57 ± 6.74 μg 13.23 ± 7.68% RDA T1: 18.08 ± 8.20 μg 17.38 ± 11.58% RDA T2: 16.53 ± 6.84 μg 15.79 ± 6.90% RDA | - | NS | - |
3.5. Associations of the GFD with Anthropometric Characteristics
3.6. Associations of a GFD with Body Composition and Bone Mineral Density
3.7. Associations of a GFD with Food Habits
4. Discussion
4.1. Summary of the Primary and Secondary Outcomes
4.2. Literature Documentation
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Gastroenterology Organisation (WGO). Available online: https://www.worldgastroenterology.org/guidelines/celiac-disease (accessed on 1 January 2024).
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2019, 70, 1. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [PubMed]
- Pham-Short, A.; Donaghue, K.C.; Ambler, G.; Phelan, H.; Twigg, S.; Craig, M.E. Screening for Celiac Disease in Type 1 Diabetes: A Systematic Review. Pediatrics 2015, 136, e170–e176. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, A.; Capriati, T.; Bizzarri, C.; Ferretti, F.; Ancinelli, M.; Romano, F.; Perilli, A.; Laureti, F.; Locatelli, M. Autoimmune Diseases and Celiac Disease Which Came First: Genotype or Gluten? Expert Rev. Clin. Immunol. 2015, 12, 67–77. [Google Scholar] [CrossRef]
- Du, Y.; Shan, L.-F.; Cao, Z.-Z.; Feng, J.-C.; Cheng, Y. Prevalence of Celiac Disease in Patients with Down Syndrome: A Meta-Analysis. Oncotarget 2017, 9, 5387–5396. [Google Scholar] [CrossRef]
- Poddighe, D.; Romano, M.; Dossybayeva, K.; Abdukhakimova, D.; Galiyeva, D.; Demirkaya, E. Celiac Disease in Juvenile Idiopathic Arthritis and Other Pediatric Rheumatic Disorders. J. Clin. Med. 2022, 11, 1089. [Google Scholar] [CrossRef]
- Sahin, Y. Celiac Disease in Children: A Review of the Literature. World J. Clin. Pediatr. 2021, 10, 53–71. [Google Scholar] [CrossRef]
- D’heedene, M.; Vanuytsel, T.; Wauters, L. Celiac Disease: Hope for New Treatments beyond a Gluten-Free Diet. Clin. Nutr. 2024, 43, 1240–1249. [Google Scholar] [CrossRef]
- Francavilla, R.; Cristofori, F.; Stella, M.; Borrelli, G.; Naspi, G.; Castellaneta, S. Treatment of Celiac Disease: From Gluten-Free Diet to Novel Therapies. Minerva Pediatr. 2014, 66, 501–516. [Google Scholar]
- Elli, L.; Ferretti, F.; Orlando, S.; Vecchi, M.; Monguzzi, E.; Roncoroni, L.; Schuppan, D. Management of Celiac Disease in Daily Clinical Practice. Eur. J. Intern. Med. 2019, 61, 15–24. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac Disease: Understanding the Gluten-Free Diet. Eur. J. Nutr. 2016, 56, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Calado, J.; Verdelho Machado, M. Celiac Disease Revisited. GE-Port. J. Gastroenterol. 2021, 29, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Andrewski, E.; Cheng, K.; Vanderpool, C. Nutritional Deficiencies in Vegetarian, Gluten-Free, and Ketogenic Diets. Pediatr. Rev. 2022, 43, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Bathrellou, E.; Kontogianni, M.D.; Panagiotakos, D.B. Celiac Disease and Non-Celiac Gluten or Wheat Sensitivity and Health in Later Life: A Review. Maturitas 2018, 112, 29–33. [Google Scholar] [CrossRef]
- Wahab, P.J.; Meijer, J.W.R.; Mulder, C.J.J. Histologic Follow-up of People With Celiac Disease on a Gluten-Free Diet. Am. J. Clin. Pathol. 2002, 118, 459–463. [Google Scholar] [CrossRef]
- Nardo, G.D.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Raucci, U.; Parisi, P. Nutritional Deficiencies in Children with Celiac Disease Resulting from a Gluten-Free Diet: A Systematic Review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef]
- Macedo, L.; Catarino, M.; Festas, C.; Alves, P. Vulnerability in Children with Celiac Disease: Findings from a Scoping Review. Children 2024, 11, 729. [Google Scholar] [CrossRef]
- Sue, A.; Dehlsen, K.; Ooi, C.Y. Paediatric Patients with Coeliac Disease on a Gluten-Free Diet: Nutritional Adequacy and Macro- and Micronutrient Imbalances. Curr. Gastroenterol. Rep. 2018, 20, 2. [Google Scholar] [CrossRef]
- Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients 2021, 13, 2877. [Google Scholar] [CrossRef]
- Alzaben, A.S.; Turner, J.; Shirton, L.; Samuel, T.M.; Persad, R.; Mager, D. Assessing Nutritional Quality and Adherence to the Gluten-Free Diet in Children and Adolescents with Celiac Disease. Can. J. Diet. Pract. Res. 2015, 76, 56–63. [Google Scholar] [CrossRef]
- Ballestero Fernández, C.; Varela-Moreiras, G.; Úbeda, N.; Alonso-Aperte, E. Nutritional Status in Spanish Children and Adolescents with Celiac Disease on a Gluten Free Diet Compared to Non-Celiac Disease Controls. Nutrients 2019, 11, 2329. [Google Scholar] [CrossRef]
- Elliott, C. The Nutritional Quality of Gluten-Free Products for Children. Pediatrics 2018, 142, e20180525. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Agostoni, C. Nutritional Aspects of Gluten-Free Products. J. Sci. Food Agric. 2015, 95, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Mehtab, W.; Agarwal, S.; Agarwal, H.; Ahmed, A.; Agarwal, A.; Prasad, S.; Chauhan, A.; Bhola, A.; Singh, N.; Ahuja, V.; et al. Gluten-Free Foods Are Expensive and Nutritionally Imbalanced than Their Gluten-Containing Counterparts. Indian J. Gastroenterol. 2024, 43, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Bathrellou, E.; Kontogianni, M.D. Nutritional Value of Cereal-Based Gluten-Free Products and Comparison to That of Gluten Containing Counterparts in the Greek Market. Int. J. Food Sci. Nutr. 2024, 75, 664–672. [Google Scholar] [CrossRef]
- Ehteshami, M.; Shakerhosseini, R.; Sedaghat, F.; Hedayati, M.; Eini-Zinab, H.; Hekmatdoost, A. The Effect of Gluten Free Diet on Components of Metabolic Syndrome: A Randomized Clinical Trial. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 2979–2984. [Google Scholar] [CrossRef]
- Diamanti, A.; Capriati, T.; Basso, M.; Panetta, F.; Di Ciommo Laurora, V.; Bellucci, F.; Cristofori, F.; Francavilla, R. Celiac Disease and Overweight in Children: An Update. Nutrients 2014, 6, 207–220. [Google Scholar] [CrossRef]
- De Giuseppe, R.; Bergomas, F.; Loperfido, F.; Giampieri, F.; Preatoni, G.; Calcaterra, V.; Cena, H. Could Celiac Disease and Overweight/Obesity Coexist in School-Aged Children and Adolescents? A Systematic Review. Child. Obes. 2023, 20, 35. [Google Scholar] [CrossRef]
- Barone, M.; Iannone, A.; Cristofori, F.; Dargenio, V.N.; Indrio, F.; Verduci, E.; Di Leo, A.; Francavilla, R. Risk of Obesity during a Gluten-Free Diet in Pediatric and Adult Patients with Celiac Disease: A Systematic Review with Meta-Analysis. Nutr. Rev. 2022, 81, 252–266. [Google Scholar] [CrossRef]
- PRISMA. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Website. Available online: https://www.prisma-statement.org/ (accessed on 11 November 2024).
- Higgins, J.P.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Lemeris, C.; Akl, E.A.; Bateson, T.F.; Berkman, N.D.; et al. A Tool to Assess Risk of Bias in Non-Randomized Follow-up Studies of Exposure Effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef]
- Allowaymi, S.S.; Binobead, M.A.; Alshammari, G.M.; Alrasheed, A.; Mohammed, M.A.; Yahya, M.A. Nutritional Status of Saudi Children with Celiac Disease Following the Ministry of Health’s Gluten-Free Diet Program. Nutrients 2022, 14, 2792. [Google Scholar] [CrossRef] [PubMed]
- Balamtekin, N.; Aksoy, Ç.; Baysoy, G.; Uslu, N.; Demir, H.; Köksal, G.; Saltık-Temizel, İ.N.; Özen, H.; Gürakan, F.; Yüce, A. Is Compliance with Gluten-Free Diet Sufficient? Diet Composition of Celiac Patients. Turk. J. Pediatr. 2015, 57, 374–379. [Google Scholar] [PubMed]
- Ferrara, P.; Cicala, M.; Tiberi, E.; Spadaccio, C.; Marcella, L.; Gatto, A.; Calzolari, P.; Castellucci, G. High Fat Consumption in Children with Celiac Disease. Acta Gastro-Enterol. Belg. 2009, 72, 296–300. [Google Scholar]
- Forchielli, M.L.; Fernicola, P.; Diani, L.; Scrivo, B.; Salfi, N.C.; Pessina, A.C.; Lima, M.; Conti, V.; Pession, A. Gluten-Free Diet and Lipid Profile in Children With Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Forchielli, M.L.; Diani, L.; Labriola, F.; Bolasco, G.; Rocca, A.; Salfi, N.C.; Leone, A.; Miserocchi, C.; Andreozzi, L.; Levi della Vida, F.; et al. Gluten Deprivation: What Nutritional Changes Are Found During the First Year in Newly Diagnosed Coeliac Children? Nutrients 2019, 12, 60. [Google Scholar] [CrossRef]
- Kozioł-Kozakowska, A.; Salamon, D.; Grzenda-Adamek, Z.; Krawczyk, A.; Duplaga, M.; Gosiewski, T.; Kowalska-Duplaga, K. Changes in Diet and Anthropometric Parameters in Children and Adolescents with Celiac Disease—One Year of Follow-Up. Nutrients 2021, 13, 4306. [Google Scholar] [CrossRef]
- Larretxi, I.; Simon, E.; Benjumea, L.; Miranda, J.; Bustamante, M.A.; Lasa, A.; Eizaguirre, F.J.; Churruca, I. Gluten-Free-Rendered Products Contribute to Imbalanced Diets in Children and Adolescents with Celiac Disease. Eur. J. Nutr. 2018, 58, 775–783. [Google Scholar] [CrossRef]
- Lionetti, E.; Antonucci, N.; Marinelli, M.; Bartolomei, B.; Franceschini, E.; Gatti, S.; Catassi, G.N.; Verma, A.K.; Monachesi, C.; Catassi, C. Nutritional Status, Dietary Intake, and Adherence to the Mediterranean Diet of Children with Celiac Disease on a Gluten-Free Diet: A Case-Control Prospective Study. Nutrients 2020, 12, 143. [Google Scholar] [CrossRef]
- Mager, D.R.; Qiao, J.; Turner, J. Vitamin D and K Status Influences Bone Mineral Density and Bone Accrual in Children and Adolescents with Celiac Disease. Eur. J. Clin. Nutr. 2011, 66, 488–495. [Google Scholar] [CrossRef]
- Nestares, T.; Martín-Masot, R.; Labella, A.; Aparicio, V.A.; Flor-Alemany, M.; López-Frías, M.; Maldonado, J. Is a Gluten-Free Diet Enough to Maintain Correct Micronutrients Status in Young Patients with Celiac Disease? Nutrients 2020, 12, 844. [Google Scholar] [CrossRef]
- Rujner, J.; Socha, J.; Syczewska, M.; Wojtasik, A.; Kunachowicz, H.; Stolarczyk, A. Magnesium Status in Children and Adolescents with Coeliac Disease without Malabsorption Symptoms. Clin. Nutr. 2004, 23, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Sila, S.; Mašić, M.; Kranjčec, D.; Niseteo, T.; Marić, L.; Radunić, A.; Hojsak, I.; Jadrešin, O.; Mišak, Z. Quality of Diet of Patients with Coeliac Disease in Comparison to Healthy Children. Children 2022, 9, 1595. [Google Scholar] [CrossRef] [PubMed]
- Szaflarska-Popławska, A.; Dolińska, A.; Kuśmierek, M. Nutritional Imbalances in Polish Children with Coeliac Disease on a Strict Gluten-Free Diet. Nutrients 2022, 14, 3969. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.; Fabiano, V.; Dilillo, D.; Picca, M.; Cravidi, C.; Brambilla, P. Intakes of Nutrients in Italian Children with Celiac Disease and the Role of Commercially Available Gluten-Free Products. J. Hum. Nutr. Diet. 2012, 26, 436–444. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Tian, D.; Zhou, J.; Dong, S. Relationship between Vitamin D Levels and Pediatric Celiac Disease: A Systematic Review and Meta-Analysis. BMC Pediatr. 2024, 24, 185. [Google Scholar] [CrossRef]
- Dehghani, S.M.; Ilkhanipour, H.; Samipour, L.; Niknam, R.; Shahramian, I.; Parooie, F.; Salarzaei, M.; Tahani, M. Investigation of the Factors Affecting Bone Mineral Density in Children with Celiac Disease. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 138. [Google Scholar] [CrossRef]
- Bianchi, P.I.; Aronico, N.; Santacroce, G.; Broglio, G.; Lenti, M.V.; Di Sabatino, A. Nutritional Consequences of Celiac Disease and Gluten-Free Diet. Gastroenterol. Insights 2024, 15, 878–894. [Google Scholar] [CrossRef]
- García-Manzanares, Á.; Lucendo, A.J. Review: Nutritional and Dietary Aspects of Celiac Disease. Nutr. Clin. Pract. 2011, 26, 163–173. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.; et al. Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten Free Diet and Nutrient Deficiencies: A Review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.-J.; Wang, Y. Use of Percentiles and Z-Scores in Anthropometry. In Handbook of Anthropometry; Preedy, V., Ed.; Springer: New York, NY, USA, 2012; pp. 29–48. [Google Scholar] [CrossRef]
- Delavar, M.; Karimian, P.; Ebrahimi, H.; Jafarnejad, S. Effects of Vitamin D on Bone Density in Healthy Children: A Systematic Review. J. Fam. Med. Prim. Care 2022, 11, 870. [Google Scholar] [CrossRef]
- Mosca, C.; Thorsteinsdottir, F.; Abrahamsen, B.; Rumessen, J.J.; Händel, M.N. Newly Diagnosed Celiac Disease and Bone Health in Young Adults: A Systematic Literature Review. Calcif. Tissue Int. 2022, 110, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, M.V.; Bentley, J.L.; Higgins, S.; Kindler, J.M.; Esco, M.R.; MacDonald, H.V. Celiac Disease and Bone Health in Children and Adolescents: A Systematic Review and Meta-Analysis. J. Clin. Densitom. 2020, 23, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Dourado, D.; Cristina, D.; Kawano, M.M.; Tianeze, C.; Pereira, M. Effect of a Gluten-Free Diet on Bone Mineral Density in Children and Adolescents with Celiac Disease: Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2022, 64, 5192–5202. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M.; Leffler, D.A. Nutritional Consequences of Celiac Disease and the Gluten-Free Diet. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 123–129. [Google Scholar] [CrossRef]
- Ghunaim, M.; Seedi, A.; Alnuman, D.; Aljohani, S.; Aljuhani, N.; Almourai, M.; Alsuhaymi, S. Impact of a Gluten-Free Diet in Adults With Celiac Disease: Nutritional Deficiencies and Challenges. Cureus 2024, 16, 74983. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.D.; Ahuja, V.; Makharia, G.K. Who to Screen and How to Screen for Celiac Disease. World J. Gastroenterol. 2022, 28, 4493–4507. [Google Scholar] [CrossRef]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional Differences Between a Gluten-Free Diet and a Diet Containing Equivalent Products with Gluten. Plant Foods Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Crespo-Escobar, P.; Martínez-Barona, S.; Fornés-Ferrer, V.; Donat, E.; Ribes-Koninckx, C. Differences in the Macronutrient and Dietary Fibre Profile of Gluten-Free Products as Compared to Their Gluten-Containing Counterparts. Eur. J. Clin. Nutr. 2019, 73, 930–936. [Google Scholar] [CrossRef]
- Newberry, C.; McKnight, L.; Sarav, M.; Pickett-Blakely, O. Going Gluten Free: The History and Nutritional Implications of Today’s Most Popular Diet. Curr. Gastroenterol. Rep. 2017, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Bizzarri, B.; Gaiani, F.; Semeraro, F.; Iuliano, S.; Di Mario, F.; Nouvenne, A.; Kayali, S. De ’Angelis Gl Growth Changes after Gluteen Free Diet in Pediatric Celiac Patients: A Literature-Review. PubMed 2018, 89, 5–10. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Ni, Y.; Yi, C.; Fang, Y.; Ning, Q.; Shen, B.; Zhang, K.; Liu, Y.; Yang, L.; et al. Global Prevalence of Overweight and Obesity in Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2024, 178, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Mędza, A.; Szlagatys-Sidorkiewicz, A. Nutritional Status and Metabolism in Celiac Disease: Narrative Review. J. Clin. Med. 2023, 12, 5107. [Google Scholar] [CrossRef]
- Capristo, E.; Mingrone, G.; Addolorato, G.; Greco, A.V.; Corazza, G.R.; Gasbarrini, G. Differences in Metabolic Variables between Adult Coeliac Patients at Diagnosis and Patients on a Gluten-Free Diet. Scand. J. Gastroenterol. 1997, 32, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Capristo, E.; Addolorato, G.; Mingrone, G.; De Gaetano, A.; Greco, A.V.; Tataranni, P.A.; Gasbarrini, G. Changes in Body Composition, Substrate Oxidation, and Resting Metabolic Rate in Adult Celiac Disease Patients after a 1-y Gluten-Free Diet Treatment. Am. J. Clin. Nutr. 2000, 72, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Meazza, C.; Pagani, S.; Gertosio, C.; Bozzola, E.; Bozzola, M. Celiac Disease and Short Stature in Children. Expert Rev. Endocrinol. Metab. 2014, 9, 535–542. [Google Scholar] [CrossRef]
- Almahmoud, E.; Alkazemi, D.U.Z.; Al-Qabandi, W. Growth Stunting and Nutritional Deficiencies among Children and Adolescents with Celiac Disease in Kuwait: A Case–Control Study. Children 2024, 11, 1042. [Google Scholar] [CrossRef]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and Vitamin D: Skeletal and Extraskeletal Health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef]
- Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, Calcium Homeostasis and Aging. Bone Res. 2016, 4, 16041. [Google Scholar] [CrossRef]
- Deora, V.; Aylward, N.; Sokoro, A.; El-Matary, W. Serum Vitamins and Minerals at Diagnosis and Follow-up in Children With Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, J.M.; Heynen, L.; Vreugdenhil, A.C.E. Nutrient Deficiencies in Children with Celiac Disease during Long Term Follow-Up. Clin. Nutr. 2023, 42, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- McGrogan, L.; Mackinder, M.; Stefanowicz, F.; Aroutiounova, M.; Catchpole, A.; Wadsworth, J.; Buchanan, E.; Cardigan, T.; Duncan, H.; Hansen, R.; et al. Micronutrient Deficiencies in Children with Coeliac Disease; a Double-Edged Sword of Both Untreated Disease and Treatment with Gluten-Free Diet. Clin. Nutr. 2021, 40, 2784–2790. [Google Scholar] [CrossRef]
- Moon, R.J.; Davies, J.H.; Cooper, C.; Harvey, N.C. Vitamin D, and Maternal and Child Health. Calcif. Tissue Int. 2020, 106, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Posterick, A.; Ayars, C.L. Celiac Disease Dietary Adherence on the Rural–Urban Continuum. Nutrients 2023, 15, 4535. [Google Scholar] [CrossRef]
- Elsahoryi, N.A.; Ibrahim, M.O.; Alhaj, O.A. Adherence to the Gluten-Free Diet Role as a Mediating and Moderating of the Relationship between Food Insecurity and Health-Related Quality of Life in Adults with Celiac Disease: Cross-Sectional Study. Nutrients 2024, 16, 2229. [Google Scholar] [CrossRef]
- Khalifeh, F.; Riasatian, M.S.; Ekramzadeh, M.; Honar, N.; Jalali, M. Assessing the Prevalence of Food Insecurity among Children with Celiac Disease: A Cross-Sectional Study. J. Food Secur. 2019, 7, 192–195. [Google Scholar]
- Meyer, S.; Monachesi, C.; Barchetti, M.; Lionetti, E.; Catassi, C. Cross-Cultural Participation in Food-Related Activities and Quality of Life among Children with Celiac Disease. Children 2023, 10, 1300. [Google Scholar] [CrossRef]
- Fernández Miaja, M.; Díaz Martín, J.J.; Jiménez Treviño, S.; Suárez González, M.; Bousoño García, C. Study of Adherence to the Gluten-Free Diet in Coeliac Patients. An. Pediatría 2021, 94, 377–384. [Google Scholar] [CrossRef]
- White, L.E.; Bannerman, E.; Gillett, P.M. Coeliac Disease and the Gluten-Free Diet: A Review of the Burdens; Factors Associated with Adherence and Impact on Health-Related Quality of Life, with Specific Focus on Adolescence. J. Hum. Nutr. Diet. 2016, 29, 593–606. [Google Scholar] [CrossRef]
- Al-Majali, M.A.; Burayzat, S.; Tayyem, R.F. Dietary Mangement of Type 1 Diabetes Mellitus with Celiac Disease. Curr. Diabetes Rev. 2022, 18, 111–119. [Google Scholar] [CrossRef]
- Mearin, M.L.; Agardh, D.; Antunes, H.; Al-toma, A.; Auricchio, R.; Castillejo, G.; Catassi, C.; Ciacci, C.; Discepolo, V.; Dolinsek, J.; et al. ESPGHAN Position Paper on Management and Follow-up of Children and Adolescents With Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Hill, I.D.; Fasano, A.; Guandalini, S.; Hoffenberg, E.; Levy, J.; Reilly, N.; Verma, R. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-Related Disorders. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Whitton, C.; Ramos-García, C.O.; Kirkpatrick, S.I.; Healy, J.D.; Dhaliwal, S.S.; Boushey, C.J.; Collins, C.; Rollo, M.E.; Kerr, D.A. A Systematic Review Examining Contributors to Misestimation of Food and Beverage Intake Based on Short-Term Self-Report Dietary Assessment Instruments Administered to Adults. Adv. Nutr. 2022, 13, 2620–2665. [Google Scholar] [CrossRef] [PubMed]
- Hooson, J.; Hutchinson, J.; Warthon-Medina, M.; Hancock, N.; Greathead, K.; Knowles, B.; Vargas-Garcia, E.; Gibson, L.E.; Bush, L.A.; Margetts, B.; et al. A Systematic Review of Reviews Identifying UK Validated Dietary Assessment Tools for Inclusion on an Interactive Guided Website for Researchers: Www.Nutritools.Org. Crit. Rev. Food Sci. Nutr. 2019, 60, 1265–1289. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.L.; Ho, Y.Y.; Rollo, M.E.; Collins, C.E. Validity of Dietary Assessment Methods When Compared to the Method of Doubly Labeled Water: A Systematic Review in Adults. Front. Endocrinol. 2019, 10, 850. [Google Scholar] [CrossRef]
| Study (Author, Year) | Risk of Bias | |||||||
|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall | |
| Allowaymi et al., 2022 [33] | − | +++ | − | − | + | ++ | + | +++ |
| Balamtekin et al., 2015 [34] | + | ++ | − | − | − | ++ | − | ++ |
| Ballestero Fernández et al., 2019 [22] | + | − | − | − | − | − | − | + |
| Ferrara et al., 2009 [35] | +++ | +++ | ++ | + | − | ++ | ++ | +++ |
| Forchielli et al., 2015 [36] | − | − | − | − | − | − | − | − |
| Forchielli et al., 2019 [37] | − | − | − | − | − | − | − | − |
| Kozioł-Kozakowska et al., 2015 [38] | ++ | +++ | − | + | +++ | + | − | +++ |
| Larretxi et al., 2018 [39] | − | +++ | − | − | ++ | − | − | +++ |
| Lionnetti et al., 2020 [40] | + | + | − | − | + | − | − | + |
| Mager et al., 2011 [41] | + | − | + | + | ++ | − | − | ++ |
| Nestares et al., 2020 [42] | − | +++ | − | + | + | − | − | +++ |
| Rujner et al., 2003 [43] | + | − | − | + | ++ | − | − | ++ |
| Sila et al., 2022 [44] | ++ | +++ | − | ++ | +++ | + | − | +++ |
| Szaflarska-Popławska et al., 2022 [45] | + | + | − | − | +++ | + | − | +++ |
| Zuccotti et al., 2012 [46] | + | + | + | − | − | − | − | + |
| Study | Type of study | Population | Duration of GFD | Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Age a | Sex b | Energy | Protein | Carbohydrate | Fat | Fiber | |||
| Allowaymi et al., 2022 [33] | Cross-sectional | 66 (CD) | 10.4 ± 4.1 y (6–16 y) | F: 37 M: 29 | Short term ≥6 months | ↓ (p ≤ 0.001) (compared to DRI) G: 1296 kcal B: 1400 kcal DRI: 1800 kcal | ↑ (p ≤ 0.001) (compared to DRI) G: 42.94 g B: 47.37 g DRI: 34 g | ↑ (p ≤ 0.001) (compared to DRI) G: 172 g B: 192.8 g DRI: 130 g | NS | ↓ (p ≤ 0.001) (compared to DRI) G: 12.85 g B: 12.51 g DRI: 25 g |
| Nestares et al., 2020 [42] | Cross-sectional case–control study | 68 (CD) | 8.5 ± 4.1 y (7–18 y) | F: 52 M: 16 | Short term >6 months | NS | NS | NS | NS | NS |
| 43 (HC) | 10.3 ± 4.5 y | F: 20 M: 23 | ||||||||
| Zuccotti et al., 2012 [46] | Cross-sectional case–control study | 18 (CD) | 7.6 ± 2.8 y (4–10 y) | G: 13 B: 5 | Short term ≥6 months | ↑ (p < 0.001) (compared to HCs) CD: 2141.92 ± 680.44 kcal HC: 1376.91 ± 1009.6 kcal | ↑ (p = 0.012) (compared to HCs) CD: 68.3 ± 22.7 g HC: 55.7 ± 30.0 g | ↑ (p < 0.001) (compared to HCs) CD: 286.6 ± 88.6 g HC: 149.1 ± 62.1 g | NS | NS |
| 18 (HC) | 7.0 ± 2.3 y | F: 11 M: 7 | ||||||||
| Ballestero- Fernández et al., 2019 [22] | Cross-sectional age and gender-matched study | 70 (CD) | (4–18 y) | F: 35 M: 35 | Long term ≥1 year | - | ↓ (p < 0.05) (compared to HCs) CD: 15.5% TEI HCs: 16.5% TEI | NS | NS | NS |
| 67 (HC) | F: 26 M: 41 | |||||||||
| Balamtekin et al., 2015 [34] | Cross-sectional case–control study | 28 (CD) | 10.3 ± 4.6 y (3–18 y) | F: 22 M: 6 | Long term 4.0 ± 3.3 y | ↓ (p = 0.002) (compared to HCs) CD: 1582.7 ± 419.4 Kcal HC: 1921.8 ± 321.2 kcal | ↓ (p < 0.002) (compared to HCs) CD: 45.5 ± 12.9 g HC: 6.3 ± 12.5 g | ↓ (p = 0.002) (compared to HCs) CD: 190.0 ± 68.2 g HC: 244.8 ± 53.4 g | NS | ↓ (p = 0.001) (compared to HCs) CD: 13.8 ± 7.0 g HC: 20.1 ± 5.7 g |
| 25 (HC) | 9.5 ± 3.4 y | F: 18 M: 7 | ||||||||
| Ferrara et al., 2009 [35] | Retrospective | 50 (CD) | 10.68 y (6–16 y) | F: 32 M: 18 | Long term ≥1 year | NS | - | - | ↑ (p < 0.008) (compared to HCs) CD: 72.5 ± 37.2 g HC: 52.9 ± 35.4 g | - |
| 50 (HC) | 10.74 y (6–17 y) | F: 33 M: 17 | ||||||||
| Forchielli et al., 2015 [37] | Longitudinal study | 205 (CD) | 10.7 ± 4.2 y (1–18 y) | F: 132 M: 73 | Long term 6.2 ± 4.1 y | ↓ (p ≤ 0.0001) (compared to LARN) CD: 1761.6 ± 453.6 kcal LARN: 2054.5 ± 568.4 kcal | ↑ (p ≤ 0.0001) (compared to LARN) CD: 66.2 ± 23.8 g LARN: 29 ± 12 g | NS | NS | NS |
| Larretxi et al., 2018 [39] | Cross-sectional | 83 (CD) | 9.2 ± 3.8 y (3–18 y) | F: 53 M: 30 | Long term 6.43 ± 4.18 y | NS | ↓ (p ≤ 0.001) (compared to GCD) GF:79.8 ± 17.0 g GC:83.5 ± 17.2 g | ↓ (p ≤ 0.001) (compared to GCD) GF:219.0 ± 47.1 g GC:223.2 ± 42.2 g | ↑ (p ≤ 0.001) (compared to GCD) GF:86.2 ± 19.6 g GC: 83.2 ± 19.5 g | - |
| Lionnetti et al., 2020 [40] | Case–Control Prospective Study | 120 (CD) | 10.5 (4.4–15.5 y) | F: 72 M: 48 | Long term ≥2 years | NS | NS | ↓ (p = 0.001) (compared to HCs) CD:209.7 ± 25.6 g HC:260.5 ± 18.3 g | ↑ (p = 0.015) (compared to HCs) CD: 78.1 ± 7.3 g HC: 64.4 ± 5.2 g | ↓ (p = 0.015) (compared to HCs) CD: 12.6 ± 1.5 g HC: 15 ± 1.9 g |
| 100 (HC) | 10.1 (4.7–14.5 y) | F: 56 M: 44 | ||||||||
| Sila et al., 2022 [44] | Cross-sectional case–control study | 76 (CD) | 9.0 ± 4.3 y | F: 43 M: 33 | Long term 34.1 ± 25.4 y | ↑ (p < 0.001) (compared to HCs) CD: 1740.3 ± 482.2 kcal HC: 1454.8 ± 423.9 kcal | ↑ (p = 0.021) (compared to HCs) CD:67.0 ± 19.4 g HC:62.1 ± 17.1 g | - | ↑ (p < 0.001) (compared to HCs) CD: 70.0 ± 26.7 g HC: 52.4 ± 19.7 g | NS |
| 590 (HC) | 9.9 ± 0.1 y (6–14 y) | F: 317 M: 273 | ||||||||
| Szaflarska-Popławska et al., 2022 [45] | Single-center prospective cohort study | 48 (CD) | 11.8 ± 3.6 y | F: 33 M: 15 | Long term 5.02 ± 3.87 y | NS In line with the reference values | NS 190.3% DRI | NS 189.4% DRI | NS In line with the reference values | NS In line with the reference values |
| 50 (HC) | 10.2 ± 3.8 y | F: 26 M: 24 | ||||||||
| Rujner et al., 2004 [43] | Cross-sectional case–control study | 41 (CD) | 5.9–18.3 y | F: 29 M: 18 | Long term 2.7–17.3 y | ↓ (compared to HCs) 139.2% RDI (CD) 150.5% RDI (HCs) | ↓ (compared to HCs) 113% RDI (CD) 141.2% RDI (HCs) | - | ↑ (compared to HCcs) 163% RDI (CD) 153.8% RDI (HCs) | - |
| 8 (HC) | 6.4–17.3 y | F: 4 M: 4 | ||||||||
| Forchielli et al., 2019 [37] | Prospective | 79 (CD) | 7.9 ± 3.8 y (1–18 y) | F: 52 M: 27 | Diagnosis (T0) Short term 6 months (T1) Long term 12 months (T2) | ↓ at diagnosis ↓↓ after 12 months (p ≤ 0.001) (compared to recommendations) T0: 1786 ± 401.8 kcal T2: 1698.33 ± 377.46 kcal Recommendations: 1925.9 ± 504.1 kcal | ↑ diagnosis ↑ 12 months (p < 0.001) (compared to recommendations) T0: 65 ± 21.9 g T2: 62 ± 19.3 g Recommendations: 26.2 ± 10.5 g | NS | NS | ↑ 12 months (p = 0.046) (compared to time of diagnosis) T0: 12.2 ± 4 g T2: 13.1 ± 5.2 g |
| Mager et al., 2011 [41] | Prospective | 43 (CD) | 9.4 ± 4.2 y (3–17 y) | F: 30 M: 13 | Diagnosis (T0) Long term 12 months (T2) | ↓ (p < 0.05) (after 12 months) T0: 1813 ± 680 kcal T2: 1611 ± 318 kcal | ↓ (p < 0.05) (after 12 months) T0: 61.5 ± 27.2 g T2: 56.7 ± 10.8 g | - | - | - |
| Kozioł-Kozakowska et al., 2015 [38] | Prospective | 40 (CD) | 8.4 ± 3.5 y (7–17.5 y) | F: 28 M: 12 | Diagnosis (T0) Short term 6 months (T1) Long term 12 months (T2) | ↑ after 6 mo ↑↑ after 12 mo (p = 0.02) (compared to time of diagnosis) T0: 1440.59 ± 367.44 kcal T1: 1551.30 ± 384.14 kcal T2: 1629.11 ± 368.09 kcal No significant difference compared to EER | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papoutsaki, M.; Katsagoni, C.N.; Papadopoulou, A. Short- and Long-Term Nutritional Status in Children and Adolescents with Celiac Disease Following a Gluten-Free Diet: A Systematic Review. Nutrients 2025, 17, 487. https://doi.org/10.3390/nu17030487
Papoutsaki M, Katsagoni CN, Papadopoulou A. Short- and Long-Term Nutritional Status in Children and Adolescents with Celiac Disease Following a Gluten-Free Diet: A Systematic Review. Nutrients. 2025; 17(3):487. https://doi.org/10.3390/nu17030487
Chicago/Turabian StylePapoutsaki, Maria, Christina N. Katsagoni, and Alexandra Papadopoulou. 2025. "Short- and Long-Term Nutritional Status in Children and Adolescents with Celiac Disease Following a Gluten-Free Diet: A Systematic Review" Nutrients 17, no. 3: 487. https://doi.org/10.3390/nu17030487
APA StylePapoutsaki, M., Katsagoni, C. N., & Papadopoulou, A. (2025). Short- and Long-Term Nutritional Status in Children and Adolescents with Celiac Disease Following a Gluten-Free Diet: A Systematic Review. Nutrients, 17(3), 487. https://doi.org/10.3390/nu17030487







