Excessive Iodine Intake During Lactation Is Not Related to the Incidence of Thyroid Disease: A 3-Year Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
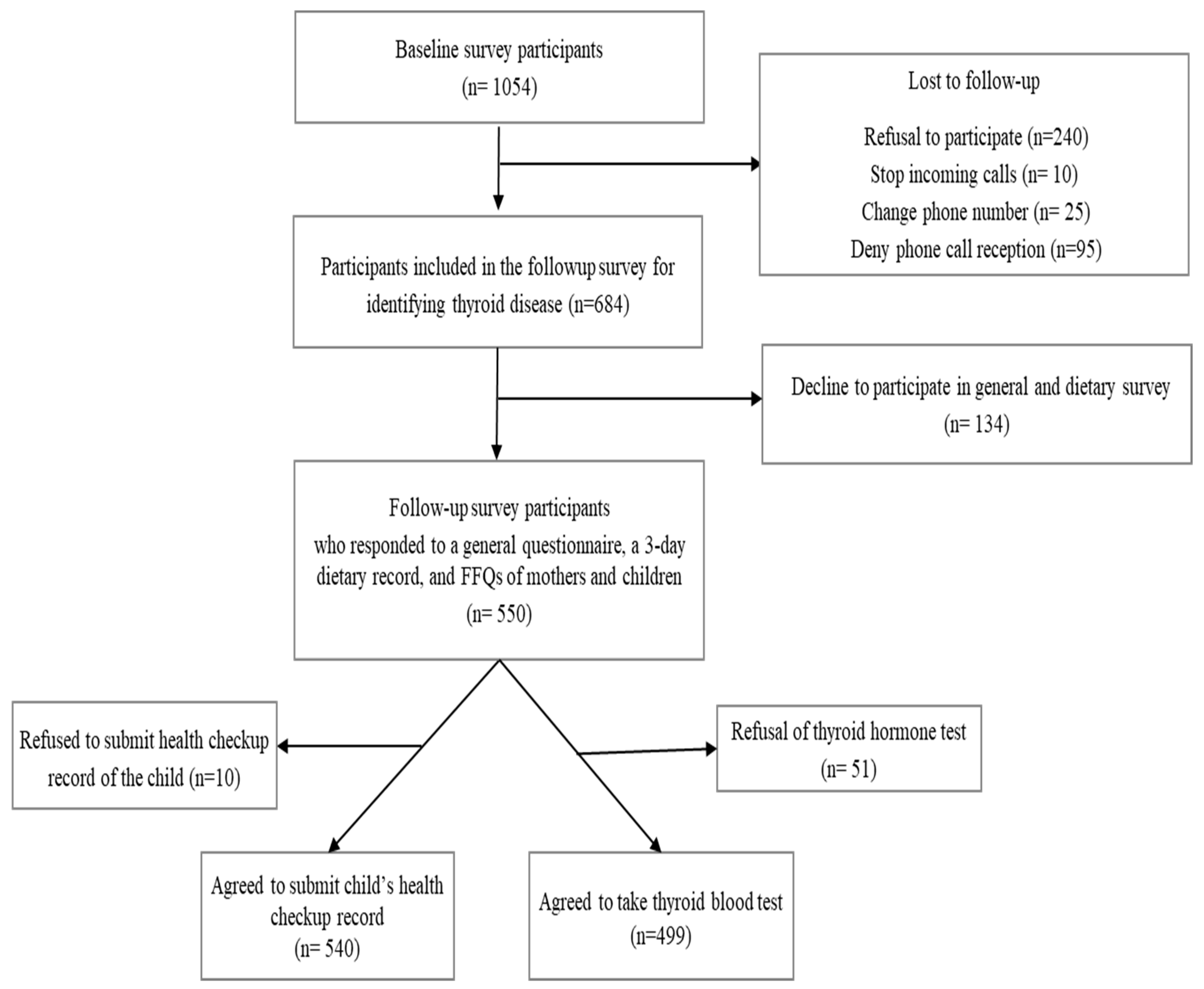
2.1. Participants
2.2. Covariates
2.3. Nutrient and Iodine Intake Assessment
2.4. Thyroid Hormone Measurement and Diagnosis of Thyroid Disease and Subclinical Hypothyroidism
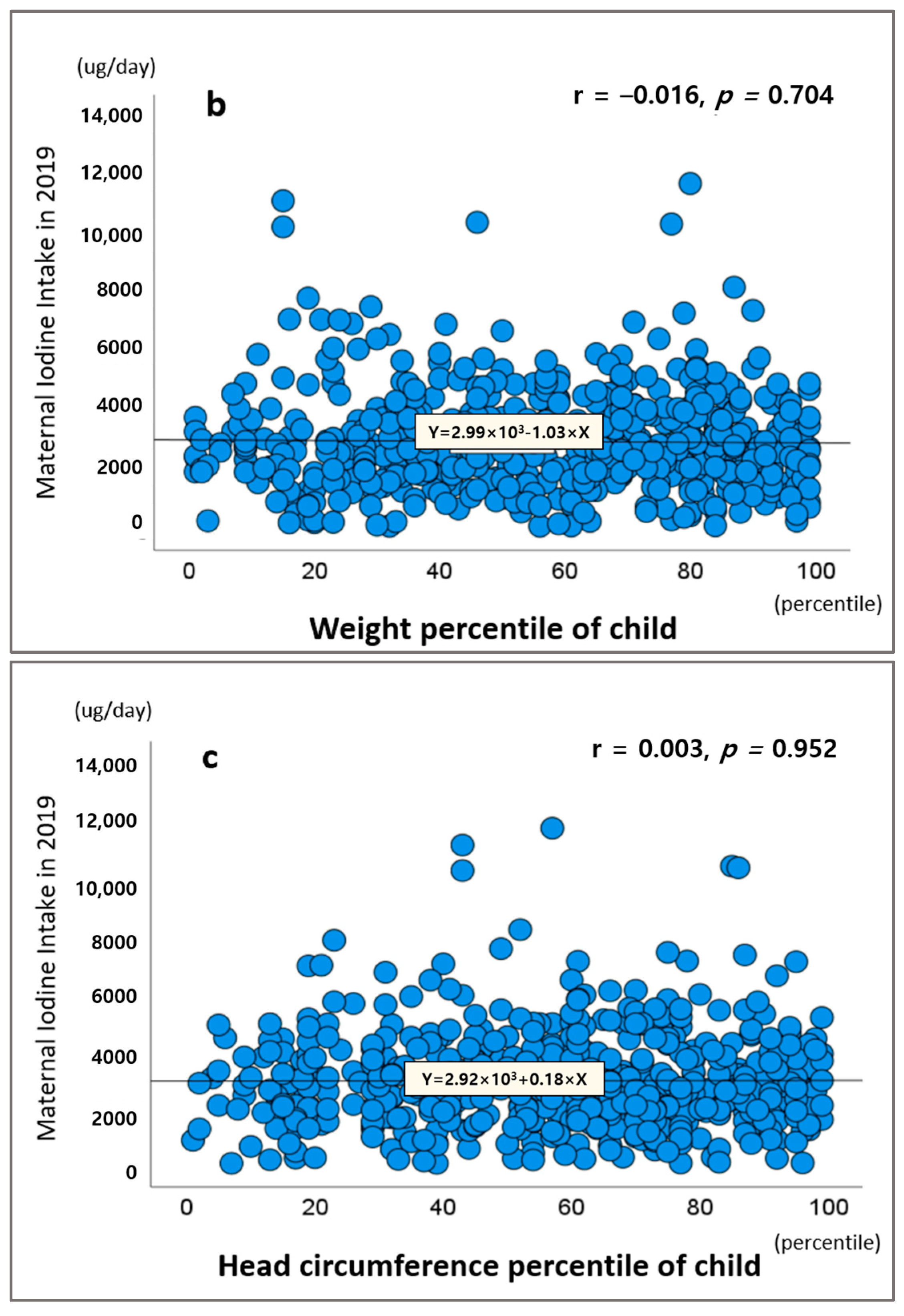
2.5. Thyroid Dysfunction and Growth and Development of Children
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tatar, B. Place-making landscape and materialities: Whales and social practices in Ulsan, Korea. J. Korean Cult. Anthropol. 2017, 50, 405–446. [Google Scholar]
- Wolmarans, D.W. Maintaining euthyroidism: Fundamentals of thyroid hormone physiology, iodine metabolism and hypothyroidism. S. Afr. Fam. Pract. 2017, 59, 11–21. [Google Scholar] [CrossRef]
- Boelaert, K.; Franklyn, J. Thyroid hormone in health and disease. J. Endocrinol. 2005, 187, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, F.R. Iodine and thyroid hormones during pregnancy and postpartum. Gynecol. Endocrinol. 2007, 23, 414–428. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Andersson, M. Assessment of iodine nutrition in populations: Past, present, and future. Nutr. Rev. 2012, 70, 553–570. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Hussein, I.; Al Ghannami, S.; El Badawi, S.; Al Hamad, N.M.; Hajj, B.A.; Al-Thani, M.; Winichagoon, P.; Pongcharoen, T.; van der Haar, F. Estimation of the prevalence of inadequate and excessive iodine intakes in school-age children from the adjusted distribution of urinary iodine concentrations from population surveys. J. Nutr. 2016, 146, 1204–1211. [Google Scholar] [CrossRef]
- Skeaff, S.A. Iodine deficiency in pregnancy: The effect on neurodevelopment in the child. Nutrients 2011, 3, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.R. Iodine and thyroid function. Ann. Pediatr. Endocrinol. 2014, 19, 8–12. [Google Scholar] [CrossRef]
- Pennington, J. A review of iodine toxicity reports. J. Am. Diet. Assoc. 1990, 90, 1571–1581. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Chung, H.R.; Shin, C.H.; Yang, S.W.; Choi, C.W.; Kim, B.I. Subclinical hypothyroidism in Korean preterm infants associated with high levels of iodine in breast milk. J. Clin. Endocrinol. Metab. 2009, 94, 4444–4447. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, H.N.; Ha, J. Association high-iodine-containing seaweed soup consumption after birth and subclinical hypothyroidism in Korean women: Korea National Health and Nutrition Examination Survey IV (2013–2015). Int. J. Thyroidol. 2019, 12, 105–112. [Google Scholar] [CrossRef]
- Choue, R.; Yim, J.C.; Cho, Y.; Lee, W. The effects of dietary iodine intake on the postpartum thyrioditis (PPT) manifestation. Korean J. Nutr. 1997, 30, 1195–1202. [Google Scholar]
- Cho, Y.W.; Kang, M.S.; Cha, Y.S.; Kook, J.H.; Kim, Y.R.; Park, P.W.; Lee, W.H.; Lim, J.E.; Cho, Y.W. Clinical Report of Effects of Pre and Post-Partum Thyroiditis (PPT). Endocrinol. Metab. 1997, 12, 541–549. [Google Scholar]
- Kim, W.B.; Yim, C.H.; Park, K.S.; Moon, B.S.; Lee, J.H.; Jun, H.W.; Jin, H.J.; Kim, S.Y.; Cho, B.Y.; Lee, H.G. The Incidence of Postpartum Thyroiditis and Effect of High Iodine Intake on it in Korean Women. Endocrinol. Metab. 1998, 13, 339–350. [Google Scholar]
- Lee, D.-K.; Lee, H.; Lee, H.; Yoon, T.; Park, S.-J.; Lee, H.-J. Nationwide Representative Survey of Dietary Iodine Intake and Urinary Excretion in Postpartum Korean Women. Nutrients 2021, 13, 3955. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Vanderpump, M. Subclinical thyroid disease. Br. Med. Bull. 2013, 107, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, M.; Wang, C.-H.; Pak, Y.; Chiu, K.C.; Gianoukakis, A.G. Parity and risk of thyroid autoimmunity based on the NHANES (2001–2002, 2007–2008, 2009–2010, and 2011–2012). J. Clin. Endocrinol. Metab. 2017, 102, 3437–3442. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, S.S.; Tabatabaee, A.; Aminorroaya, A.; Amini, M.; Feizi, A.; Janghorbani, M.; Tabatabaei, A.; Meamar, R.; Adibi, A.; Abyar, M. Isfahan thyroid cohort study (ITCS). Arch. Iran. Med. 2021, 24, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-R.; Paik, H.-Y.; Song, Y.; Park, Y.J. An iodine database for common Korean foods and the association between iodine intake and thyroid disease in Korean adults. Int. J. Thyroidol. 2015, 8, 170–182. [Google Scholar] [CrossRef]
- Ju, D.L.; Cho, S.W.; Chung, C.W.; Lee, Y.A.; Cheon, G.J.; Park, Y.J.; Shin, C.H.; Jun, J.K.; Chung, J.-K.; Park, S.K.; et al. High intakes of iodine among women during pregnancy and the postpartum period has no adverse effect on thyroid function. Eur. J. Nutr. 2023, 62, 239–249. [Google Scholar] [CrossRef]
- Orito, Y.; Oku, H.; Kubota, S.; Amino, N.; Shimogaki, K.; Hata, M.; Manki, K.; Tanaka, Y.; Sugino, S.; Ueta, M. Thyroid function in early pregnancy in Japanese healthy women: Relation to urinary iodine excretion, emesis, and fetal and child development. J. Clin. Endocrinol. Metab. 2009, 94, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Aakre, I.; Bjøro, T.; Norheim, I.; Strand, T.A.; Barikmo, I.; Henjum, S. Development of thyroid dysfunction among women with excessive iodine intake–A 3-year follow-up. J. Trace Elem. Med. Biol. 2015, 31, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, S.; Mikeda, T.; Okada, T.; Nakamura, K.; Kotani, T.; Hishinuma, A. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid 2004, 14, 1077–1083. [Google Scholar] [CrossRef]
- Shi, X.; Han, C.; Li, C.; Mao, J.; Wang, W.; Xie, X.; Li, C.; Xu, B.; Meng, T.; Du, J. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: A cross-sectional study of 7190 pregnant women in China. J. Clin. Endocrinol. Metab. 2015, 100, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Konno, N.; Makita, H.; Yuri, K.; Iizuka, N.; Kawasaki, K. Association between dietary iodine intake and prevalence of subclinical hypothyroidism in the coastal regions of Japan. J. Clin. Endocrinol. Metab. 1994, 78, 393–397. [Google Scholar] [PubMed]
- Konno, N.; Yuri, K.; Taguchi, H.; Miura, K.; Taguchi, S.; Hagiwara, K.; Murakami, S. Screening for thyroid diseases in an iodine sufficient area with sensitive thyrotrophin assays, and serum thyroid autoantibody and urinary iodide determinations. Clin. Endocrinol. 1993, 38, 273–281. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, Y.S.; Kim, J.Y.; Hong, K.H.; Park, Y.K. Association between iodine nutrition status and thyroid disease-related hormone in Korean adults: Korean National Health and Nutrition Examination Survey VI (2013–2015). Nutrients 2019, 11, 2757. [Google Scholar] [CrossRef] [PubMed]
- Eng, P.H.; Cardona, G.R.; Fang, S.L.; Previti, M.; Alex, S.; Carrasco, N.; Chin, W.W.; Braverman, L.E. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology 1999, 140, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Markou, K.; Georgopoulos, N.; Kyriazopoulou, V.; Vagenakis, A. Iodine-induced hypothyroidism. Thyroid. 2001, 11, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Cerqueira, C.; Ovesen, L.; Rasmussen, L.B.; Perrild, H.; Andersen, S.; Pedersen, I.B.; Carlé, A. Iodine intake as a determinant of thyroid disorders in populations. Best. Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 13–27. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E. Iodine-induced thyroid dysfunction. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Eftychia, G.K.; Roupas, N.D.; Markou, K.B. Effect of excess iodine intake on thyroid on human health. Minerva Med. 2017, 108, 136–146. [Google Scholar]
- Noahsen, P.; Rex, K.F.; Bülow Pedersen, I.; Mulvad, G.; Florian-Sørensen, H.C.; Pedersen, M.L.; Andersen, S. Adaptation to a high iodine intake in Greenland Inuit suggested by thyroid disease pattern. Thyroid 2021, 31, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare; The Korean Nutrition Society. 2020 Dietary Reference Intakes for Koreans; Ministry of Health and Welfare: Sejong, Republic of Korea, 2020. [Google Scholar]
- Ministry of Health Labour and Welfare of Japan. Dietary Reference Intakes for Japanese; Ministry of Health Labour and Welfare of Japan: Tokyo, Japan, 2020. [Google Scholar]


| Variables | Normal (n = 661) | Thyroid Disease (n = 23) | p-Value | |||
|---|---|---|---|---|---|---|
| Age (years) | 32.5 ± 3.8 (1) | 30.5 ± 3.6 | 0.018 † | |||
| Body mass index (kg/m2) | 24.1 ± 3.3 | 24.3 ± 3.2 | 0.631 † | |||
| Child characteristics at birth | Gestational (weeks) | 38.6 ± 2.6 | 38.7 ± 1.5 | 0.780 † | ||
| Height (cm) | 50.1 ± 3.7 | 50.4 ± 3.1 | 0.383 † | |||
| Weight (kg) | 3.24 ± 0.40 | 3.34 ± 0.40 | 0.067 † | |||
| Fertility | Primiparity | 476 | (72.0) (2) | 19 | (82.6) | 0.264 ‡ |
| Multiparity | 185 | (28.0) | 4 | (17.4) | ||
| Feeding type | Breastfeeding | 137 | (20.7) | 6 | (26.1) | 0.656 § |
| Mixed feeding | 479 | (72.5) | 15 | (65.2) | ||
| Formula feeding | 45 | (6.8) | 2 | (8.7) | ||
| Family history of thyroid disease | Yes | 108 | (16.3) | 6 | (26.1) | 0.250 § |
| No | 553 | (83.7) | 17 | (73.9 | ||
| Education | Middle School | 3 | (0.5) | 0 | (0.0) | 0.650 § |
| High School | 57 | (8.6) | 1 | (4.3) | ||
| College graduation | 526 | (79.6) | 21 | (91.3) | ||
| Graduate School | 75 | (11.3) | 1 | (4.3) | ||
| Household income (USD) | <2000 | 42 | (6.4) | 2 | (8.7) | 0.305 § |
| 2000–4000 | 373 | (56.4) | 10 | (43.5) | ||
| 4000–6000 | 159 | (24.1) | 9 | (39.1) | ||
| >6000 | 87 | (13.2) | 2 | (8.7) | ||
| Variables | Normal (n = 661) | Thyroid Disease (n = 23) | p-Value † | ||||
| Mean | ± | SD | Mean | ± | SD | ||
| Calorie intake in 2019 (kcal) | 2077.2 ± 605.3 | 2073.4 ± 635.9 | 0.790 | ||||
| Calorie intake in 2019 (kcal/kg) | 33.6 ± 11.1 | 33.6 ± 11.9 | 0.846 | ||||
| Iodine intake in 2019 (μg) | 2960.2 ± 1743.2 | 2494.1 ± 1340.3 | 0.210 | ||||
| Normal (n = 530) | Thyroid Disease (n = 20) | ||||||
| Calorie intake in 2022 (kcal) | 1231.0 ± 671.0 | 1413.4 ± 647.5 | 0.134 | ||||
| Calorie intake in 2022 (kcal/kg) | 26.1 ± 6.7 | 28.1 ± 7.5 | 0.181 | ||||
| Iodine intake in 2022 (μg) | 368.5 ± 461.5 | 524.5 ± 479.4 | 0.774 | ||||
| Difference in calorie intake between 2019 and 2022 (kcal) | 517.7 ± 640.4 | 463.5 ± 715.2 | 0.948 | ||||
| Difference in iodine intake between 2019 and 2022 (μg) | 2605.7 ± 1783.0 | 1938.2 ± 1668.2 | 0.101 | ||||
| Postpartum Dietary Iodine Intake (μg) | ||||
|---|---|---|---|---|
| ≤2400 | >2400 | ≤3000 | >3000 | |
| Median iodine intake (μg) | 1686 | 3630 | 1940 | 4075 |
| Number of cases/total | 13/288 | 10/396 | 15/403 | 8/281 |
| Model 1 RR (95% CI) | 1.00 | 0.43 (0.10–1.82) | 1.00 | 0.81(0.19–3.48) |
| Model 2 RR (95% CI) | 1.00 | 0.60 (0.13–2.67) | 1.00 | 1.01 (0.23–4.45) |
| Model 3 RR (95% CI) | 1.00 | 0.92 (0.19–4.51) | 1.00 | 1.66 (0.33–8.22) |
| Average Dietary Iodine Intake from Baseline and Follow-Up (μg) | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Median iodine intake (μg) | 756 | 1265 | 1758 | 2631 |
| Number of cases/total | 3/124 | 3/125 | 2/125 | 6/125 |
| Model 1 RR (95% CI) | 1.00 | 2.78 (0.46–16.70) | 0.98 (0.24–3.93) | 2.72 (0.46–15.92) |
| p for trend | 0.2646 | |||
| Model 2 RR (95% CI) | 1.00 | 2.87 (0.48–17.29) | 0.87 (0.21–3.55) | 2.29 (0.39–13.46) |
| p for trend | 0.2601 | |||
| Model 3 RR (95% CI) | 1.00 | 2.45 (0.39–15.31) | 0.58 (0.12–2.79) | 1.05 (0.19–5.80) |
| p for trend | 0.3964 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-J.; Lee, D.-K.; Lee, H.-J. Excessive Iodine Intake During Lactation Is Not Related to the Incidence of Thyroid Disease: A 3-Year Follow-Up Study. Nutrients 2025, 17, 476. https://doi.org/10.3390/nu17030476
Park S-J, Lee D-K, Lee H-J. Excessive Iodine Intake During Lactation Is Not Related to the Incidence of Thyroid Disease: A 3-Year Follow-Up Study. Nutrients. 2025; 17(3):476. https://doi.org/10.3390/nu17030476
Chicago/Turabian StylePark, Seon-Joo, Do-Kyung Lee, and Hae-Jeung Lee. 2025. "Excessive Iodine Intake During Lactation Is Not Related to the Incidence of Thyroid Disease: A 3-Year Follow-Up Study" Nutrients 17, no. 3: 476. https://doi.org/10.3390/nu17030476
APA StylePark, S.-J., Lee, D.-K., & Lee, H.-J. (2025). Excessive Iodine Intake During Lactation Is Not Related to the Incidence of Thyroid Disease: A 3-Year Follow-Up Study. Nutrients, 17(3), 476. https://doi.org/10.3390/nu17030476







