Pyroptosis: A Novel Therapeutic Target for Bioactive Compounds in Human Disease Treatment? A Narrative Review
Abstract
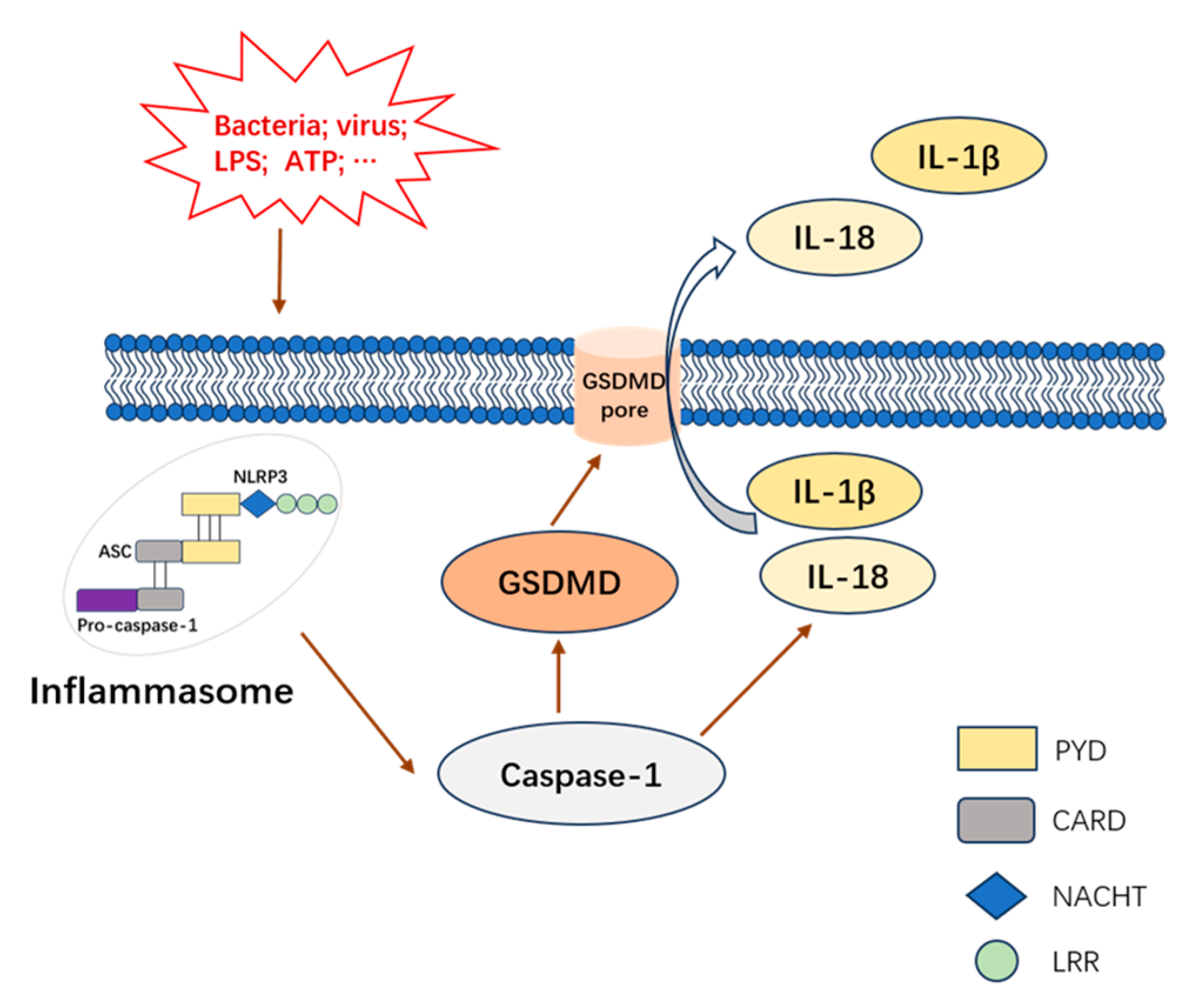
1. Introduction
2. Bibliographic Search
3. Bioactive Compounds Regulate Pyroptosis in Inflammatory Disease
| Bioactive Compound | Derived from | In Vivo/Vitro | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| 1′-Acetoxychavicol acetate | Alpinia | In vitro | 0.1, 1, 2.5 μM | Inhibition of mitochondrial ROS generation Prevention of oxidized mitochondrial DNA release Decrease in NLRP3, caspase-1, ASC, IL-1β, and GSDMD cleavage | [27] |
| In vivo | 1.5 mg/kg | Decrease in NLRP3 and caspase-1 | |||
| Celastrol | Tripterygium wilfordii Hook | In vitro | 12.5, 25, 50 nM | Reduction in IL-1β and IL-18, NLRP3 inflammasome, caspase-1, LDH leakage Decrease in ROS and NF-κB | [26] |
| DHA | Food | In vitro | 30 µM | Suppression of IL-1β | [32] |
| 200 µM | Increase in pyroptosis Increase in IL-1β, caspase-1 | ||||
| Punicalin | Leaves of Terminalia catappa L and pomegranate husk | In vitro | 20, 50, 100 µM | Decrease in ROS Decrease in NLRP3, caspase 1, ASC and GSDMD-N, IL-1β, and IL-18 | [29] |
| Resveratrol | Grapes, peanuts, and Reynoutria japonica | In vitro | 7.5, 15, 30 µM | Reduction in caspase-1, NLRP3, IL-1β, and IL-18 Increase in mitochondrial membrane potential Inhibition of P62 and Pink1 Increase in TOMM20, Parkin, and LC3B-II Increase in mitophagy | [33] |
| In vivo | 15 mg/kg | Improvement of GA Improvement of peritonitis Decrease in IL-1β and IL-18 | |||
| Scutellarin | Erigeron breviscapus | In vitro | 12.5, 25, 50 µM | Inhibition of caspase-11p26 and GSDMD-NT Reduction in pyroptosis. Suppression of NLRP3, ASC, IL-1β, and caspase-1p10 Increase in Ser/Thr phosphorylation of PKA | [30] |
| In vivo | 50, 100 mg/kg | Decrease in IL-1β | |||
| Toonaones D, limonoids | Toona ciliata M. Roem. | In vitro | 2.5, 5, 10 µM | Inhibition of GMDMD, caspase-1, and IL-1β | [28] |
| Chikusetsu saponin IVa | Panax japonicus | In vitro | 10, 20, 40 µM | Inhibition of NLRP3, ASC, caspase-1, and IL-1β Decrease in LDH release Suppression of NF-κB | [31] |
| In vivo | 50, 100 mg/kg | Improvement of lipid homeostasis Inhibition of inflammation in adipose tissue Decrease in chemokines and cytokines Inhibition of the accumulation of adipose tissue macrophages Decrease in IL-1β, caspase-1, NLRP3, and ASC Suppression of NF-κB | |||
| Ellagic acid | Galla chinensis and Pericarpium granati | In vivo | 10, 50 mg/kg | Decrease in TNFα, IL-6, IL-17A and IL-10, NLRP3, and caspase 1 Improvement of neuroinflammation, demyelination, and axonal damage Improvement of MBP, GFAP, and Iba1 immunoreactivity. | [34] |
| Shionone | Aster tataricus | In vitro | 2.5, 5, 10 µg/mL | Reduction in ASC, pro-caspase-1, GSDMD, GSDMD-N, caspase-1, NF-κB, and NLRP3 | [35] |
| In vivo | 50, 100 mg/kg | Improvement of bladder wet weight, score of hemorrhage and edema Reduction in ASC, pro-caspase-1, GSDMD, GSDMD-N, caspase-1, NF-κB, and NLRP3 |
4. Bioactive Compounds Regulate Pyroptosis in Bacterial Infection
| Bioactive Compound | Derived from | In Vivo/Vitro | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| Oil of Artemisia argyi H.Lév. and Vaniot | Artemisia argyi H. Lév. and Vaniot | In vitro | SDAO: 22.5, 33.75, 45 μg/mL SFEAO: 68, 102, 136 μg/ml | Inhibition of NLRP3, caspase-1, NF-κB, and IL-1β | [40] |
| Piperine | Black pepper (Piper nigrum Linn) | In vitro | 40, 80, 160 μM | Induction of pyroptosis Suppression of IL-1β or HMGB1 Suppression of AMPK | [38] |
| In vivo | 20 mg/kg | Improvement of peritoneal Reduction in serum IL-1β | |||
| Scutellarin | Erigeron breviscapus | In vitro | 0.1, 0.2, 0.4 mM | Decrease in ASC, caspase-1, IL-1β, and NLRP3 PKA pathway activation | [41] |
| In vivo | 100, 200 mg/kg | Improvement of survival mice Decrease in serum IL-1β | |||
| Taraxasterol | Dandelion (Taraxacum mongolicum Hand. -Mazz.) | In vitro | 25, 50, 100 μM | Decrease in ASC, GSDMD, IL-1β, and caspase-1 Suppression of mTORC1 and mTORC2 | [42] |
| In vivo | 20 mg/kg | Improvement of survival mice Decrease in serum IL-1β | |||
| Schisandrin A, B, and C | Schisandra chinensis (Turcz.) Baill. | In vitro | 10 μM | Inhibition of NLRP3 inflammasome, IL-1β Decrease in mitochondrial ROS | [45] |
| Berberine | Rhizoma coptidis and Cortex phellodendri | In vitro | 0.75, 1.5, 3.0 μM | Increase in caspase-1p10, IL-1β Activation of AMPK signal pathway | [46] |
| In vivo | 100 mg/kg | Decrease in peritoneal live bacterial Improvement of survival mice Increase in IL-1β Increase in neutrophil recruitment in the peritoneal cavity |
5. Bioactive Compounds Regulate Pyroptosis in Cancer
| Bioactive Compound | Derived from | In Vivo/Vitro | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| Dioscin | Polygonatum zanlanscianense, Dioscorea nipponica Makino, and Dioscorea zingiberensis Wright | In vitro | 2, 4 μΜ in MG63 and U2OS 2.5, 5 μΜ in MNNG/HOS | Increase in caspase-3, cleaved GSDME-N Inhibition of cell proliferation Activation of JNK/p38 pathway | [54] |
| In vivo | 12, 24 mg/kg | Inhibition of OS proliferation | |||
| DHA | Food | In vitro | 50, 100, 200 μM | Increase in GSDMD, IL-1β, and caspase-1 Increase in HMGB1 Formation of membrane pore | [51] |
| Nobiletin | Citrus nobilis Lour., C. aurantium L., and C. reticulata Blanco. | In vitro | 80 μM | Increase in IL-1β, IL-18, NLRP3, ASC, and cleaved caspase 1 | [53] |
| Tanshinone IIA | Salvia miltiorrhiz | In vitro | 2, 4, 8 μM | Decrease in cell proliferation Increase in caspase-3 and caspase-9 Increase in cleaved GSDMD, caspase-1, IL-1β, and IL-18 | [55] |
| Curcumin | Turmeric and zedoary | In vitro | 10, 20, 30 μM | Increase in GSDME-N-terminus Increase in ROS | [56] |
| Curcumin | Turmeric | In vitro | 40 μM | Induction of caspase-1, HMGB1, IL-18, and IL-1β Decrease in NF-κB and toll-like receptors | [57] |
| Dihydroartemisinin | Artemisia annua | In vitro | 5, 20, 40 μM | Increase in caspase-3, AIM2, GSDME, HMGB1IL-1β, and IL-18 | [52] |
| In vivo | 4 mg/kg | Inhibition of breast xenograft tumors |
6. Bioactive Compounds Regulate Pyroptosis in Diabetes
| Bioactive Compound | Derived from | In Vivo/Vitro | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| Epigallocatechin-3-gallate | green tea | In vitro | 20, 30, 40 μM | Decrease in IL-1β and NLRP3 inflammasome | [59] |
| In vivo | 50 mg/kg | Inhibition of NLRP3 inflammasome Improvement of glucose tolerance | |||
| Quercetin-Rich Guava Juice and Trehalose | Psidium guajav | In vivo | Guava juice+ Trehalose: 4 + 2, 8 + 4, 20 + 1 mL/kg | Improvement of oral glucose tolerance test (OGTT), homeostasis model assessment of IR (HOMA-IR), and homeostasis model assessment of the function of β cell (HOMA-β) and plasma insulin Decrease in ROS, 4-hydroxynonenal, caspase-3, LC3-B, and IL-1β | [60] |
| Curcumin | Curcuma longa | In vitro | 10 μM | Decrease in IL-18, ASC, GSDMD-N, cleaved-caspase-1, and NLRP3 Regulation of TREM2/TLR4/NF-κB signaling | [61] |
| In vivo | 50 mg/kg | Improvement of neuronal cell death and cognitive deficits Decrease in GSDMD-N, NLRP3, IL-18, cleaved-caspase-1, and ASC, Regulation of TREM2/TLR4/NF-κB signaling | |||
| Sinapic acid | Brassica alba (L.) Boiss, Brassica juncea (L.) Czern. et Coss | In vitro | 1 μM | Decrease in ASC, NRLP3, and caspase-1 Inhibition of lncRNA-MALAT1 | [62] |
| In vivo | 5, 10, 50 mg/kg | Decrease in serum ET-1 and IL-1β Reduction of ASC, NRLP3, and caspase-1 | |||
| Ginsenoside Rb2 (Rb2) | ginsenosides | In vitro | 50 μM | Improvement of IR Decrease in caspase-1, ASC, NLRP3, IL-1β, and GSDMD Reduction of the phosphorylation of p65 and IκBα | [63] |
| In vivo | 40 mg/kg | Improvement of body weight, fat accumulation, and IR Decrease in caspase-1, ASC, NLRP3, IL-1β, and GSDMD Reduction in the phosphorylation of p65 and IκBα |
7. Bioactive Compounds Regulate Pyroptosis in Tissue Injury
| Bioactive Compound | Derived from | In Vivo/Vitro | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| DHA/AA | Food | In vitro | 50 μM | Inhibition of IL-18, IL-1β, and NLRP3 inflammasome | [64] |
| In vivo | 50 mg/kg | Decrease in AL, AST, and LDH Inhibition of caspase-1 and NLRP3 | |||
| DHA | Food | In vitro | 25 μM | Increase of cell viability Decrease in IL-1β, IL-18, NLRP3, caspase-1, and ASC Activation of PI3K/Akt pathway | [65] |
| In vivo | 300 mg/kg | Decrease in serum AST, ALT, IL-1β, and IL-18 Decrease in liver tissue MDA, caspase-1, ASC, and NLRP3 Increase in liver tissue GSH, SOD, and CAT | |||
| Limonin | Citrus fruit | In vitro | 10, 25, 50 μM | Decrease in ROS generation, LDH level, caspase-1, NLRP3, caspase-1, GSDMD, and IL-1β | [68] |
| In vivo | 50, 100 mg/kg | Decrease in serum ALT, AST, and LDH Increase in liver tissue GSH | |||
| Betulinic acid | Chinese herbal | In vivo | 20 mg/kg | Improvement of functional recovery Decrease in GSDMD, NLRP3, caspase-1, ASC, IL-1β, and IL-18 Induction of autophagy Decrease in ROS | [69] |
| Ligustrazine | Rhizoma Chuanxiong | In vitro | 10, 20, 40 μM | Decrease in NLRP3, caspase-1, ACS, IL-1β, and GSDMD-N Inhibition of Txnip/Trx/NF-κB pathway Decrease in ALT, AST, MDA, and NO Decrease in ROS generation Increase in SOD, GSH, and CAT | [67] |
| In vivo | 20, 40 mg/kg | Improvement of liver structure, fibrosis, and cell death Decrease in NLRP3, ASC, caspase-1, GSDMD TNF-α, IL-6, and IL-1β Decrease in serum ALT and AST Decrease in liver MDA and NO Increase in liver SOD, GSH, and CAT Decrease in ROS generation Inhibition of Txnip/Trx/NF-κB pathway | |||
| Andrographolide | Andrographis paniculata | In vitro | 1, 3, 10 μM | Decrease in NLRP3 inflammasome Inhibition of NF-κB pathway Decrease in IL-1β, TNF-αIL-6, and LDH | [71] |
| In vivo | 0.5, 1, 2 mg/kg | Decrease in neuronal cell death and degeneration Improvement of neurobehavioral disorders and brain edema Decrease in TNF-α and IL-6 Inhibition of NLRP3 inflammasome | |||
| p-coumaric acid | fruits, vegetables, and cereals | In vivo | 50, 100, 200 mg/kg | Improvement of intestinal barrier morphology and apoptosis Increase in villus height and the ratio villus height/crypt depth Decrease in AIM2, NLRP3, and caspase-1 | [72] |
| Lycopene | Fruits and vegetables with red color | In vivo | 5 mg/kg | Decrease in NLRP3, caspase-1, IL-1β, IL-18, and ASC Increase in GSDMD Inhibition of NF-κB | [74] |
| Curcumin | Curcuma longa | In vitro | 20, 40, 80 μM | Decrease in NLRP3, cleaved caspase 1, GSDMD, GSDMD-N, and cleaved IL-1β Inhibition of NF-κB and SIRT1 | [75] |
| In vivo | 100, 200 mg/kg | Improvement of histopathological injury Decrease in myeloperoxidase (MPO), chemokine (C-C motif) ligand 7 (CCL7), IL-6, and TNF-α Inhibition of SIRT1 | |||
| Kaempferol | tea, kale, broccoli, cabbage, grapefruit | In vitro | 25, 50, 100 μM | Decrease in microglia activation, ROS, and NADPH oxidase 4 Inhibition of MAPK pathway and NF-κB p65 translocation Decrease in NLRP3 caspase-1 p10, ASC, N-GSDMD, IL-18, and IL-1β | [70] |
| In vivo | 25, 50, 100 mg/kg | Improvement of tissue damage and hindlimb motor function Decrease in oxidative stress and microglia activation | |||
| Gastrodin | Tianma | In vitro | 5 μg/mL | Inhibition of cleaved caspase-1, NLRP3, IL-1β, and IL-18 Modulation of lncRNA NEAT1/miR-22-3p pathway | [72] |
| In vivo | 50 mg/kg | Improvement of neurological scores Decrease in the area of cerebral infarction Inhibition of cleaved caspase-1, NLRP3, IL-1β, and IL-18 | |||
| Vitamin D | Food | In vitro | 10−8, 10−7, 10−6 mol/L | Improvement and lipid accumulation Decrease in NLRP3 inflammasome, GSDMD-N, and IL-1β | [66] |
| In vivo | 5 μg/kg | Decrease in NLRP3 inflammasome, IL-1β, and IL-18 Decrease in serum AST, ALT, and triglyceride (TG) Improvement of gut microbiota dysbiosis |
8. Bioactive Compounds Regulate Pyroptosis in Heart Disease
| Bioactive Compound | Derived from | In Vivo/Vitro | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| Pinocembrin | Boesenbergia pandurate | In vitro | 1 μM | Inhibition of NLRP3 inflammasome, Nrf2, and Sirt3 | [76] |
| In vivo | 5 mg/kg | Improvement of cardiac fibrosis and cardiac function Reduction in left ventricular internal dimension in diastole (LVIDd), left ventricular internal dimension in systole (LVIDs), left ventricular fractional shortening (LVFS), and left ventricular ejection fraction (LVEF), Decrease in CK-MB, LDH, IL-18, and IL-1β | |||
| Astragaloside IV | Astragalus | In vitro | 100 μM | Decrease in NLRP3, GSDMD-N, cleaved caspase-1, cleaved IL-18, and cleaved IL-1β | [78] |
| In vivo | 40 mg/kg | Improvement of poor ventricular remodeling, myocardial fibrosis, and myocardial hypertrophy Decrease in ROS, NLRP3 inflammasome, and GSDMD Decrease in macrophages and neutrophils | |||
| Sweroside | Swertia pseudochinensis Hara | In vitro | 50 μM | Reduction in LDH, CK-MB, MDA, and ROS Increase in SOD and glutathione peroxidase (GSH-Px) Decrease in NLRP3, ASC, IL-1β, and cleaved caspase-1 Inhibition of Keap1 and Nrf2 | [77] |
| In vivo | 20, 50, 100 mg/kg | Reduction in infarct size Improvement of cardiac function | |||
| Ginsenoside Rb1 | ginseng, panax notoginseng, and American ginseng | In vitro | 25, 50, 100 μM | Inhibition of calcium transients Improvement of contractile function and field potential | [79] |
| In vivo | 10, 20, 40 mg/kg | Inhibition of apoptosis and pyroptosis Improvement of myocardial damage |
9. Bioactive Compounds Regulate Pyroptosis in Other Diseases
| Bioactive Compound | Disease | In Vivo/Vitro | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| Baeckein E | Gout | In vitro | 0.4, 0.8, 1.6 μM | Decrease in IL-1β and NLRP3 inflammasome Improvement of mitochondrial damage Suppression of MAPK/NF-κB pathway | [82] |
| In vivo | 12.5, 50 mg/kg | Suppression of NLRP3 inflammasome and ankle swelling Inhibition of NF-κB | |||
| Glycyrrhizin | COVID-19 | In vitro | 1 mM | Decrease in IL-1β, IL-6, IL-8, and ferritin SARS-CoV-2 replication inhibition | [88] |
| Dihydromyricetin | Atherosclerosis | In vitro | 300 μM | Decrease in LDH, IL-1β, and caspase-1 cleavage Suppression of intracellular and mitochondrial ROS Activation of the Nrf2 | [85] |
| Vitexin | Nephrolithiasis | In vitro | 10, 20 mg/L | Decrease in LDH Inhibition of pyroptosis-related proteins Increase in pan-cytokeratin (Pan-ck) expression Decrease in Vimentin and alpha-smooth muscle actin (α-SMA) expression Downregulation of Wnt/β-catenin pathway Suppression of TNF-α and IL-1β | [81] |
| In vivo | 10, 20 mg/kg | Improvement of crystal deposition and kidney tissue injury Decrease in MDA Increase in SOD, GSH, and CAT Reduction in GSDMD, NLRP3, cleaved caspase-1, and IL-1β Suppression of CD44 and OPN expression Improvement of MCP-1 expression and F4/80-positive macrophage infiltration | |||
| Schisandrin | Alzheimer’s disease | In vitro | 10 μM | Suppression of neuronal apoptosis and pyroptosis | [86] |
| In vivo | 2 mg/kg | Improvement of cognitive impairment Inhibition of Aβ production Suppression of neuronal apoptosis and pyroptosis Inhibition of NLRP1, ASC, caspase-1, IL-18, and IL-1β | |||
| Isoliquiritin | Depression | In vitro | 50 μM | Increase in miRNA-27a Decrease in p-NF-κB, SYK, NLRP3, cleaved caspase-1, IL-1β, and GSDMD-N | [87] |
| In vivo | 10, 30 mg/kg | Improvement of sucrose preference and neuronal cells disorder Increase in miRNA-27a and NeuN Decrease in p-NF-κB and SYK Inhibition of GSDMD-N, NLRP3 inflammasome, IL-1β, and TNF-α | |||
| Clinical | No treatment | Decrease in miRNA-27a |
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grosso, G. Effects of Polyphenol-Rich Foods on Human Health. Nutrients 2018, 10, 1089. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, R.; Chen, J.; Li, S.; Huang, K.; Cao, H.; Farag, M.A.; Battino, M.; Daglia, M.; Capanoglu, E.; et al. Health benefits of saponins and its mechanisms: Perspectives from absorption, metabolism, and interaction with gut. Crit. Rev. Food Sci. Nutr. 2024, 64, 9311–9332. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Y.; Bajpai, V.K.; El-Kammar, H.A.; Simal-Gandara, J.; Cao, H.; Cheng, K.W.; Wang, M.; Arroo, R.R.J.; Zou, L.; et al. Advance toward isolation, extraction, metabolism and health benefits of kaempferol, a major dietary flavonoid with future perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 2773–2789. [Google Scholar] [CrossRef]
- Bobis, O.; Moise, A.R.; Ballesteros, I.; Reyes, E.S.; Durán, S.S.; Sánchez-Sánchez, J.; Cruz-Quintana, S.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Eucalyptus honey: Quality parameters, chemical composition and health-promoting properties. Food Chem. 2020, 325, 126870. [Google Scholar] [CrossRef]
- Singh, L.; Wani, A.W.; Shaifali; Sadawarti, R.K.; Kaur, H.; Bashir, O.; Majeed, J.; Mirza, A.A.; Sharma, J.; Ali, S.; et al. A comprehensive review on neurotrophic receptors and their implications in brain health: Exploring the neuroprotective potential of berries. J. Berry Res. 2024, 18785093241301881. [Google Scholar] [CrossRef]
- Kargar, A.; Kızıltan, G. Plant-based diets: Obesity prejudice and body self-perception relations in young females. Med. J. Nutr. Metab. 2024, 17, 53–63. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–467. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Cervantes, J.; Nagata, T.; Uchijima, M.; Shibata, K.; Koide, Y. Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages. Cell. Microbiol. 2008, 10, 41–52. [Google Scholar] [CrossRef]
- Kelk, P.; Johansson, A.; Claesson, R.; Hänström, L.; Kalfas, S. Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 2003, 71, 4448–4455. [Google Scholar] [CrossRef]
- Li, P.; Allen, H.; Banerjee, S.; Franklin, S.; Herzog, L.; Johnston, C.; McDowell, J.; Paskind, M.; Rodman, L.; Salfeld, J.; et al. Mice deficient in IL-1 β-converting enzyme are defective in production of mature IL-1 β and resistant to endotoxic shock. Cell 1995, 80, 401–411. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Rogers, C.; Fernandes-Alnemri, T.; Mayes, L.; Alnemri, D.; Cingolani, G.; Alnemri, E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017, 8, 14128. [Google Scholar] [CrossRef]
- Orning, P.; Weng, D.; Starheim, K.; Ratner, D.; Best, Z.; Lee, B.; Brooks, A.; Xia, S.; Wu, H.; Kelliher, M.A.; et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 2018, 362, 1064–1069. [Google Scholar] [CrossRef]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Li, P.; Nilson, R.; Tang, A.Y.; Rongvaux, A.; Bunnell, S.C.; Shao, F.; Green, D.R.; et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. USA 2018, 115, E10888–E10897. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.M.Y.; Ansara, J.; et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; Chen, X.; Wang, Z.; Liang, X.; Zhang, T.; Liu, M.; Zhou, N.; Lv, J.; Tang, K.; et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol. 2020, 5, eaax7969. [Google Scholar] [CrossRef]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020, 368, eaaz7548. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef]
- Xin, W.; Wang, Q.; Zhang, D.; Wang, C. A new mechanism of inhibition of IL-1β secretion by celastrol through the NLRP3 inflammasome pathway. Eur. J. Pharmacol. 2017, 814, 240–247. [Google Scholar] [CrossRef]
- Sok, S.P.M.; Ori, D.; Wada, A.; Okude, H.; Kawasaki, T.; Momota, M.; Nagoor, N.H.; Kawai, T. 1′-Acetoxychavicol acetate inhibits NLRP3-dependent inflammasome activation via mitochondrial ROS suppression. Int. Immunol. 2021, 33, 373–386. [Google Scholar] [CrossRef]
- Shi, Q.-Q.; Zhang, X.-J.; Wang, T.-T.; Zhang, Y.; Zeb, M.A.; Zhang, R.-H.; Li, X.-L.; Xiao, W.-L. Toonaones A-I, limonoids with NLRP3 inflammasome inhibitory activity from Toona ciliata M. Roem. Phytochem. 2021, 184, 112661. [Google Scholar] [CrossRef]
- Shen, R.; Yin, P.; Yao, H.; Chen, L.; Chang, X.; Li, H.; Hou, X. Punicalin Ameliorates Cell Pyroptosis Induced by LPS/ATP Through Suppression of ROS/NLRP3 Pathway. J. Inflamm. Res. 2021, 14, 711–718. [Google Scholar] [CrossRef]
- Ye, J.; Zeng, B.; Zhong, M.; Li, H.; Xu, L.; Shu, J.; Wang, Y.; Yang, F.; Zhong, C.; Ye, X.; et al. Scutellarin inhibits caspase-11 activation and pyroptosis in macrophages via regulating PKA signaling. Acta Pharm. Sin. B 2021, 11, 112–126. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, C.; Wang, T.; He, Y.; Zhou, Z.; Dun, Y.; Zhao, H.; Ren, D.; Wang, J.; Zhang, C. Chikusetsu saponin IVa ameliorates high fat diet-induced inflammation in adipose tissue of mice through inhibition of NLRP3 inflammasome activation and NF-κB signaling. Oncotarget 2017, 8, 31023–31040. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.; Chandrasaharan, K.; Zhao, X.; Chayaburakul, K.; Ong, W.Y.; Herr, D.R. Metabolism of Docosahexaenoic Acid (DHA) Induces Pyroptosis in BV-2 Microglial Cells. Neuromol. Med. 2018, 20, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Chen, S.; Wu, X.; Zhu, J.; Li, J. Resveratrol Relieves Gouty Arthritis by Promoting Mitophagy to Inhibit Activation of NLRP3 Inflammasomes. J. Inflamm. Res. 2021, 14, 3523–3536. [Google Scholar] [CrossRef] [PubMed]
- Kiasalari, Z.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Azadi-Ahmadabadi, E.; Esmaeil-Jamaat, E.; Fahanik-Babaei, J.; Fakour, M.; Fereidouni, F.; Ghasemi-Tarie, R.; Jalalzade-Ogvar, S.; et al. Ellagic acid ameliorates neuroinflammation and demyelination in experimental autoimmune encephalomyelitis: Involvement of NLRP3 and pyroptosis. J. Chem. Neuroanat. 2021, 111, 101891. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yin, H.; Fan, L.; Zhou, Y.; Tang, X.; Fei, X.; Tang, H.; Peng, J.; Zhang, J.; Xue, Y.; et al. Shionone alleviates NLRP3 inflammasome mediated pyroptosis in interstitial cystitis injury. Int. Immunopharmacol. 2021, 90, 107132. [Google Scholar] [CrossRef]
- Xiao, Y.; Cai, W. Autophagy and Bacterial Infection. Adv. Exp. Med. Biol. 2020, 1207, 413–423. [Google Scholar]
- Ceballos-Olvera, I.; Sahoo, M.; Miller, M.A.; Del Barrio, L.; Re, F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1β is deleterious. PLoS Pathog. 2011, 7, e1002452. [Google Scholar] [CrossRef]
- Liang, Y.D.; Bai, W.J.; Li, C.G.; Xu, L.H.; Wei, H.X.; Pan, H.; He, X.H.; Ouyang, D.Y. Piperine Suppresses Pyroptosis and Interleukin-1β Release upon ATP Triggering and Bacterial Infection. Front. Pharmacol. 2016, 7, 390. [Google Scholar] [CrossRef]
- Pompilio, A.; Scocchi, M.; Mangoni, M.L.; Shirooie, S.; Serio, A.; da Costa, Y.F.G.; Alves, M.S.; Karatoprak, G.Ş.; Süntar, I.; Khan, H.; et al. Bioactive compounds: A goldmine for defining new strategies against pathogenic bacterial biofilms? Crit. Rev. Microbiol. 2023, 49, 117–149. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Q.; Wu, Y.; Zeng, Q.; Song, X.; Guo, Y.; Zhou, P.; Wang, Y.; Liao, X.; Wang, Q.; et al. The Essential Oil of Artemisia argyi H.Lév. and Vaniot Attenuates NLRP3 Inflammasome Activation in THP-1 Cells. Front. Pharmacol. 2021, 12, 712907. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Y.Y.; Zeng, C.Y.; Li, C.G.; Xu, L.H.; Yan, L.; Bai, W.J.; Zha, Q.B.; Ouyang, D.Y.; He, X.H. Scutellarin Suppresses NLRP3 Inflammasome Activation in Macrophages and Protects Mice against Bacterial Sepsis. Front. Pharmacol. 2017, 8, 975. [Google Scholar] [CrossRef]
- Yang, F.; Ye, X.J.; Chen, M.Y.; Li, H.C.; Wang, Y.F.; Zhong, M.Y.; Zhong, C.S.; Zeng, B.; Xu, L.H.; He, X.H.; et al. Inhibition of NLRP3 Inflammasome Activation and Pyroptosis in Macrophages by Taraxasterol Is Associated with Its Regulation on mTOR Signaling. Front. Immunol. 2021, 12, 632606. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alkazmi, L.; Habotta, O.A.; Batiha, G.E. High-mobility group box 1 (HMGB1) in COVID-19: Extrapolation of dangerous liaisons. Inflammopharmacology 2022, 30, 811–820. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Modulation of inflammasome pathways by bacterial and viral pathogens. J. Immunol. 2011, 187, 597–602. [Google Scholar] [CrossRef]
- Guo, M.; An, F.; Yu, H.; Wei, X.; Hong, M.; Lu, Y. Comparative effects of schisandrin A, B, and C on Propionibacterium acnes-induced, NLRP3 inflammasome activation-mediated IL-1β secretion and pyroptosis. Biomed. Pharmacother. 2017, 96, 129–136. [Google Scholar] [CrossRef]
- Li, C.G.; Yan, L.; Jing, Y.Y.; Xu, L.H.; Liang, Y.D.; Wei, H.X.; Hu, B.; Pan, H.; Zha, Q.B.; Ouyang, D.Y.; et al. Berberine augments ATP-induced inflammasome activation in macrophages by enhancing AMPK signaling. Oncotarget 2017, 8, 95–109. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Wegiel, B.; Larsen, R.; Gallo, D.; Chin, B.Y.; Harris, C.; Mannam, P.; Kaczmarek, E.; Lee, P.J.; Zuckerbraun, B.S.; Flavell, R.; et al. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J. Clin. Investig. 2014, 124, 4926–4940. [Google Scholar] [CrossRef]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef]
- Du, T.; Gao, J.; Li, P.; Wang, Y.; Qi, Q.; Liu, X.; Li, J.; Wang, C.; Du, L. Pyroptosis, metabolism, and tumor immune microenvironment. Clin. Transl. Med. 2021, 11, e492. [Google Scholar] [CrossRef]
- Pizato, N.; Luzete, B.C.; Kiffer, L.; Corrêa, L.H.; de Oliveira Santos, I.; Assumpção, J.A.F.; Ito, M.K.; Magalhães, K.G. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci. Rep. 2018, 8, 1952. [Google Scholar]
- Li, Y.; Wang, W.; Li, A.; Huang, W.; Chen, S.; Han, F.; Wang, L. Dihydroartemisinin induces pyroptosis by promoting the AIM2/caspase-3/DFNA5 axis in breast cancer cells. Chem. Biol. Interact. 2021, 340, 109434. [Google Scholar] [CrossRef]
- Wang, J.G.; Jian, W.J.; Li, Y.; Zhang, J. Nobiletin promotes the pyroptosis of breast cancer via regulation of miR-200b/JAZF1 axis. Kaohsiung J. Med. Sci. 2021, 37, 572–582. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, W.; Cheng, C.; Mo, F.; Chen, L.; Peng, G.; Cai, X.; Wang, J.; Yang, S.; Liu, X. Dioscin inhibits the growth of human osteosarcoma by inducing G2/M-phase arrest, apoptosis, and GSDME-dependent cell death in vitro and in vivo. J. Cell. Physiol. 2020, 235, 2911–2924. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, W.; Wang, J. Tanshinone IIA regulates microRNA-125b/foxp3/caspase-1 signaling and inhibits cell viability of nasopharyngeal carcinoma. Mol. Med. Rep. 2021, 23, 371. [Google Scholar] [CrossRef]
- Liang, W.F.; Gong, Y.X.; Li, H.F.; Sun, F.L.; Li, W.L.; Chen, D.Q.; Xie, D.P.; Ren, C.X.; Guo, X.Y.; Wang, Z.Y.; et al. Curcumin Activates ROS Signaling to Promote Pyroptosis in Hepatocellular Carcinoma HepG2 Cells. In Vivo 2021, 35, 249–257. [Google Scholar] [CrossRef]
- Miller, J.M.; Thompson, J.K.; MacPherson, M.B.; Beuschel, S.L.; Westbom, C.M.; Sayan, M.; Shukla, A. Curcumin: A double hit on malignant mesothelioma. Cancer Prev. Res. 2014, 7, 330–340. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, D.; Tang, C.; Lu, Y.; Huang, S.; Peng, C.; Hu, X. Pyroptosis in diabetes and diabetic nephropathy. Clin. Chim. Acta 2022, 531, 188–196. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Hu, X.; Xu, Q.; Zhang, Y.; Liu, H.; Diao, Y.; Zhang, X.; Li, L.; Yu, J.; et al. Epigallocatechin-3-gallate prevents inflammation and diabetes -Induced glucose tolerance through inhibition of NLRP3 inflammasome activation. Int. Immunopharmacol. 2021, 93, 107412. [Google Scholar] [CrossRef]
- Lin, C.F.; Kuo, Y.T.; Chen, T.Y.; Chien, C.T. Quercetin-Rich Guava (Psidium guajava) Juice in Combination with Trehalose Reduces Autophagy, Apoptosis and Pyroptosis Formation in the Kidney and Pancreas of Type II Diabetic Rats. Molecules 2016, 21, 334. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Zhao, Y.; Zhang, Y.; Zhang, X.; Guan, J.; Liu, Y.; Fu, J. Curcumin protects against cognitive impairments in a rat model of chronic cerebral hypoperfusion combined with diabetes mellitus by suppressing neuroinflammation, apoptosis, and pyroptosis. Int. Immunopharmacol. 2021, 93, 107422. [Google Scholar] [CrossRef]
- Han, Y.; Qiu, H.; Pei, X.; Fan, Y.; Tian, H.; Geng, J. Low-dose Sinapic Acid Abates the Pyroptosis of Macrophages by Downregulation of lncRNA-MALAT1 in Rats with Diabetic Atherosclerosis. J. Cardiovasc. Pharmacol. 2018, 71, 104–112. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Y.; Hu, X.; Yang, L.; Chen, X.; Li, Q.; Gu, X. Ginsenoside Rb2 improves insulin resistance by inhibiting adipocyte pyroptosis. Adipocyte 2020, 9, 302–312. [Google Scholar] [CrossRef]
- Fan, G.; Li, Y.; Chen, J.; Zong, Y.; Yang, X. DHA/AA alleviates LPS-induced Kupffer cells pyroptosis via GPR120 interaction with NLRP3 to inhibit inflammasome complexes assembly. Cell Death Dis. 2021, 12, 73. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, F.; Cao, Y.; Zhang, J.; Shi, P.; Sun, X.; Zhang, F.; Tong, L. DHA attenuates hepatic ischemia reperfusion injury by inhibiting pyroptosis and activating PI3K/Akt pathway. Eur. J. Pharmacol. 2018, 835, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, X.; Jin, S.; Ma, Z.; Wang, H.; Ao, N.; Yang, J.; Du, J. Vitamin D ameliorates high-fat-diet-induced hepatic injury via inhibiting pyroptosis and alters gut microbiota in rats. Arch. Biochem. Biophys. 2021, 705, 108894. [Google Scholar] [CrossRef]
- Ma, X.; Ruan, Q.; Ji, X.; Yang, J.; Peng, H. Ligustrazine alleviates cyclophosphamide-induced hepatotoxicity via the inhibition of Txnip/Trx/NF-κB pathway. Life Sci. 2021, 274, 119331. [Google Scholar] [CrossRef]
- Yang, R.; Yu, H.; Chen, J.; Zhu, J.; Song, C.; Zhou, L.; Sun, Y.; Zhang, Q. Limonin Attenuates LPS-Induced Hepatotoxicity by Inhibiting Pyroptosis via NLRP3/Gasdermin D Signaling Pathway. J. Agric. Food Chem. 2021, 69, 982–991. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Zhuang, R.; Zhang, H.; Wang, Y.; Hu, X.; Xu, Y.; Li, J.; Li, Y.; Wang, X.; et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int. J. Biol. Sci. 2021, 17, 1138–1152. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Sun, B.; Jiang, W.; Liao, C.; Dai, X.; Chen, Y.; Chen, J.; Ding, R. Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Free Radic. Biol. Med. 2021, 168, 142–154. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Zhang, D.; Li, H.; Shen, H.; Ding, X.; Chen, G. Andrographolide ameliorates intracerebral hemorrhage induced secondary brain injury by inhibiting neuroinflammation induction. Neuropharmacology 2018, 141, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Ouyang, B.; Ji, X.Y.; Liu, M.F. Gastrodin Alleviates Cerebral Ischaemia/Reperfusion Injury by Inhibiting Pyroptosis by Regulating the lncRNA NEAT1/miR-22-3p Axis. Neurochem. Res. 2021, 46, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; He, Q.; Chen, Y.Z.; Du, Y.F.; Guo, Y.X.; Xu, J.Y.; Qin, L.Q. p-Coumaric acid ameliorates ionizing radiation-induced intestinal injury through modulation of oxidative stress and pyroptosis. Life Sci. 2021, 278, 119546. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Li, X.W.; Zhu, S.Y.; Li, M.Z.; Zhao, Y.; Talukder, M.; Li, Y.H.; Li, J.L. Lycopene Ameliorates Di(2-ethylhexyl) Phthalate-Induced Pyroptosis in Spleen via Suppression of Classic Caspase-1/NLRP3 Pathway. J. Agric. Food Chem. 2021, 69, 1291–1299. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Cai, N.; Xu, T.; He, F. Anti-inflammatory effects of curcumin in acute lung injury: In vivo and in vitro experimental model studies. Int. Immunopharmacol. 2021, 96, 107600. [Google Scholar] [CrossRef]
- Gu, J.; Huang, H.; Liu, C.; Jiang, B.; Li, M.; Liu, L.; Zhang, S. Pinocembrin inhibited cardiomyocyte pyroptosis against doxorubicin-induced cardiac dysfunction via regulating Nrf2/Sirt3 signaling pathway. Int. Immunopharmacol. 2021, 95, 107533. [Google Scholar] [CrossRef]
- Li, J.; Zhao, C.; Zhu, Q.; Wang, Y.; Li, G.; Li, X.; Li, Y.; Wu, N.; Ma, C. Sweroside Protects Against Myocardial Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress and Pyroptosis Partially via Modulation of the Keap1/Nrf2 Axis. Front. Cardiovasc. Med. 2021, 8, 650368. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, H.; Yang, T.; Liu, Q.; Zhou, H. Astragaloside IV attenuate MI-induced myocardial fibrosis and cardiac remodeling by inhibiting ROS/caspase-1/GSDMD signaling pathway. Cell Cycle 2022, 21, 2309–2322. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Sun, H.; Sun, G.; Sun, X. Ginsenoside Rb1 ameliorates cardiotoxicity triggered by aconitine via inhibiting calcium overload and pyroptosis. Phytomedicine 2021, 83, 153468. [Google Scholar] [CrossRef]
- Wu, X.; Ren, G.; Zhou, R.; Ge, J.; Chen, F.H. The role of Ca2+ in acid-sensing ion channel 1a-mediated chondrocyte pyroptosis in rat adjuvant arthritis. Lab. Investig. 2019, 99, 499–513. [Google Scholar] [CrossRef]
- Ding, T.; Zhao, T.; Li, Y.; Liu, Z.; Ding, J.; Ji, B.; Wang, Y.; Guo, Z. Vitexin exerts protective effects against calcium oxalate crystal-induced kidney pyroptosis in vivo and in vitro. Phytomedicine 2021, 86, 153562. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, H.; An, X.; Zhang, J.; Kuang, J.; Hou, J.; Yan, M. Baeckein E suppressed NLRP3 inflammasome activation through inhibiting both the priming and assembly procedure: Implications for gout therapy. Phytomedicine 2021, 84, 153521. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Gao, Y.; Si, Z.; Zhang, H.; Wang, L.; Tian, C.; Yuan, M.; Yang, X.; Li, X.; Shang, H.; et al. Regulatory Mechanisms of the NLRP3 Inflammasome, a Novel Immune-Inflammatory Marker in Cardiovascular Diseases. Front. Immunol. 2019, 10, 1592. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, M.X.; Zhang, L.; Zhang, D.; Li, C.; Li, Y.L. Autophagy, Pyroptosis, and Ferroptosis: New Regulatory Mechanisms for Atherosclerosis. Front. Cell Dev. Biol. 2021, 9, 809955. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, T.; Yi, L.; Zhou, X.; Mi, M. Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. Biofactors 2018, 44, 123–136. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Guan, H.; Zhou, Y.; Liu, L. Schisandrin Inhibits NLRP1 Inflammasome-Mediated Neuronal Pyroptosis in Mouse Models of Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2021, 17, 261–268. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Tong, Y.; Zhang, X.; Zhao, J.; Gao, X.; Yong, J.; Wang, H. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J. Neuroinflamm. 2021, 18, 1. [Google Scholar] [CrossRef]
- Gowda, P.; Patrick, S.; Joshi, S.D.; Kumawat, R.K.; Sen, E. Glycyrrhizin prevents SARS-CoV-2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine 2021, 142, 155496. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Qi, Z.; Armas Diaz, Y.; Cassotta, M.; Grosso, G.; Cianciosi, D.; Zhang, D.; Zou, X.; Quiles, J.L.; Battino, M.; et al. Pyroptosis: A Novel Therapeutic Target for Bioactive Compounds in Human Disease Treatment? A Narrative Review. Nutrients 2025, 17, 461. https://doi.org/10.3390/nu17030461
Yang B, Qi Z, Armas Diaz Y, Cassotta M, Grosso G, Cianciosi D, Zhang D, Zou X, Quiles JL, Battino M, et al. Pyroptosis: A Novel Therapeutic Target for Bioactive Compounds in Human Disease Treatment? A Narrative Review. Nutrients. 2025; 17(3):461. https://doi.org/10.3390/nu17030461
Chicago/Turabian StyleYang, Bei, Zexiu Qi, Yasmany Armas Diaz, Manuela Cassotta, Giuseppe Grosso, Danila Cianciosi, Di Zhang, Xiaobo Zou, José L. Quiles, Maurizio Battino, and et al. 2025. "Pyroptosis: A Novel Therapeutic Target for Bioactive Compounds in Human Disease Treatment? A Narrative Review" Nutrients 17, no. 3: 461. https://doi.org/10.3390/nu17030461
APA StyleYang, B., Qi, Z., Armas Diaz, Y., Cassotta, M., Grosso, G., Cianciosi, D., Zhang, D., Zou, X., Quiles, J. L., Battino, M., & Giampieri, F. (2025). Pyroptosis: A Novel Therapeutic Target for Bioactive Compounds in Human Disease Treatment? A Narrative Review. Nutrients, 17(3), 461. https://doi.org/10.3390/nu17030461














