Enhancing Gut Microbiome and Metabolic Health in Mice Through Administration of Presumptive Probiotic Strain Lactiplantibacillus pentosus PE11
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Cultures
2.2. Animals
2.3. Serum Biochemical Analyses
2.4. Histology
2.5. Gene Expression Analysis
2.6. Analysis of Microbial Communities
2.6.1. DNA Extraction
2.6.2. Amplicon-Based Next-Generation Sequencing
2.6.3. Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Body Weight
3.2. Histological Analysis
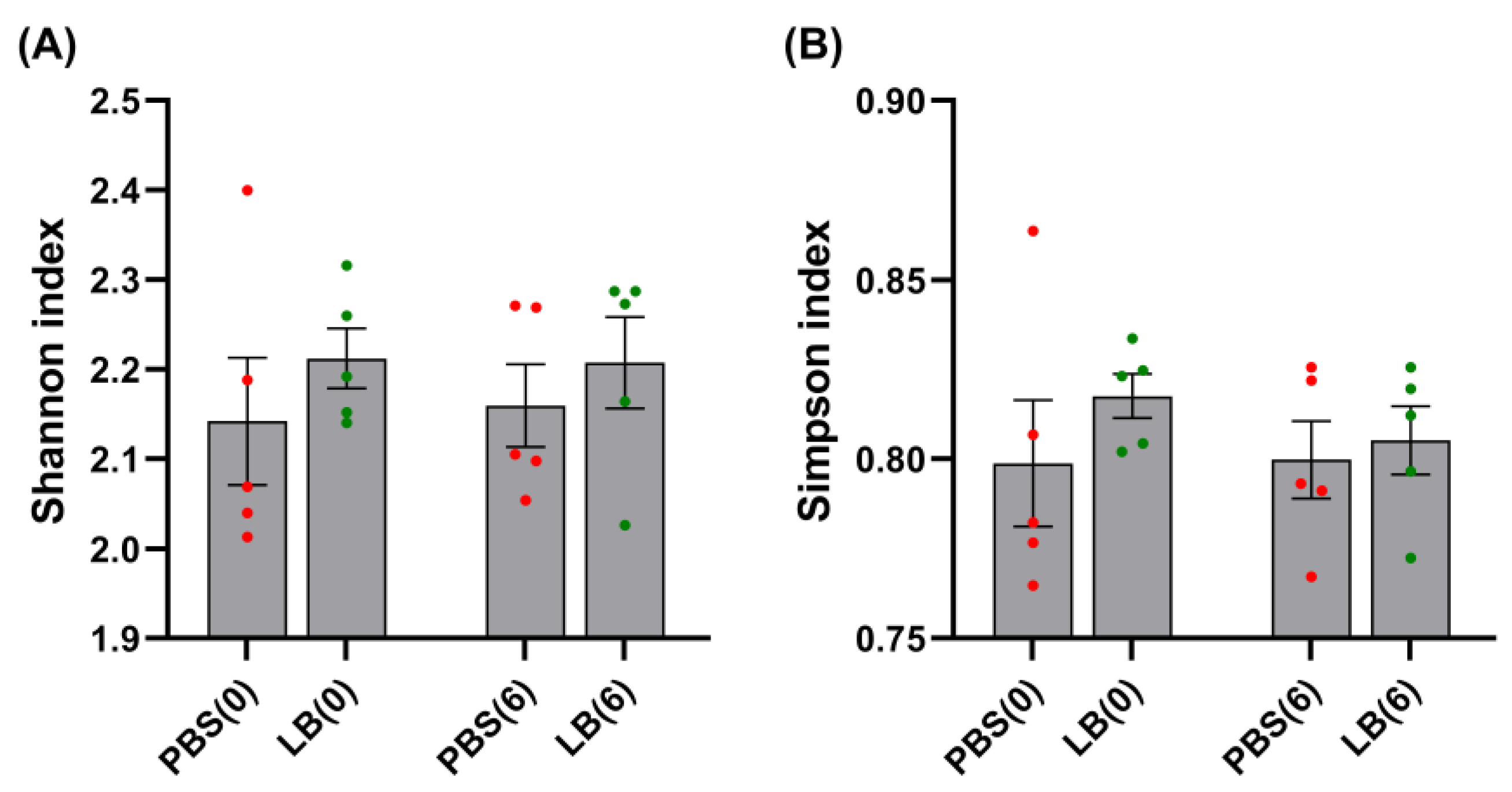
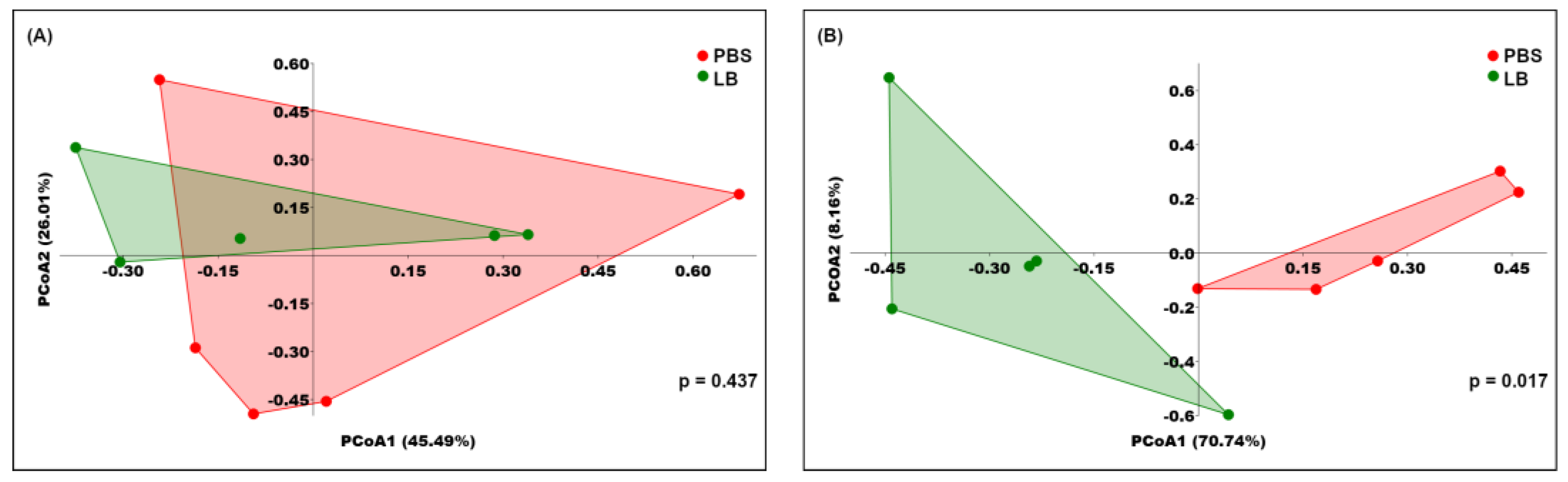
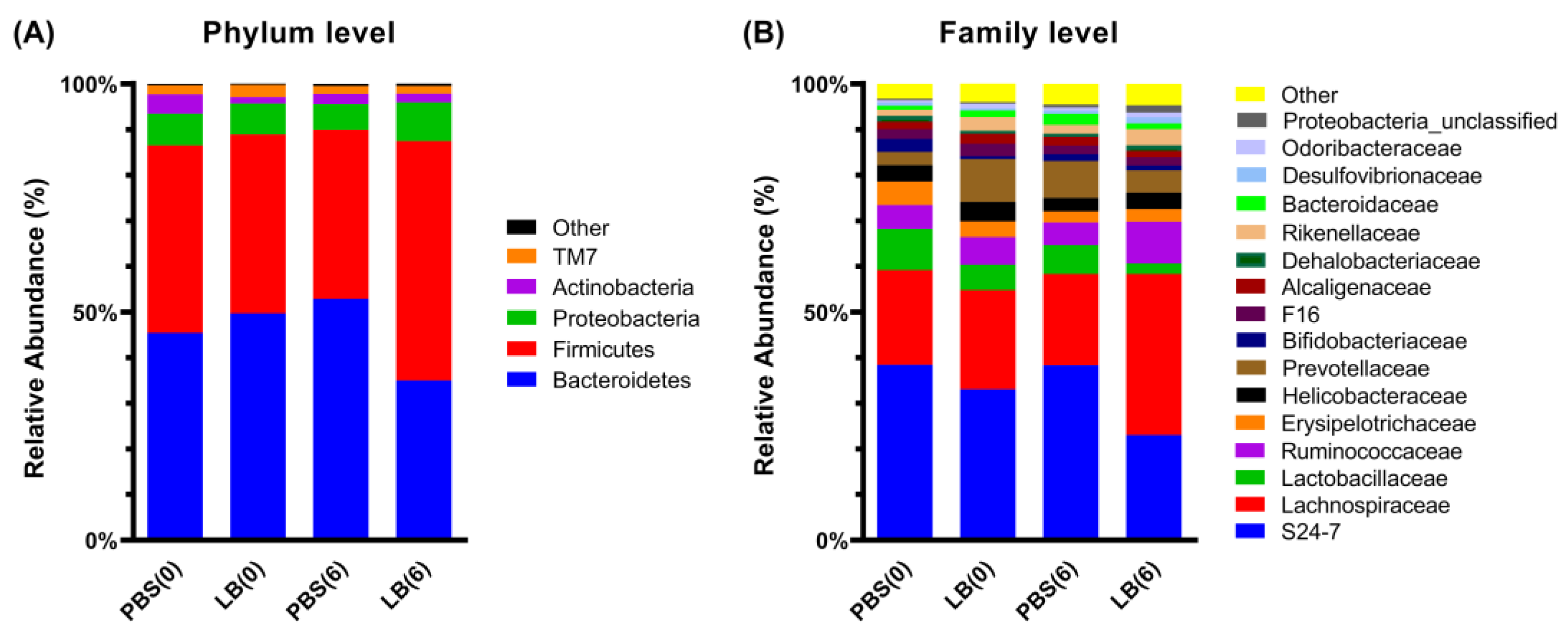
3.3. Analysis of Fecal Microbial Community
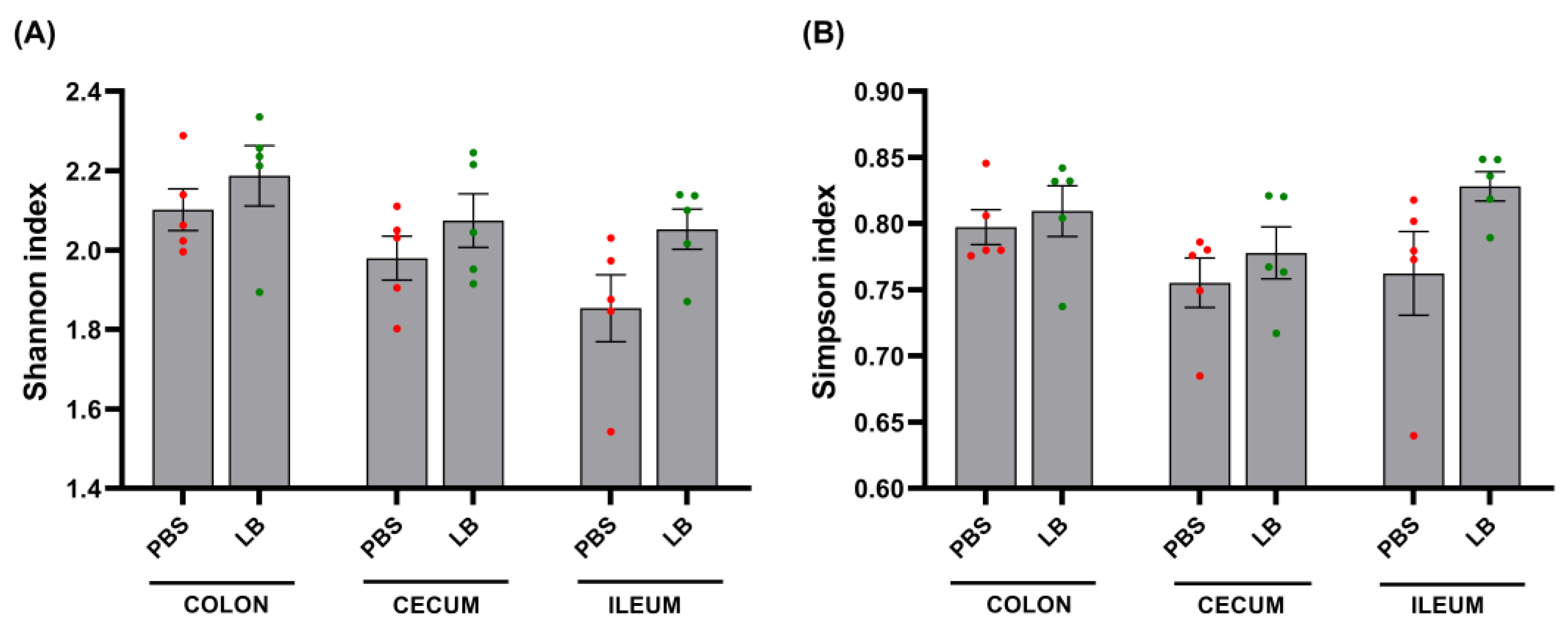
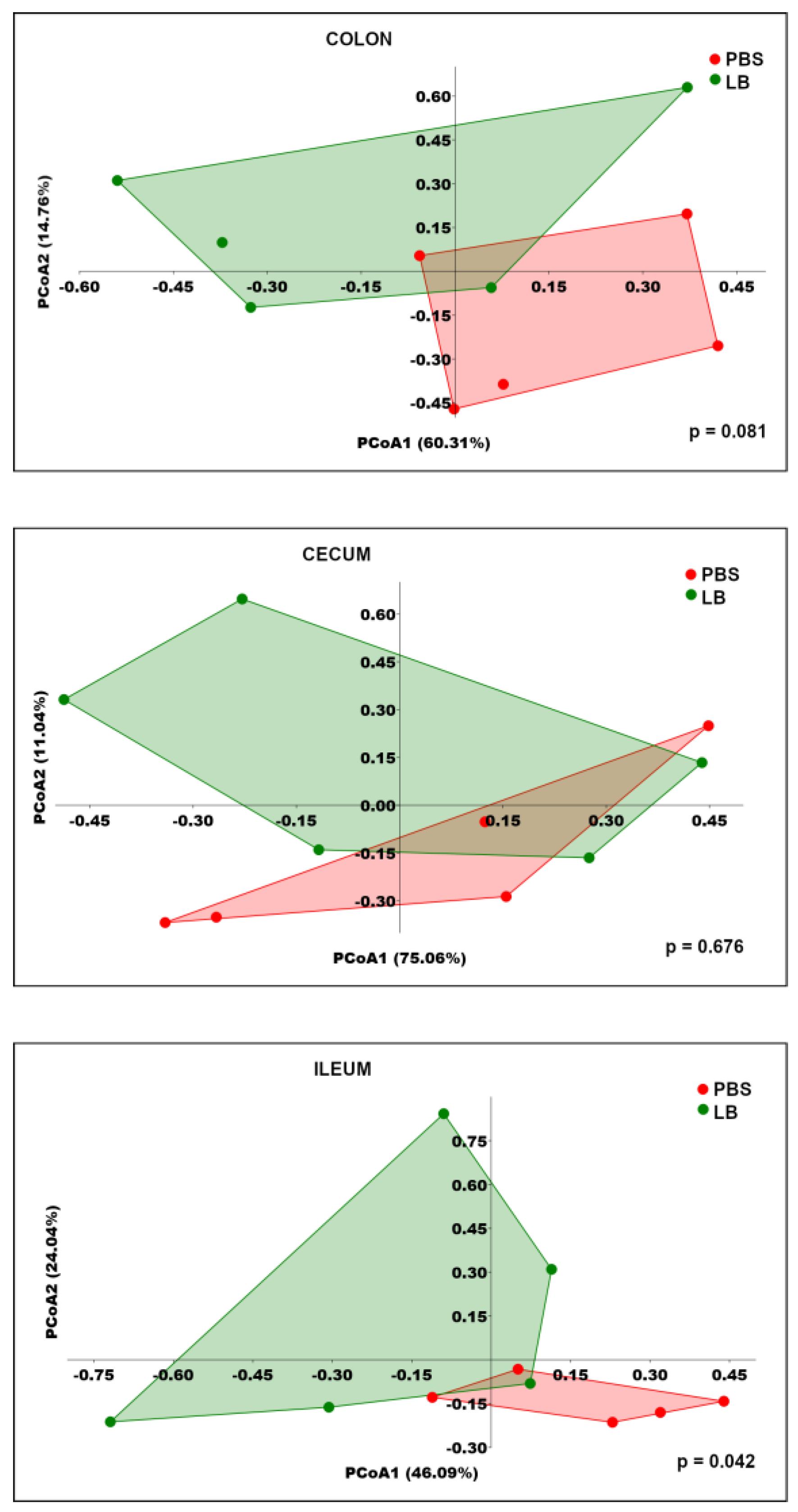
3.4. Analysis of GI Microbial Communities
3.5. Gene Expression Analysis
3.6. Serum Biochemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef]
- Lkhagva, E.; Chung, H.-J.; Hong, J.; Tang, W.H.W.; Lee, S.-I.; Hong, S.-T.; Lee, S. The Regional Diversity of Gut Microbiome along the GI Tract of Male C57BL/6 Mice. BMC Microbiol. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of Gut Microbiota in Infectious and Inflammatory Diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef] [PubMed]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Asha; Sharma, K.K. Gut–Organ Axis: A Microbial Outreach and Networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Rau, S.; Gregg, A.; Yaceczko, S.; Limketkai, B. Prebiotics and Probiotics for Gastrointestinal Disorders. Nutrients 2024, 16, 778. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Merenstein, D.J.; Tancredi, D.J.; Karl, J.P.; Krist, A.H.; Lenoir-Wijnkoop, I.; Reid, G.; Roos, S.; Szajewska, H.; Sanders, M.E. Is There Evidence to Support Probiotic Use for Healthy People? Adv. Nutr. 2024, 15, 100265. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front Med. 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Piquer-Esteban, S.; Ruiz-Ruiz, S.; Arnau, V.; Diaz, W.; Moya, A. Exploring the Universal Healthy Human Gut Microbiota around the World. Comput. Struct. Biotechnol. J. 2021, 20, 421–433. [Google Scholar] [CrossRef]
- Somalou, P.; Ieronymaki, E.; Feidaki, K.; Prapa, I.; Stylianopoulou, E.; Spyridopoulou, K.; Skavdis, G.; Grigoriou, M.E.; Panas, P.; Argiriou, A.; et al. Novel Wild-Type Pediococcus and Lactiplantibacillus Strains as Probiotic Candidates to Manage Obesity-Associated Insulin Resistance. Microorganisms 2024, 12, 231. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F.M. Assumption-Free Analysis of Quantitative Real-Time Polymerase Chain Reaction (PCR) Data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Ganger, M.T.; Dietz, G.D.; Ewing, S.J. A Common Base Method for Analysis of qPCR Data and the Application of Simple Blocking in qPCR Experiments. BMC Bioinf. 2017, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tegopoulos, K.; Tsirka, T.; Stekas, C.; Gerasimidi, E.; Skavdis, G.; Kolovos, P.; Grigoriou, M.E. Spatiotemporal Dynamics of Assyrtiko Grape Microbiota. Microorganisms 2024, 12, 577. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive Statistical, Functional and Integrative Analysis of Microbiome Data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Zaplana, T.; Miele, S.; Tolonen, A.C. Lachnospiraceae Are Emerging Industrial Biocatalysts and Biotherapeutics. Front. Bioeng. Biotechnol. 2024, 11, 1324396. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Z.; Xu, C.; Kan, S.; Chen, D. Disturbances of the Gut Microbiota and Microbiota-Derived Metabolites in Inflammatory Bowel Disease. Nutrients 2022, 14, 5140. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, Q.; Wang, Q.; Chen, Y.; Lv, L.; Zheng, B.; Yan, R.; Jiang, H.; Shen, J.; Wang, S.; et al. The Impact of Dietary Fibers on Clostridioides Difficile Infection in a Mouse Model. Front. Cell Infect. Microbiol. 2022, 12, 1028267. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Donde, H.; Ghare, S.; Stocke, K.; Zhang, J.; Vadhanam, M.; Reddy, S.; Gobejishvili, L.; Chilton, P.; Joshi-Barve, S.; et al. Decrease in Acetyl-CoA Pathway Utilizing Butyrate-Producing Bacteria Is a Key Pathogenic Feature of Alcohol-Induced Functional Gut Microbial Dysbiosis and Development of Liver Disease in Mice. Gut Microbes 2021, 13, 1946367. [Google Scholar] [CrossRef]
- Gao, Y.; Davis, B.; Zhu, W.; Zheng, N.; Meng, D.; Walker, W.A. Epithelial Biology and Secretion: Short-Chain Fatty Acid Butyrate, a Breast Milk Metabolite, Enhances Immature Intestinal Barrier Function Genes in Response to Inflammation in Vitro and in Vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G521. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Taminiau, B.; Rodriguez, C.; Daube, G. Gut Microbiota Composition Associated with Clostridioides Difficile Colonization and Infection. Pathogens 2022, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; He, X.; Chen, H.; He, Q.; Yao, Z.; Li, Y.; Yang, H.; Simpson, S. Oil Tea Improves Glucose and Lipid Levels and Alters Gut Microbiota in Type 2 Diabetic Mice. Nutr. Res. 2018, 57, 67–77. [Google Scholar] [CrossRef]
- Koontanatechanon, A.; Wongphatcharachai, M.; Nonthabenjawan, N.; Jariyahatthakij, P.; Khorporn, T.; Parnsen, W.; Keattisin, B.; Leksrisompong, P.; Srichana, P.; Prasopdee, S.; et al. Effects of Omega-3-Rich Pork Lard on Serum Lipid Profile and Gut Microbiome in C57BL/6NJ Mice. Int. J. Food Sci. 2022, 2022, 9269968. [Google Scholar] [CrossRef] [PubMed]
- Bellés, A.; Abad, I.; Sánchez, L.; Grasa, L. Whey and Buttermilk-Based Formulas Modulate Gut Microbiota in Mice with Antibiotic-Induced Dysbiosis. Mol. Nutr. Food Res. 2023, 67, e2300248. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, W.; Zhu, L.; Zhai, S.; Qin, S.; Du, Z. Effects of Phycocyanin in Modulating the Intestinal Microbiota of Mice. Microbiologyopen 2019, 8, e00825. [Google Scholar] [CrossRef]
- Ahn, J.-S.; Lkhagva, E.; Jung, S.; Kim, H.-J.; Chung, H.-J.; Hong, S.-T. Fecal Microbiome Does Not Represent Whole Gut Microbiome. Cell Microbiol. 2023, 2023, 6868417. [Google Scholar] [CrossRef]
- Gu, S.; Chen, D.; Zhang, J.-N.; Lv, X.; Wang, K.; Duan, L.-P.; Nie, Y.; Wu, X.-L. Bacterial Community Mapping of the Mouse Gastrointestinal Tract. PLoS ONE 2013, 8, e74957. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Cesari, V.; Gargari, G.; Rossi, U.; Biddau, C.; Lecchi, C.; Fiore, W.; Arioli, S.; Toschi, I.; Guglielmetti, S. Probiotics Modulate Mouse Gut Microbiota and Influence Intestinal Immune and Serotonergic Gene Expression in a Site-Specific Fashion. Front. Microbiol. 2021, 12, 706135. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zeng, D.; Yang, M.; Wen, B.; Lai, J.; Zhou, Y.; Sun, H.; Xiong, L.; Wang, J.; Lin, Y.; et al. Probiotic Clostridium Butyricum Improves the Growth Performance, Immune Function, and Gut Microbiota of Weaning Rex Rabbits. Probiotics Antimicro Prot. 2019, 11, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ji, Y.; Jung, H.-Y.; Park, H.; Kang, J.; Choi, S.-H.; Shin, H.; Hyun, C.-K.; Kim, K.-T.; Holzapfel, W.H. Lactobacillus Plantarum HAC01 Regulates Gut Microbiota and Adipose Tissue Accumulation in a Diet-Induced Obesity Murine Model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.S.; Kim, H.N.; Park, H.J.; Lee, J.E.; Yeo, S.Y.; Yang, J.S.; Park, S.Y.; Yoon, H.S.; Cho, G.S.; Franz, C.M.a.P.; et al. Modulation of the Murine Microbiome with a Concomitant Anti-Obesity Effect by Lactobacillus Rhamnosus GG and Lactobacillus Sakei NR28. Benef. Microbes 2012, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef]
- Bertrand, P.P.; Bertrand, R.L. Serotonin Release and Uptake in the Gastrointestinal Tract. Auton. Neurosci. 2010, 153, 47–57. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, C.; Qin, X.; Zhou, B.; Liu, X.; Liu, T.; Xie, R.; Liu, J.; Wang, B.; Cao, H. Saccharomyces Boulardii, a Yeast Probiotic, Inhibits Gut Motility through Upregulating Intestinal Serotonin Transporter and Modulating Gut Microbiota. Pharmacol. Res. 2022, 181, 106291. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Clemente, C.; D’Attoma, B.; Russo, F. Effects of Lactobacillus Rhamnosus GG on the Serotonergic Pathway in a Gliadin-Induced Enteropathy Animal Model. J. Funct. Foods 2020, 72, 104077. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Jin, Y.-J.; Ganesan, R.; Park, H.J.; Min, B.H.; Jeong, M.K.; Yoon, S.J.; Choi, M.R.; Sharma, S.P.; Jang, Y.J.; et al. Multistrain Probiotics Alleviate Diarrhea by Modulating Microbiome-Derived Metabolites and Serotonin Pathway. Probiotics Antimicro. Prot. 2024, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Guo, S.; Ye, D.; Rawat, M.; Ma, T.Y. TNF-α Modulation of Intestinal Tight Junction Permeability Is Mediated by NIK/IKK-α Axis Activation of the Canonical NF-κB Pathway. Am. J. Pathol. 2016, 186, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Archer, A.C.; Muthukumar, S.P.; Halami, P.M. Lactobacillus Fermentum MCC2759 and MCC2760 Alleviate Inflammation and Intestinal Function in High-Fat Diet-Fed and Streptozotocin-Induced Diabetic Rats. Probiotics Antimicrob. Proteins 2021, 13, 1068–1080. [Google Scholar] [CrossRef]
- Liu, Q.; Jian, W.; Wang, L.; Yang, S.; Niu, Y.; Xie, S.; Hayer, K.; Chen, K.; Zhang, Y.; Guo, Y.; et al. Alleviation of DSS-Induced Colitis in Mice by a New-Isolated Lactobacillus Acidophilus C4. Front. Microbiol. 2023, 14, 1137701. [Google Scholar] [CrossRef]
- Karanchi, H.; Muppidi, V.; Wyne, K. Hypertriglyceridemia; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lee, Y.; Siddiqui, W.J. Cholesterol Levels; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lim, P.S.; Loke, C.F.; Ho, Y.W.; Tan, H.Y. Cholesterol Homeostasis Associated with Probiotic Supplementation in Vivo. J. Appl. Microbiol. 2020, 129, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Cui, Y.; Yin, Y.-N.; Zhao, X.; Yang, J.-W.; Wang, Z.-G.; Fu, N.; Tang, Y.; Wang, X.-H.; Liu, X.-W.; et al. Effects of Two Lactobacillus Strains on Lipid Metabolism and Intestinal Microflora in Rats Fed a High-Cholesterol Diet. BMC Complement. Altern. Med. 2011, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, X.; Wang, J.; Wu, F.; Sui, Y.; Yang, L.; Wang, Z. Lactobacillus Plantarum Strains as Potential Probiotic Cultures with Cholesterol-Lowering Activity. J. Dairy. Sci. 2013, 96, 2746–2753. [Google Scholar] [CrossRef]
- Choi, M.J.; Yu, H.; Kim, J.I.; Seo, H.; Kim, J.G.; Kim, S.-K.; Lee, H.S.; Cheon, H.G. Anti-Obesity Effects of Lactiplantibacillus Plantarum SKO-001 in High-Fat Diet-Induced Obese Mice. Eur. J. Nutr. 2023, 62, 1611–1622. [Google Scholar] [CrossRef]
- Damodharan, K.; Lee, Y.S.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.-W. Preliminary Probiotic and Technological Characterization of Pediococcus Pentosaceus Strain KID7 and in Vivo Assessment of Its Cholesterol-Lowering Activity. Front. Microbiol. 2015, 6, 768. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Hemeda, S.A.; Albadrani, G.M.; Fadl, S.E.; Elgendey, F. Ameliorating Effect of Probiotic on Nonalcoholic Fatty Liver Disease and Lipolytic Gene Expression in Rabbits. Sci. Rep. 2023, 13, 6312. [Google Scholar] [CrossRef] [PubMed]
- Melia, S.; Juliyarsi, I.; Kurnia, Y.F.; Aritonang, S.N.; Purwati, E.; Sukma, A.; Fitria, N.; Susmiati, S.; Meinapuri, M.; Pratama, Y.E.; et al. Effect of Fermented Milk Pediococcus Acidilactici BK01 on Cholesterol and Microbiota in Wistar Mice Intestine. J. Adv. Vet. Anim. Res. 2023, 10, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.J.; Kim, C.E.; Jeon, M.-H.; Lee, S.-J.; Lee, J.M.; Lee, E.-W.; Hasan, M.T. Characterization of Pediococcus Acidilactici FS2 Isolated from Korean Traditional Fermented Seafood and Its Blood Cholesterol Reduction Effect in Mice. J. Funct. Foods 2021, 87, 104847. [Google Scholar] [CrossRef]
- Skrypnik, K.; Bogdański, P.; Łoniewski, I.; Reguła, J.; Suliburska, J. Effect of Probiotic Supplementation on Liver Function and Lipid Status in Rats. Acta Sci. Pol. Technol. Aliment. 2018, 17, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, F.; Keshavarz Azizi Raftar, S.; Shahryari, A.; Behrouzi, A.; Yaghoubfar, R.; Lari, A.; Moradi, H.R.; Khatami, S.; Omrani, M.D.; Vaziri, F.; et al. Comparative Effects of Alive and Pasteurized Akkermansia Muciniphila on Normal Diet-Fed Mice. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Moriles, K.E.; Zubair, M.; Azer, S.A. Alanine Aminotransferase (ALT) Test; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y. Akkermansia Muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl. Environ. Microbiol. 2020, 86, e03004-19. [Google Scholar] [CrossRef]
- Luo, M.; Yan, J.; Wu, L.; Wu, J.; Chen, Z.; Jiang, J.; Chen, Z.; He, B. Probiotics Alleviated Nonalcoholic Fatty Liver Disease in High-Fat Diet-Fed Rats via Gut Microbiota/FXR/FGF15 Signaling Pathway. J. Immunol. Res. 2021, 2021, 2264737. [Google Scholar] [CrossRef] [PubMed]
- Al-muzafar, H.M.; Amin, K.A. Probiotic Mixture Improves Fatty Liver Disease by Virtue of Its Action on Lipid Profiles, Leptin, and Inflammatory Biomarkers. BMC Complement. Altern. Med. 2017, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, C.; Zhao, S.; Wang, X.; Wang, J.; Zhang, H.; Wang, Y.; Zhao, G. Probiotic Bifidobacterium Lactis V9 Attenuates Hepatic Steatosis and Inflammation in Rats with Non-Alcoholic Fatty Liver Disease. AMB Express 2020, 10, 101. [Google Scholar] [CrossRef]
- Klempfner, R.; Erez, A.; Sagit, B.-Z.; Goldenberg, I.; Fisman, E.; Kopel, E.; Shlomo, N.; Israel, A.; Tenenbaum, A. Elevated Triglyceride Level Is Independently Associated With Increased All-Cause Mortality in Patients With Established Coronary Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.P.S.; Choi, K.C.; Cockram, C.S.; Ho, C.S.; Chan, M.H.M.; Ozaki, R.; Wong, G.W.K.; Ko, G.T.C.; So, W.Y.; Tong, P.C.Y.; et al. Independent Associations of Alanine Aminotransferase (ALT) Levels with Cardiovascular Risk Factor Clustering in Chinese Adolescents. J. Hepatol. 2008, 49, 115–122. [Google Scholar] [CrossRef] [PubMed]


| Biochemical Parameter | PBS Group Control | LB Group Lb. pentosus PE11 | p-Value |
|---|---|---|---|
| ALT (U/L) | 75 (53–145) | 33 (17–43) | 0.008 |
| ALP (U/L) | 76 (60–96) | 87 (78–93) | 0.286 |
| BUN (mg/dL) | 43 (39–92) | 42 (37–47) | 0.413 |
| CRE (mg/dL) | 0.31 (0.27–0.39) | 0.29 (0.28–0.31) | 0.460 |
| HDL (mg/dL) | 54 (33–66) | 40 (39–57) | 0.278 |
| LDL (mg/dL) | 5 (4–8) | 5 (4–6) | 0.762 |
| TC (mg/dL) | 81 (72–100) | 67 (65–94) | 0.056 |
| TG (mg/dL) | 91 (79–101) | 57 (37–67) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farmakioti, I.; Stylianopoulou, E.; Siskos, N.; Karagianni, E.; Kandylas, D.; Vasileiou, A.R.; Fragkiskatou, F.; Somalou, P.; Tsaroucha, A.; Ypsilantis, P.; et al. Enhancing Gut Microbiome and Metabolic Health in Mice Through Administration of Presumptive Probiotic Strain Lactiplantibacillus pentosus PE11. Nutrients 2025, 17, 442. https://doi.org/10.3390/nu17030442
Farmakioti I, Stylianopoulou E, Siskos N, Karagianni E, Kandylas D, Vasileiou AR, Fragkiskatou F, Somalou P, Tsaroucha A, Ypsilantis P, et al. Enhancing Gut Microbiome and Metabolic Health in Mice Through Administration of Presumptive Probiotic Strain Lactiplantibacillus pentosus PE11. Nutrients. 2025; 17(3):442. https://doi.org/10.3390/nu17030442
Chicago/Turabian StyleFarmakioti, Ioanna, Electra Stylianopoulou, Nikistratos Siskos, Evangelia Karagianni, Dionysios Kandylas, Andreas Rafail Vasileiou, Fragkiski Fragkiskatou, Paraskevi Somalou, Alexandra Tsaroucha, Petros Ypsilantis, and et al. 2025. "Enhancing Gut Microbiome and Metabolic Health in Mice Through Administration of Presumptive Probiotic Strain Lactiplantibacillus pentosus PE11" Nutrients 17, no. 3: 442. https://doi.org/10.3390/nu17030442
APA StyleFarmakioti, I., Stylianopoulou, E., Siskos, N., Karagianni, E., Kandylas, D., Vasileiou, A. R., Fragkiskatou, F., Somalou, P., Tsaroucha, A., Ypsilantis, P., Panas, P., Kourkoutas, Y., Skavdis, G., & Grigoriou, M. E. (2025). Enhancing Gut Microbiome and Metabolic Health in Mice Through Administration of Presumptive Probiotic Strain Lactiplantibacillus pentosus PE11. Nutrients, 17(3), 442. https://doi.org/10.3390/nu17030442







