Abstract
Background/Objectives: Colorectal cancer (CRC) is a leading cause of cancer-related morbidity and mortality worldwide. Sleep duration, diet, and obesity have each been identified as modifiable risk factors linked to CRC. However, their joint effect on CRC incidence is underexplored. This study investigated the association between sleep duration and CRC incidence and explored the joint effects of sleep duration, a pro-inflammatory diet, and obesity on CRC incidence in the Multiethnic Cohort (MEC). Methods: This prospective cohort study analyzed 193,027 participants from Hawaii and California enrolled in the MEC between 1993 and 1996. Sleep duration was self-reported and categorized as short (≤6 h), normal (7–8 h), or long (≥9 h). Diet was self-reported via FFQ and inflammatory potential was assessed using the energy-adjusted Dietary Inflammatory Index (E-DII). CRC cases were identified via cancer registries. Cox proportional hazards models estimated the hazard ratios (HRs) for CRC risk. Results: After 23.8 years of follow-up, 5825 CRC cases were identified. A pro-inflammatory diet combined with suboptimal sleep increased CRC risk by 12% (short sleep duration, aHR: 1.12; 95% CI: 1.02–1.24) and 22% (long sleep duration, aHR: 1.22, 95% CI: 1.05–1.43). Furthermore, long sleep duration was associated with a 10% increase in CRC risk (aHR: 1.10; 95% CI: 1.01–1.22) compared with normal sleep, while short sleep showed no significant association overall. Obese individuals with short or long sleep had significantly higher CRC risk (short sleep aHR: 1.35; 95% CI: 1.21–1.51; long sleep aHR: 1.36; 95% CI: 1.14–1.64) compared with non-obese individuals with corresponding sleep durations. Conclusions: Long sleep duration and a combination of suboptimal sleep duration and a pro-inflammatory dietary pattern or obesity amplifies the risk.
1. Introduction
Colorectal cancer (CRC) ranks as the third most frequently diagnosed cancer globally, representing around 10% of all cancer cases, and is the second leading cause of cancer-related mortality worldwide [1]. The American Cancer Society estimates that in the United States, there will be approximately 153,000 new cases and about 53,000 deaths attributed to CRC in 2024 [2]. The distribution of CRC varies significantly among different subpopulations in the United States, with notable disparities in incidence and mortality rates across racial and ethnic groups. A previous study linking the Multiethnic Cohort (MEC) with the Surveillance, Epidemiology, and End Results (SEER) program revealed that Japanese-American men and women, as well as African-American women, had a higher risk of colorectal cancer compared with White individuals [3]. Consistent with these findings, other studies have reported that African Americans have the highest incidence and mortality rates of CRC compared with other major racial and ethnic groups in the country [4,5].
Advancements in screening and treatment modalities have significantly reduced overall CRC incidence and mortality, particularly among older populations [6]. However, sustaining and enhancing these preventive efforts requires a deeper understanding of lifestyle factors linked to chronic inflammation, given its central role in CRC development [7]. While non-modifiable risk factors such as age, sex, genetic predisposition, and family history remain important determinants of CRC risk, lifestyle factors like diet [8] and sleep [9] stand out as those with strong inflammatory underpinnings. Indeed, there exists a direct and bidirectional relationship between inflammatory cytokines and sleep [10,11].
Sleep is a known physiological necessity. Adults aged 18 to 60 years are advised to get approximately 7 to 8 h of sleep each night on a regular basis to achieve optimal health [12]. A study evaluating 15-year trends in self-reported sleep duration among adults revealed significant racial and ethnic disparities [13]. Such deviations have been associated with adverse outcomes. For example, a meta-analysis by Chen et al. found that insufficient sleep heightened cancer risk among Asians, while excessive sleep was linked to CRC [14]. Similarly, a meta-analysis by Lu et al. found that long sleep duration increased the risk of CRC [15]. An increased risk of other types of cancers has been linked to inadequate sleep duration. For example, a nine-year prospective cohort study in China reported a 27% higher cancer risk, especially for both digestive and respiratory cancers, among those sleeping fewer than six hours nightly [16]. The NIH-AARP Diet and Health Study found that men sleeping only 5–6 h per night faced a significantly higher risk of stomach cancer [17]. These findings highlight the critical role of consistent, optimal sleep duration in reducing cancer risk, though further research is needed to understand the mechanisms behind these associations.
Diet is another factor that is strongly associated with chronic inflammation. There is a consistent association between consumption of pro-inflammatory diets and an elevated risk of cancer [18,19]. Furthermore, pro-inflammatory dietary patterns are linked to obesity [20], which, in turn, is implicated in at least 13 different cancer types [21]. Lifestyle factors that are closely related to inflammation may interact in ways that amplify CRC risk. These interactions are particularly relevant given variations in dietary habits, sleep patterns, and obesity levels across different ethnic groups [13,22,23]. This study examined the association between sleep duration and CRC incidence within the MEC. It also examined the joint effect of sleep duration and diet-associated inflammation, as well as sleep duration and obesity, on CRC incidence, recognizing the interconnected role of these factors in modulating inflammation and influencing cancer development.
2. Materials and Methods
2.1. Study Population
The composition and recruitment criteria of the MEC has been described previously [24]. This prospective cohort study included men and women 45–75 years of age, primarily from five racial and ethnic groups: White/European American, Japanese American, Native Hawaiian, Black/African American, and Latino. The cohort at baseline consisted of 215,251 participants, who were recruited between 1993 and 1996. Most African Americans and Latinos in the cohort were recruited from California, while the majority of Japanese Americans, European Americans, and Native Hawaiians were recruited from Hawaii [24]. Driver’s licenses, voter registration lists, and Medicare documents were used to identify potential participants. An initial survey was administered via a 26-page questionnaire, which included demographic, anthropometric, dietary intake, and other lifestyle factors. The MEC protocol was approved by the institutional review boards at the University of Hawaii and the University of Southern California.
2.2. Inclusion and Exclusion Criteria
Of the 215,251 participants recruited at baseline, 200,551 were eligible for this study based on their identification as members of one of the five major racial and ethnic groups. From this group, we excluded participants who did not provide data on their baseline sleep duration (n = 7118) or who developed CRC within the first 2 years of cohort entry (n = 406). After applying our inclusion and exclusion criteria, we had an analytical sample of 193,027 participants (Figure 1).
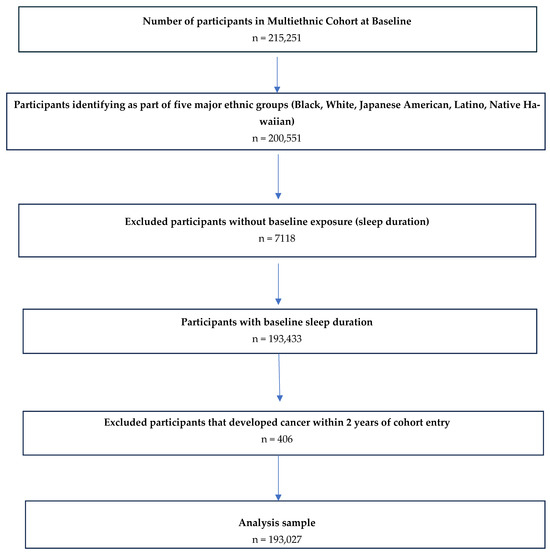
Figure 1.
Inclusion criteria utilized for final study population to examine the association between sleep duration and colorectal cancer incidence in the MEC.
2.3. Exposures
This study utilized three key exposures. First, self-reported sleep duration was analyzed as an independent exposure. Next, a combined exposure was created by jointly considering sleep duration and obesity status. Similarly, a third combined exposure was created by jointly considering sleep duration and diet quality as measured by the energy-adjusted Dietary Inflammatory Index (E-DIITM).
Self-reported sleep duration was obtained from the baseline questionnaire. Each participant was asked the following question: “On the average, during the last year, how many hours in a day did you sleep (include naps)?” Participants were provided the following responses: “5 h or less”, “6 h”, “7 h”, “8 h”, “9 h”, and “10 h or more”. All data were reported in integer hours. In accordance with the 2015 consensus statement issued by the American Academy of Sleep Medicine, we categorized 7 to 8 h as adequate (normal) sleep [25]. Furthermore, we categorized less than or equal to 6 h of sleep as short sleep duration, while 9 h or more was categorized as long sleep duration.
The inflammatory potential of the diet was calculated from self-reports of dietary intake based on the DII and the E-DII scoring algorithms, which were developed to quantify the inflammatory potential of individuals’ diets on a scale from maximally anti-inflammatory (most negative score) to maximally pro-inflammatory (most positive score). The development of the DII has been described in detail elsewhere [26]. Briefly, the DII scoring algorithm was based on a careful review of the literature through which 1943 articles identified 45 food parameters (i.e., macronutrients, including specific categories of fatty acids, carbohydrates, and proteins; micronutrients, including vitamins and minerals; flavonoids; and whole food items, including herbs and spices) as having sufficiently robust literature in relation to six inflammatory biomarkers—i.e., interleukin (IL)-1b, -4, -6, -10; tumor-necrosis factor-alpha (TNFα); and C-reactive protein (CRP). Self-report values for 28 of these food parameters were available from the validated quantitative food frequency questionnaire developed for MEC [24,27,28]. These were translated into z-scores using a global comparative database consisting of data from 11 countries by subtracting from the individual’s self-report value the mean of the global database and then dividing by the standard deviation. These scores were then converted to proportions (i.e., with values ranging from 0 to 1) and centered on 0 by doubling each and subtracting 1. These centered proportions were then multiplied by their respective coefficients (overall food parameter–specific inflammatory effect scores) to obtain DII scores for each food parameter. These were summed to obtain the overall DII score. E-DII scores were calculated using the density approach by calculating DII per 1000 kcal consumption. This employed the same procedure for scoring but relied on an energy-adjusted global comparison database [19,29]. These DII and E-DII scores have a potential range from approximately −9 to +8, i.e., from minimally to maximally pro-inflammatory, respectively. The DII and E-DII are scored similarly and scaled identically, so the scores are comparable across studies [29].
For this study, the following 28 of the 45 food parameters were used to calculate an individual’s overall E-DII/DII score: carbohydrate; protein; total fat; saturated, monounsaturated, and polyunsaturated fats; ω-3 and ω-6 FAs; alcohol; fiber; cholesterol; vitamins A, B-6, B-12, C, D, and E; thiamin; riboflavin; niacin; iron; magnesium; zinc; selenium; folate; β-carotene; isoflavones; and caffeine. Food components not included were eugenol, garlic, ginger, onion, trans fat, turmeric, green tea, black tea, falan-3-ol, flavones, flavonols, flavanones, anthocyanins, pepper, thyme, oregano, and rosemary. The decision to use the E-DII was based on overall model explanatory ability and is consistent with our previous work on CRC in the MEC [19].
Body mass index (BMI) was obtained by dividing the participants’ measured weight in kilograms by their height in square meters (BMI= weight (kg)/height (m2)), based on self-reported weight and height. We then classified them as either obese (BMI ≥ 30 kg/m2) or non-obese (BMI < 30 kg/m2).
2.4. Outcomes
The outcome of interest was invasive CRC as reported to the National Cancer Centers Surveillance, Epidemiology, and End Results (SEER) program. The date of CRC diagnosis was taken as the event time for CRC cases, which were identified from two years after participants’ entry into the cohort up to December 2019. This approach excluded individuals who developed CRC within the first two years of cohort entry to minimize potential reverse causation. Follow-up time for non-cases was up to time of death, withdrawal from the study, or no evidence of CRC by censoring time (December 2019). Mortality from cancer and other causes was determined through linkages to death certificate records in Hawaii and California. The National Death Index was also used periodically to identify deaths among cohort members who had moved to other parts of the United States [24].
2.5. Covariates
The following covariates were included based on their associations with CRC from the literature [30]: age, sex, marital status, ethnicity, education, physical activity (measured as metabolic equivalent of task (MET) for activities performed over a typical 24 h period, relative to a MET value of 1 for sitting), smoking status, family history of colon cancer, previous disease diagnosis (heart disease, stroke, and diabetes) and hormone use (women only).
2.6. Statistical Analysis
Frequencies and percentages for baseline characteristics by sleep duration were computed. Cox proportional hazards models were fitted to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for sleep duration in relation to CRC incidence. Missing data for each variable were categorized as a separate group, ensuring that participants with missing information were retained in the analysis and their contribution to the risk estimates was accounted for. Time (in years) elapsed since study entry was used as the time metric. Kaplan–Meier survival curves were inspected for visual confirmation of proportional hazards across sleep duration groups. Sleep duration of 7 to 8 h was used as the reference group. Analyses were also conducted separately for each racial and ethnic group. A joint analysis was conducted to estimate the effect of sleep duration and obesity status on risk of CRC incidence and that of sleep duration and E-DII score on risk of CRC incidence. We created a dichotomous variable for obesity. Both the median E-DII score and an E-DII score of zero were used for separate joint analyses. The median E-DII score represents the midpoint of dietary inflammatory potential in the study population, providing a population-specific benchmark for categorization. An E-DII score of 0 represents a neutral inflammatory potential, categorizing a dietary pattern as pro-inflammatory (≥0) or anti-inflammatory (<0). To test for interaction, we included a product term of sleep duration and obesity status or sleep duration and E-DII score in the Cox proportional hazards models. The statistical significance of the interaction term was assessed based on the Wald test. A p-value < 0.05 was considered evidence of interaction. All analyses were performed using Stata® version 16.
3. Results
A total of 193,027 participants were included in this study. Participant characteristics were compared based on sleep duration: 34.3% (n = 66,194) reported sleeping ≤6 h, 56.5% (n = 108,986) reported sleeping 7–8 h, and 9.2% (n = 17,847) reported sleeping ≥9 h (Table 1). Ethnic distribution varied by sleep duration, with Japanese Americans comprising the largest proportion among short sleepers (31.5%) and White/European Americans the largest proportion among long sleepers (28.1%). The mean age at cohort entry was 59.8 ± 8.8 years, and the mean age at CRC diagnosis was 74.5 ± 9.0 years. During a total of 3,875,479 person-years of follow-up (median follow-up time of 23.8 years), 5825 (3.0%) incident cases of invasive CRC were identified. Among the CRC cases, 2038 (35%) occurred in short sleepers, 3215 (55%) in normal sleepers, and 572 (10%) in long sleepers. The mean BMI was 26.6 ± 5.1 kg/m2, with approximately 39% of the participants having a BMI in the normal range (18.5–24.9 kg/m2). The mean E-DII score was −1.4 ± 2.0.

Table 1.
Baseline characteristics and colorectal cancer incidence by sleep duration in the MEC (1993–2019).
The association between short sleep duration (≤6 h) and CRC incidence was non-significant in the fully adjusted model (model 2, aHR: 1.04; 95% CI: 0.98–1.10 compared with 7–8 h). Long sleep duration (≥9 h) was associated with a 10% increased risk of CRC incidence (model 2, aHR: 1.10; 95% CI: 1.01–1.22) (Table 2). When stratified by ethnicity, both short and long sleep durations were not significantly associated with CRC incidence, except among Latinos, in whom long sleep duration was associated with a 22% risk of CRC incidence (aHR: 1.22; 95% CI: 1.01–1.48). There were no statistically significant differences in CRC incidence risk by age, sex, and E-DII quartile. However, a 17% increased risk (aHR: 1.17; 95% CI: 1.02–1.34) was observed among individuals with short sleep duration who were obese in comparison with those who had a normal BMI (Supplementary Table S1).

Table 2.
Association between sleep duration and CRC incidence in the MEC (1993–2019).
Among non-obese individuals, sleep duration was not associated with a risk of CRC incidence, with aHRs of 0.99 (95% CI: 0.93–1.06) for short sleep and 1.09 (95% CI: 0.97–1.22) for long sleep compared with normal sleep (Table 3). However, among obese participants, short sleep duration was associated with a 35% higher risk of CRC incidence (aHR: 1.35; 95% CI: 1.21–1.51), and long sleep duration was similarly associated with an increased risk (aHR: 1.36; 95% CI: 1.14–1.64) compared with non-obese individuals with normal sleep duration. Normal sleep duration in obese participants also showed a modestly increased CRC risk (aHR: 1.16; 95% CI: 1.06–1.29) compared with non-obese. Both short and long sleep durations, when combined with an E-DII score ≥ 0, were associated with an increased risk of CRC incidence. Short sleep duration combined with an E-DII score ≥ 0 was associated with a 12% increased risk of CRC incidence (aHR: 1.12; 95% CI: 1.02–1.24), while long sleep duration was associated with 22% increased risk of CRC incidence (aHR: 1.22; 95% CI: 1.05–1.43), compared with normal sleep duration combined with an E-DII score < 0.

Table 3.
Joint effects of sleep duration/obesity and sleep duration/E-DII score on CRC incidence in the MEC (1993–2019).
4. Discussion
This study examined the association between sleep duration, diet-associated inflammation, obesity, and CRC incidence using a large, ethnically diverse cohort. We found that long sleep duration (≥9 h) compared with normal sleep duration (7–8 h) was associated with an increased risk of CRC incidence after adjusting for confounders. Additionally, the joint effect of suboptimal sleep duration with obesity and diet-associated inflammation revealed a statistically significant amplification in the risk of CRC incidence.
There is consistent evidence from both cohort and case-control studies that longer sleep duration is associated with an increased risk of CRC. For example, in the Women’s Health Initiative (WHI), long sleep duration was associated with a 47% increase in the risk of CRC in postmenopausal women [31]. Furthermore, in the Health Professionals Follow-up Study (HPFS), longer sleep duration was associated with a 35% increased risk of developing CRC [32]. The risk of CRC reported in both the WHI and HPFS studies was higher than the 10% increased risk observed in our study. However, our findings are consistent with a recent meta-analysis of seven cohort studies, which reported an 11% increased risk of CRC associated with longer sleep duration [33].
The mechanism through which long sleep duration is associated with CRC incidence is not well elucidated, but several plausible pathways have been proposed. For instance, disruption of circadian rhythms could impair DNA repair processes and promote tumor genesis [34]. A long sleep duration has been associated with high levels of pro-inflammatory cytokines, which create a pro-tumorigenic environment [35]. Moreover, prolonged sleep could lead to insulin resistance, which has been implicated in CRC development [36]. Additionally, long sleep duration is also associated with depression, which may contribute to residual confounding and could be a relevant factor to consider in future research [37].
In contrast, short sleep duration as a single exposure was not associated with CRC incidence, but its joint effect with obesity revealed a 35% increased risk. Studies have shown that short sleep duration alters appetite-regulating hormones, reducing leptin and increasing ghrelin levels, which disrupts appetite and subsequently may lead to obesity [38,39]. On the other hand, obesity, which is a well-established risk factor for CRC, may promote tumorigenesis through chronic inflammation, insulin resistance, and altered adipokine regulation [40]. When coupled with short sleep duration, these mechanisms may be exacerbated, amplifying the risk of CRC. We observed a similar joint effect among individuals classified as long sleepers who were obese. Limited studies have explored this joint effect. These findings suggest that obesity may act as a modifier, amplifying the adverse effects of both insufficient and excessive sleep durations on CRC incidence.
A previous study of the MEC by Harmon et al. that explored the association between the E-DII and CRC showed that pro-inflammatory diets were associated with an increased risk of CRC incidence [19]. Consumption of pro-inflammatory, energy-dense, often high-fat diets modulate the microbiota and induce alterations in intestinal barrier function that are associated with an increase in low-grade inflammation and insulin resistance [41,42,43,44]. Gut microbiota likely plays a central role in the connection between metaflammation, a chronic low-grade inflammation in metabolically active organs, and CRC [45,46,47]. When jointly considered with suboptimal sleep duration, the combined effects of pro-inflammatory diets and both short and long sleep durations appear to exacerbate the risk. This is likely mediated through mechanisms such as gut microbiota dysbiosis, heightened systemic inflammation, disruption of circadian rhythms, and metabolic dysfunction, which together promote a pro-inflammatory and pro-carcinogenic environment [48,49,50]. These findings emphasize the importance of addressing both dietary quality and sleep health as interconnected factors in CRC prevention.
Our study had several strengths that merit discussion. First, the use of a prospective design with a long follow-up time for a large, ethnically diverse population increases the generalizability of our findings. Second, sleep duration was obtained prior to diagnosis of CRC, which reduced the likelihood of recall bias. Third, cancer diagnoses were obtained from verified registries, which reduced the likelihood of diagnostic misclassification. Fourth, the large number of covariates allowed the adjustment for many known risk factors associated with CRC. Despite these strengths, our study was limited by the self-reported nature of the sleep duration and other covariates (e.g., diet, BMI) and the absence of other essential sleep-related covariates such as sleep quality, snoring, daytime drowsiness, nightshift work, and depression. Previous studies have shown that individuals tend to overreport their self-reported sleep duration [51]. Furthermore, the exposure assessment occurred over two decades ago, and lifestyle patterns may have shifted since then. This temporal gap represents a limitation to our findings. However, given the inherent difficulty in altering deeply ingrained habits, our findings remain relevant today and provide important details on the long-term associations that are likely to persist over time. Additionally, our study was restricted to individuals at least 45 years of age. Therefore, our results are not generalizable to younger populations. Future studies should aim to understand how sleep duration affects cancer risk in populations under 45 years of age. This is especially critical, considering that early-onset CRC has been increasing at an alarming rate over the last few decades [52]. Efforts are currently underway to understand this phenomenon. For example, the Metabolic Dysregulation and Cancer Risk Program (MeDOC), a trans–National Cancer Institute research initiative, is working to advance our understanding of the underlying mechanisms that connect obesity, metabolic dysregulation, and increased cancer risk [53].
Our study highlights the important roles of sleep duration, dietary inflammation, and obesity as risk factors for CRC. Given the global rise in obesity, the widespread consumption of pro-inflammatory diets, and the high prevalence of both short and long sleep durations, these findings emphasize the urgent need for targeted public health strategies to address these interconnected risks [54,55]. Public health initiatives should focus on promoting optimal sleep duration while simultaneously advocating for the consumption of anti-inflammatory diets and supporting strategies to achieve and maintain a healthy weight, integrating these efforts into holistic approaches to reduce CRC risk.
5. Conclusions
We found that long sleep duration (≥9 h) was associated with a 10% increased risk of CRC incidence compared with participants with normal sleep duration (7–8 h). In a joint analysis, short sleep duration (≤6 h) and obesity were associated with a 35% increased risk of CRC incidence, while long sleep duration (≥9 h) and obesity were associated with a 36% increased risk, compared with those with normal sleep duration and no obesity. Furthermore, a combination of habitual short or long sleep duration and the consumption of a pro-inflammatory diet increased the risk of CRC incidence by 12% and 22%, respectively, compared with those with normal sleep duration and an anti-inflammatory diet.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17030370/s1, Table S1: Adjusted analysis of the association between sleep duration and CRC incidence stratified by age, sex, and BMI status.
Author Contributions
Conceptualization, P.T., M.D.W. and J.R.H.; methodology, P.T., L.Z., M.D.W. and J.R.H.; software, P.T., L.Z. and M.D.W.; validation, L.Z., M.D.W. and J.R.H.; formal analysis, P.T. and L.Z.; investigation, P.T., L.Z., M.D.W. and J.R.H.; resources, J.R.H.; data curation, P.T., L.Z., L.L.M., L.R.W., S.-Y.P., C.A.H., M.D.W. and J.R.H.; writing—original draft preparation, P.T.; writing—review and editing, P.T., L.Z., L.L.M., L.R.W., S.-Y.P., C.A.H., M.D.W. and J.R.H.; visualization, P.T. and L.Z.; supervision, L.Z., M.D.W. and J.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
The Multiethnic Cohort was supported by the National Institutes of Health Grant U01CA164973 (L. Le Marchand, L.R. Wilkens, C.A. Haiman). Penias Tembo was supported in part by the Cancer Epidemiology Education in Special Populations (CEESP) program through funding from the National Cancer Institute Grant R25 CA112383 (Amr Soliman-PI). Penias Tembo is a doctoral student at the University of South Carolina whose training is supported by the National Cancer Institute Funded REMEDY Study (U01CA272977-01, which is part of the MeDOC Consortium, and supports James R. Hébert, Contact MPI and Michael D. Wirth, Co-I). All funders played no role in the study design, collection, analysis, interpretation of data, and preparation of this manuscript.
Institutional Review Board Statement
The Multiethnic Cohort Study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Boards at the University of Hawaii (approval no. HSP 9575, 28/03/2024) and University of Southern California (approval no. HS-17-00714, 12/03/2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon formal request from the Multiethnic Cohort. https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs002183.v1.p1 15/01/2025. The questionnaire at baseline can be accessed at: https://www.uhcancercenter.org/for-researchers/mec-questionnaires.
Acknowledgments
We would like to acknowledge the participants of the Multiethnic Cohort for their voluntary participation in the study. We would also like to acknowledge Amr Soliman (asoliman@med.cuny.edu) for supporting Penias Tembo to lead this study as a summer fellow in the Cancer Epidemiology Education in Special Populations (CEESP) program.
Conflicts of Interest
The authors declare no conflicts of interest. However, James R. Hébert wishes to disclose that he owns a controlling interest in Connecting Health Innovations LLC (CHI), a company that has licensed the right to his invention of the Dietary Inflammatory Index (DII®) from the University of South Carolina in order to develop computer and smartphone applications for patient counseling and dietary intervention in clinical settings. This has no bearing on the use of the DII as a research tool.
Abbreviations
The following abbreviations are used in this manuscript
| aHR | Adjusted hazard ratio |
| BMI | Body mass index |
| CIs | Confidence intervals |
| CRC | Colorectal cancer |
| E-DII | Energy-adjusted Dietary Inflammatory Index |
| MEC | Multiethnic cohort |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Key Statistics for Colorectal Cancer. Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html (accessed on 31 December 2024).
- Ollberding, N.J.; Nomura, A.M.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Racial/ethnic differences in colorectal cancer risk: The multiethnic cohort study. Int. J. Cancer 2011, 129, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Kupfer, S.S.; Brim, H.; Carethers, J.M. Racial Disparity in Gastrointestinal Cancer Risk. Gastroenterology 2017, 153, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Augustus, G.J.; Ellis, N.A. Colorectal Cancer Disparity in African Americans: Risk Factors and Carcinogenic Mechanisms. Am. J. Pathol. 2018, 188, 291–303. [Google Scholar] [CrossRef]
- Murphy, C.C.; Sandler, R.S.; Sanoff, H.K.; Yang, Y.C.; Lund, J.L.; Baron, J.A. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2017, 15, 903–909.e6. [Google Scholar] [CrossRef]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef]
- Syed Soffian, S.S.; Mohammed Nawi, A.; Hod, R.; Ja’afar, M.H.; Isa, Z.M.; Chan, H.K.; Hassan, M.R.A. Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer. Nutrients 2022, 14, 1555. [Google Scholar] [CrossRef]
- Kapsimalis, F.; Basta, M.; Varouchakis, G.; Gourgoulianis, K.; Vgontzas, A.; Kryger, M. Cytokines and pathological sleep. Sleep Med. 2008, 9, 603–614. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- Wright, K.P., Jr.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef]
- Chaput, J.P.; Dutil, C.; Featherstone, R.; Ross, R.; Giangregorio, L.; Saunders, T.J.; Janssen, I.; Poitras, V.J.; Kho, M.E.; Ross-White, A.; et al. Sleep duration and health in adults: An overview of systematic reviews. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2020, 45 (Suppl. 2), S218–S231. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, C.; Mahajan, S.; Valero-Elizondo, J.; Massey, D.; Lu, Y.; Roy, B.; Riley, C.; Annapureddy, A.R.; Murugiah, K.; Elumn, J.; et al. Evaluation of Temporal Trends in Racial and Ethnic Disparities in Sleep Duration Among US Adults, 2004–2018. JAMA Netw. Open 2022, 5, e226385. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, F.; Wei, L.; Li, X.; Lyu, Z.; Feng, X.; Wen, Y.; Guo, L.; He, J.; Dai, M.; et al. Sleep duration and the risk of cancer: A systematic review and meta-analysis including dose-response relationship. BMC Cancer 2018, 18, 1149. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, N.; Yin, J.; Shi, Y.; Huang, Z. Association between sleep duration and cancer risk: A meta-analysis of prospective cohort studies. PLoS ONE 2013, 8, e74723. [Google Scholar] [CrossRef]
- Jiang, Y.; Gu, X.; Yang, X.; Sun, A.; Sun, H. Exploring the association between sleep duration and cancer risk in middle-aged and older Chinese adults: Observations from a representative cohort study (2011–2020). BMC Public Health 2024, 24, 1819. [Google Scholar] [CrossRef]
- Gu, F.; Xiao, Q.; Chu, L.W.; Yu, K.; Matthews, C.E.; Hsing, A.W.; Caporaso, N.E. Sleep Duration and Cancer in the NIH-AARP Diet and Health Study Cohort. PLoS ONE 2016, 11, e0161561. [Google Scholar] [CrossRef]
- Cho, Y.A.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Genetic Risk Score, Combined Lifestyle Factors and Risk of Colorectal Cancer. Cancer Res. Treat. 2019, 51, 1033–1040. [Google Scholar] [CrossRef]
- Harmon, B.E.; Wirth, M.D.; Boushey, C.J.; Wilkens, L.R.; Draluck, E.; Shivappa, N.; Steck, S.E.; Hofseth, L.; Haiman, C.A.; Le Marchand, L.; et al. The Dietary Inflammatory Index Is Associated with Colorectal Cancer Risk in the Multiethnic Cohort. J. Nutr. 2017, 147, 430–438. [Google Scholar] [CrossRef]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2022, 23, e13349. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Bennett, G.; O’Hara, C.; Bardon, L.A.; Gibney, E.R. Comparison of Meal Patterns Across Common Racial Groups in the UK and the USA, Examining Associations with Weight Status and Diet Quality: A Secondary Analysis of NDNS and NHANES Datasets. J. Racial Ethn. Health Disparities 2023, 12, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.; Pan, L.; Blanck, H.M. Racial and Ethnic Disparities in Adult Obesity in the United States: CDC’s Tracking to Inform State and Local Action. Prev. Chronic Dis. 2019, 16, E46. [Google Scholar] [CrossRef] [PubMed]
- Kolonel, L.N.; Henderson, B.E.; Hankin, J.H.; Nomura, A.M.; Wilkens, L.R.; Pike, M.C.; Stram, D.O.; Monroe, K.R.; Earle, M.E.; Nagamine, F.S. A multiethnic cohort in Hawaii and Los Angeles: Baseline characteristics. Am. J. Epidemiol. 2000, 151, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Stram, D.O.; Hankin, J.H.; Wilkens, L.R.; Pike, M.C.; Monroe, K.R.; Park, S.; Henderson, B.E.; Nomura, A.M.; Earle, M.E.; Nagamine, F.S.; et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am. J. Epidemiol. 2000, 151, 358–370. [Google Scholar] [CrossRef]
- Sharma, S.; Murphy, S.P.; Wilkens, L.R.; Au, D.; Shen, L.; Kolonel, L.N. Extending a multiethnic food composition table to include standardized food group servings. J. Food Compos. Anal. 2003, 16, 485–495. [Google Scholar] [CrossRef]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Jiao, L.; Duan, Z.; Sangi-Haghpeykar, H.; Hale, L.; White, D.L.; El-Serag, H.B. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br. J. Cancer 2013, 108, 213–221. [Google Scholar] [CrossRef]
- Zhang, X.; Giovannucci, E.L.; Wu, K.; Gao, X.; Hu, F.; Ogino, S.; Schernhammer, E.S.; Fuchs, C.S.; Redline, S.; Willett, W.C.; et al. Associations of self-reported sleep duration and snoring with colorectal cancer risk in men and women. Sleep 2013, 36, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.J.; Lin, C.H.; Zhou, Q.; Wang, W.L.; Qin, T.; Li, X.; Wang, Z.J. Association of sleep duration, sleep apnea, and shift work with risk of colorectal neoplasms: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2022, 13, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Z.; Nice, E.; Huang, C.; Zhang, W.; Tang, Y. Circadian rhythms and cancers: The intrinsic links and therapeutic potentials. J. Hematol. Oncol. 2022, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Zhu, X.; Storfer-Isser, A.; Mehra, R.; Jenny, N.S.; Tracy, R.; Redline, S. Sleep duration and biomarkers of inflammation. Sleep 2009, 32, 200–204. [Google Scholar] [CrossRef]
- Vigneri, P.G.; Tirrò, E.; Pennisi, M.S.; Massimino, M.; Stella, S.; Romano, C.; Manzella, L. The Insulin/IGF System in Colorectal Cancer Development and Resistance to Therapy. Front. Oncol. 2015, 5, 230. [Google Scholar] [CrossRef]
- Dong, L.; Xie, Y.; Zou, X. Association between sleep duration and depression in US adults: A cross-sectional study. J. Affect. Disord. 2022, 296, 183–188. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- van Egmond, L.T.; Meth, E.M.S.; Engström, J.; Ilemosoglou, M.; Keller, J.A.; Vogel, H.; Benedict, C. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: A laboratory study. Obesity 2023, 31, 635–641. [Google Scholar] [CrossRef]
- Rychter, A.M.; Łykowska-Szuber, L.; Zawada, A.; Szymczak-Tomczak, A.; Ratajczak, A.E.; Skoracka, K.; Kolan, M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Why Does Obesity as an Inflammatory Condition Predispose to Colorectal Cancer? J. Clin. Med. 2023, 12, 2451. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Diotallevi, C.; Fava, F.; Gobbetti, M.; Tuohy, K. Healthy dietary patterns to reduce obesity-related metabolic disease: Polyphenol-microbiome interactions unifying health effects across geography. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Brusnic, O.; Onisor, D.; Boicean, A.; Hasegan, A.; Ichim, C.; Guzun, A.; Chicea, R.; Todor, S.B.; Vintila, B.I.; Anderco, P.; et al. Fecal Microbiota Transplantation: Insights into Colon Carcinogenesis and Immune Regulation. J. Clin. Med. 2024, 13, 6578. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef]
- Qu, R.; Zhang, Y.; Ma, Y.; Zhou, X.; Sun, L.; Jiang, C.; Zhang, Z.; Fu, W. Role of the Gut Microbiota and Its Metabolites in Tumorigenesis or Development of Colorectal Cancer. Adv. Sci. 2023, 10, e2205563. [Google Scholar] [CrossRef]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Mutlu, E.; Engen, P.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. Circadian disorganization alters intestinal microbiota. PLoS ONE 2014, 9, e97500. [Google Scholar] [CrossRef]
- Zhao, E.; Tait, C.; Minacapelli, C.D.; Catalano, C.; Rustgi, V.K. Circadian Rhythms, the Gut Microbiome, and Metabolic Disorders. Gastro Hep Adv. 2022, 1, 93–105. [Google Scholar] [CrossRef]
- Tran, L.; Forsyth, C.B.; Bishehsari, F.; Voigt, R.M.; Keshavarzian, A.; Swanson, G.R. Disease Implications of the Circadian Clocks and Microbiota Interface. In Circadian Rhythms in Bacteria and Microbiomes; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Jackson, C.L.; Patel, S.R.; Jackson, W.B., 2nd; Lutsey, P.L.; Redline, S. Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 41, zsy057. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef]
- Lam, T.K.; Daschner, P.; Ishibe, N.; Wali, A.; Hall, K.; Czajkowski, S.; Mahabir, S.; Watson, J.M.; Nebeling, L.; Ross, S.; et al. Metabolic Dysregulation and Cancer Risk Program (MeDOC): A transdisciplinary approach to obesity-associated cancers. J. Natl. Cancer Inst. 2024, 116, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Liu, M.; Wang, Y.; Qin, C.; Liu, J. Global burden of sleep disturbances among older adults and the disparities by geographical regions and pandemic periods. SSM-Popul. Health 2023, 25, 101588. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).