Modulation of Gut Microbes and Hepatic Metabolites by PCP Ameliorates NASH and Fatigue-like Performance in Mice †
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Analysis of Poria cocos polysaccharides
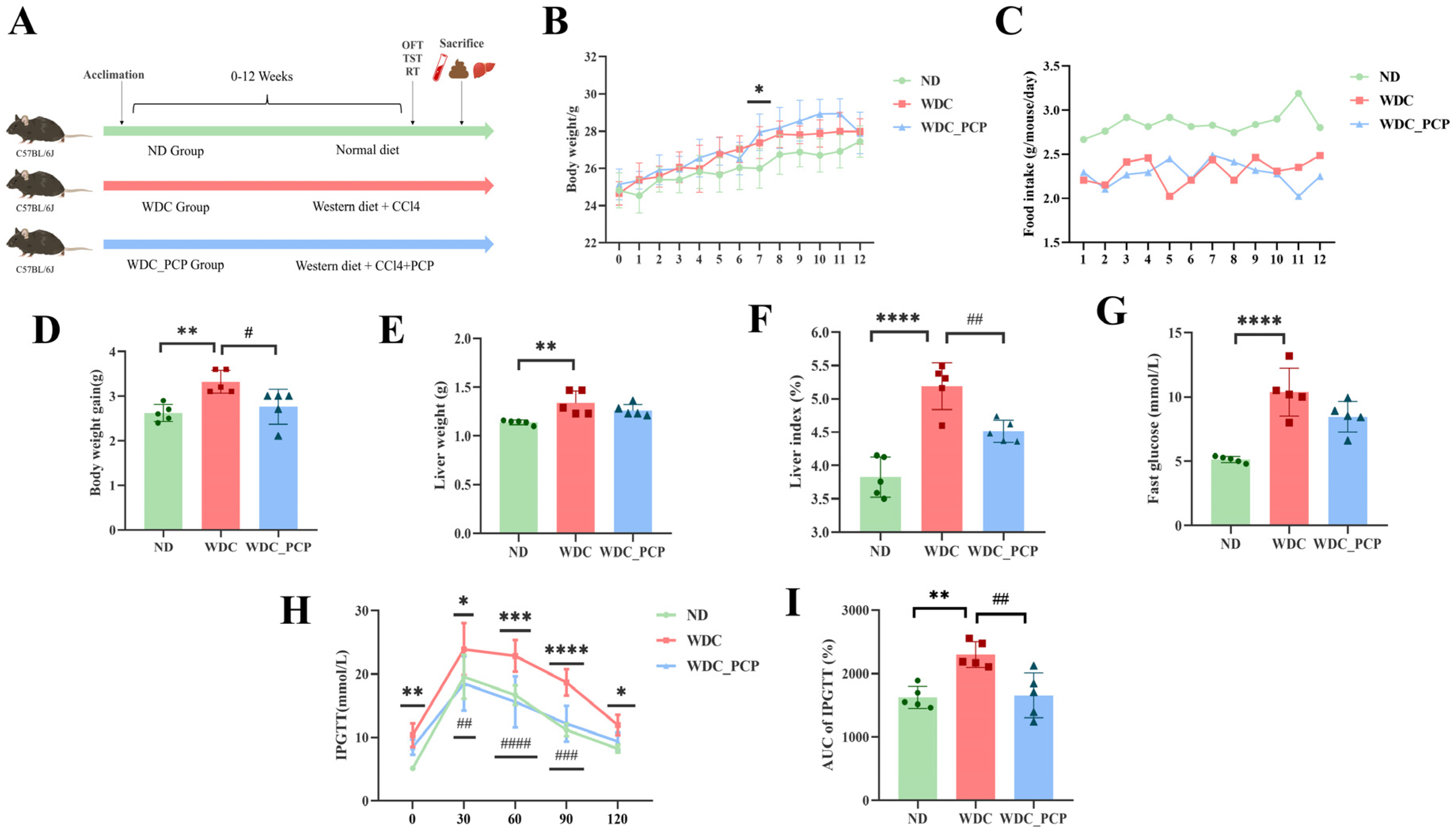
2.2. Animal Care and Experimental Design
2.3. Behavioral Tests
2.3.1. Open Field Test (OFT)
2.3.2. Tail Suspension Test (TST)
2.3.3. Rotarod Test (RT)
2.4. Histopathological Examination
2.5. Detection of Biochemical Indicators
2.6. Real-Time Quantitative Fluorescent Polymerase Chain Reaction (qRT-PCR) Analysis
2.7. High-Throughput 16S Ribosomal RNA Gene Sequencing and Analysis
2.8. Untargeted Metabolomics
2.9. Statistical Analysis
3. Results
3.1. Monosaccharide Composition Characteristics of PCP
3.2. PCP Ameliorates Weight Gain and Glucose Tolerance Abnormalities in NASH Mice
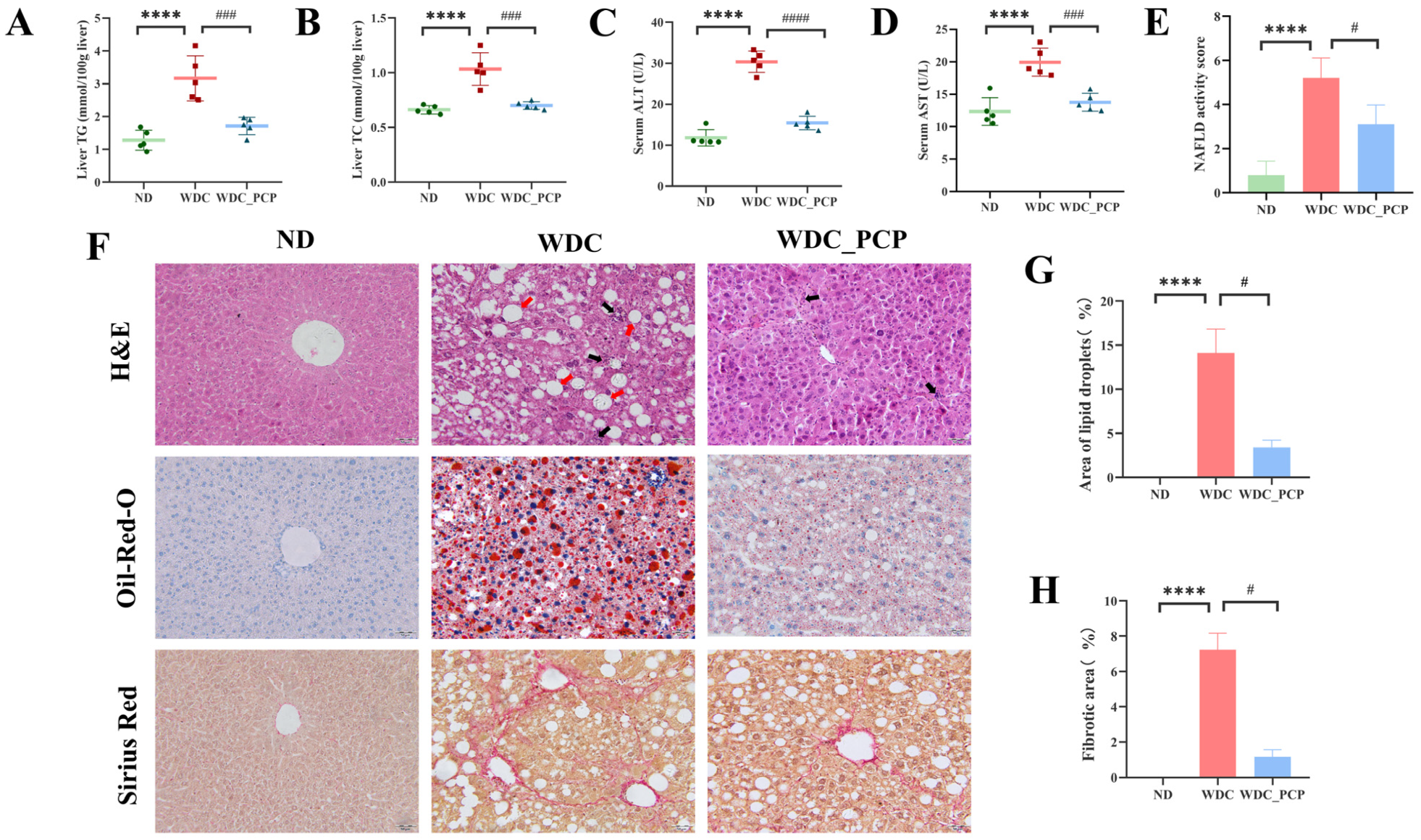
3.3. PCP Alleviates Hepatic Lipid Deposition and Liver Injury in NASH Mice
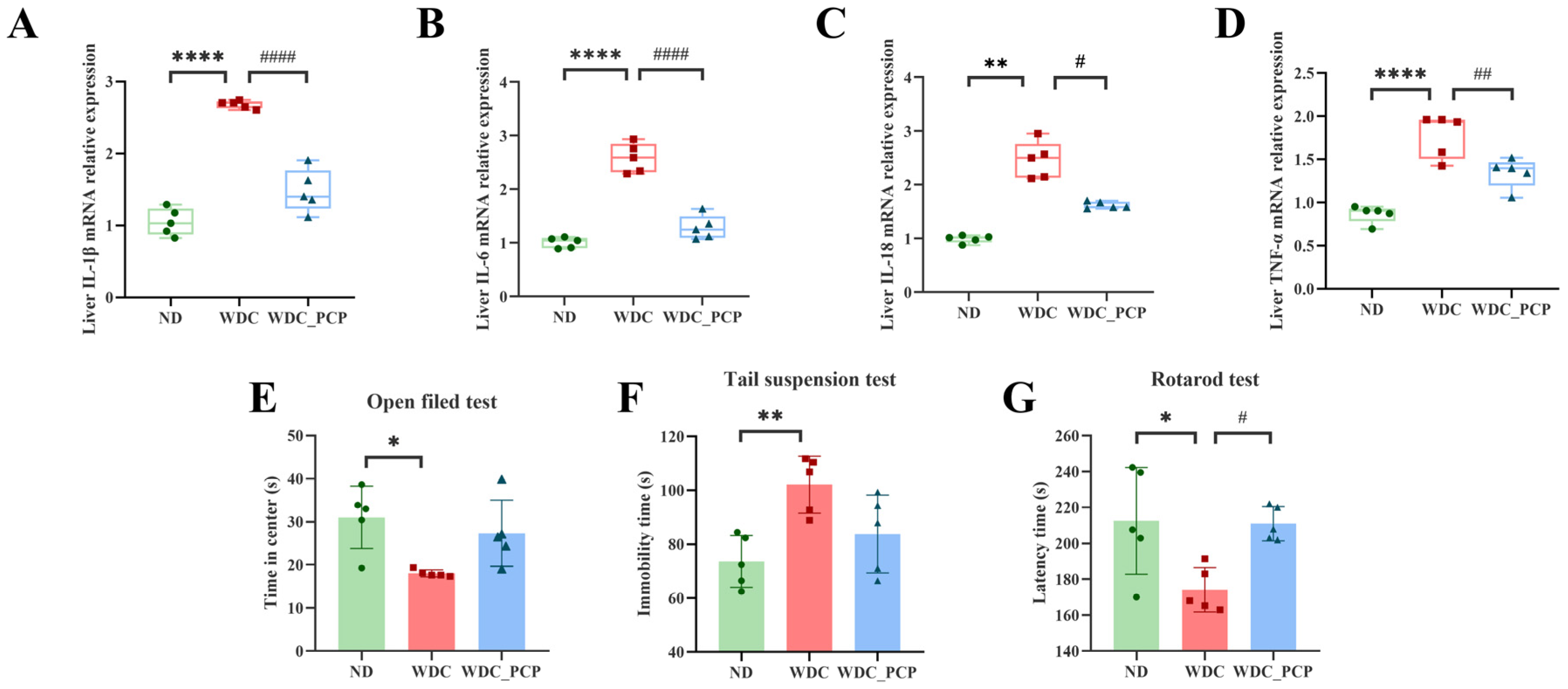
3.4. PCP Reduces the Expression of Pro-Inflammatory Factors in the Liver of NASH Mice
3.5. PCP Alleviates Fatigue-like Performance on Rotarod in NASH Mice
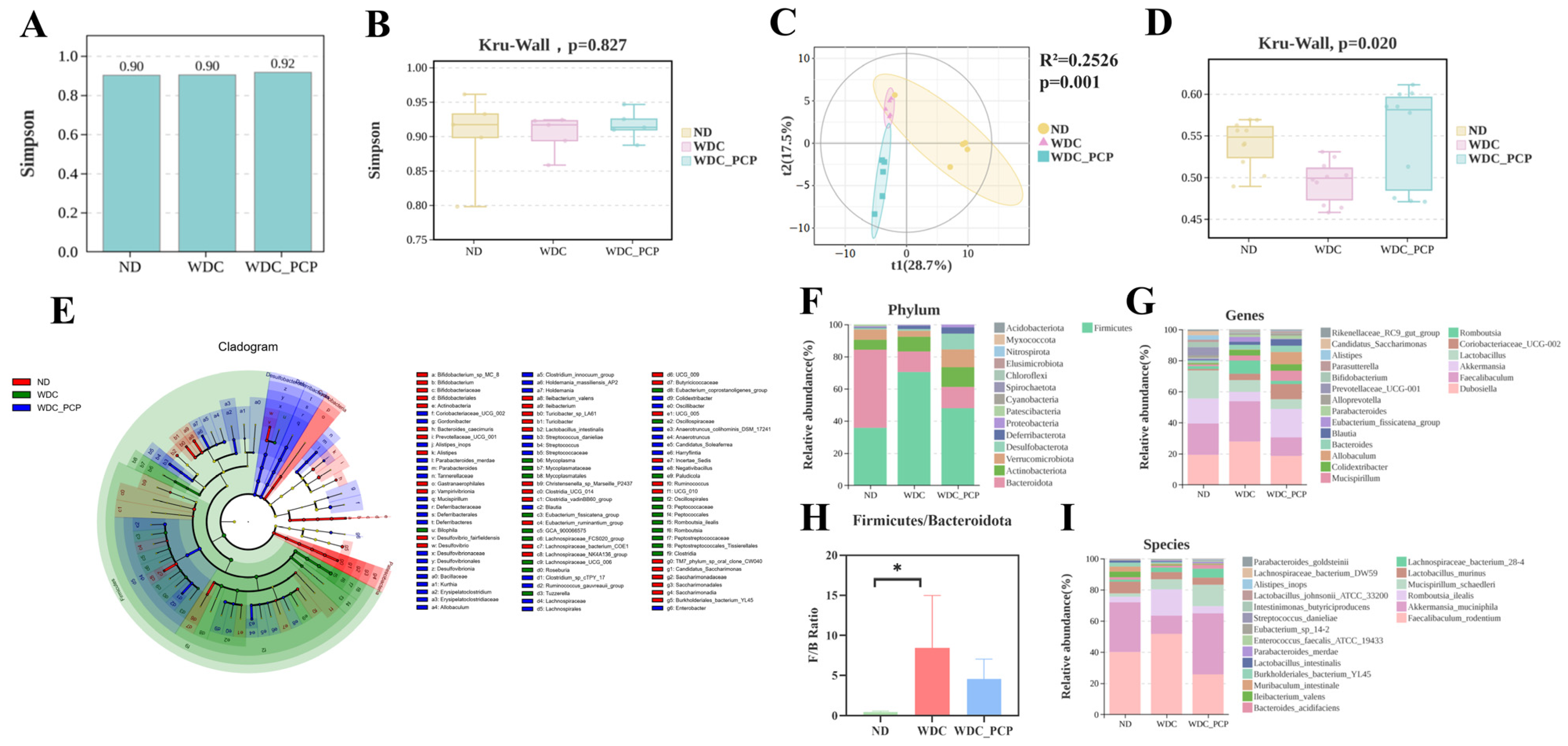
3.6. PCP Improves the Structure and Composition of Gut Microbiota in NASH Mice
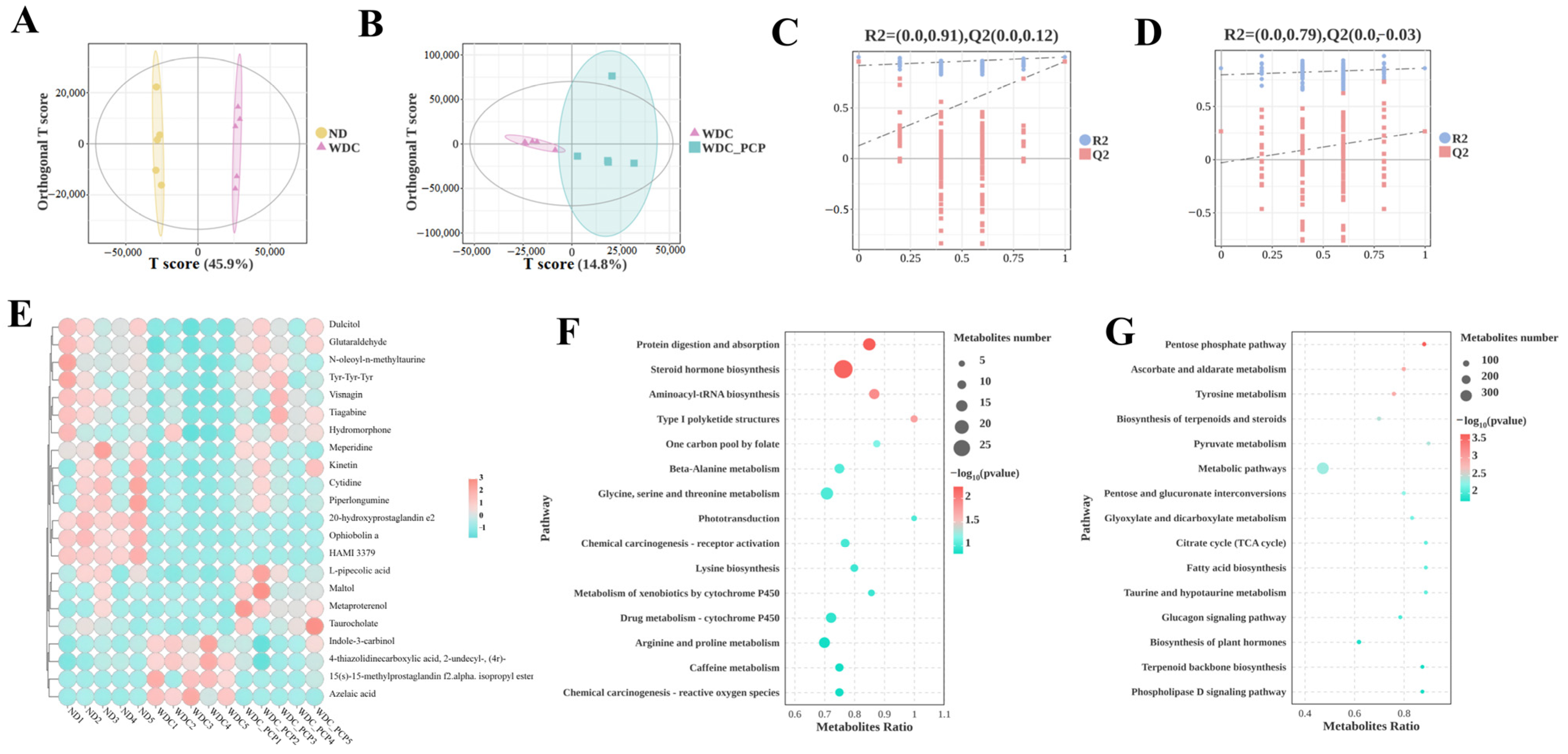
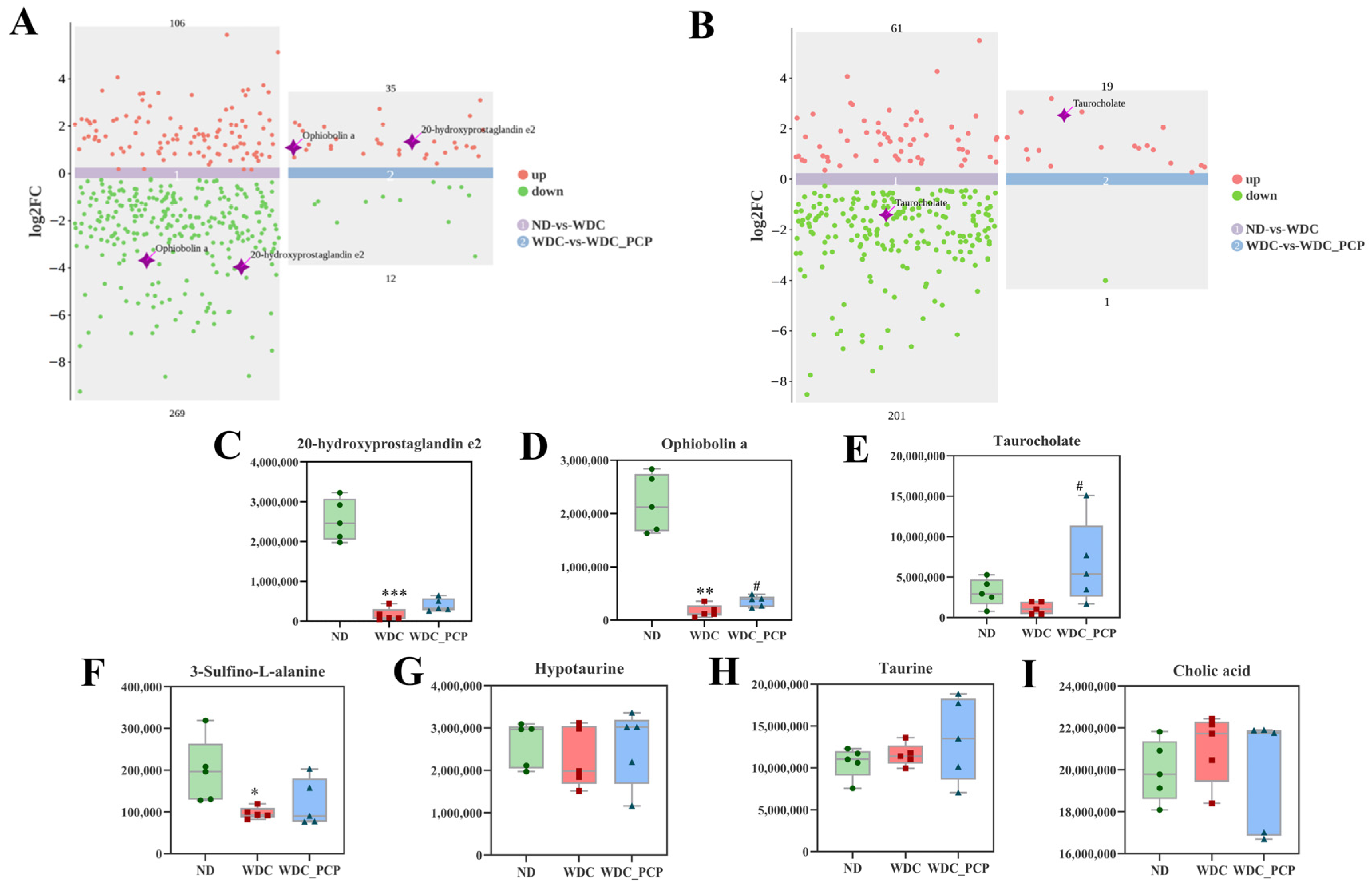
3.7. PCP Improves Hepatic Untargeted Metabolite Profiles in NASH Mice
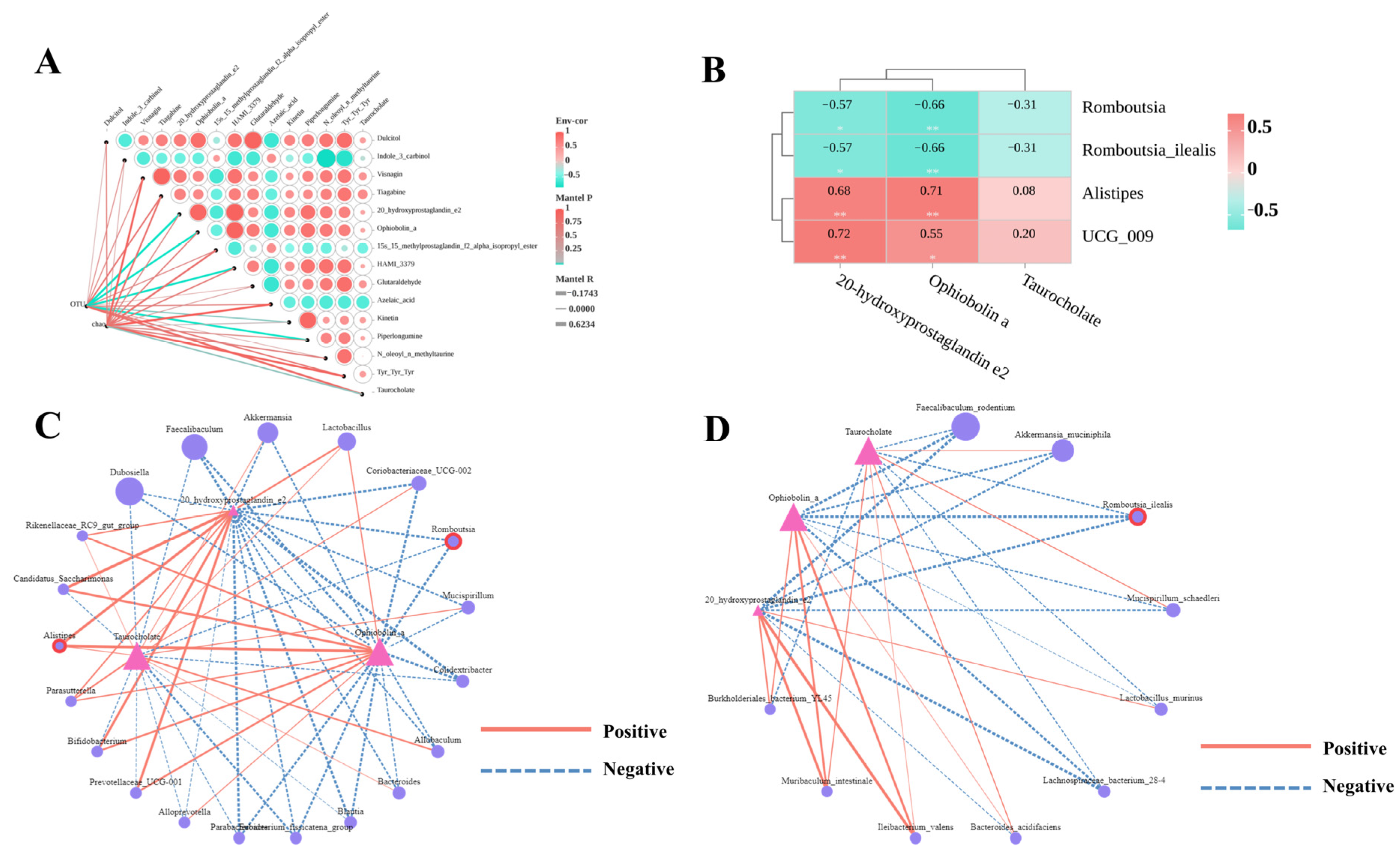
3.8. Association Between PCP-Regulated Key Differential Metabolites and Gut Microbiota
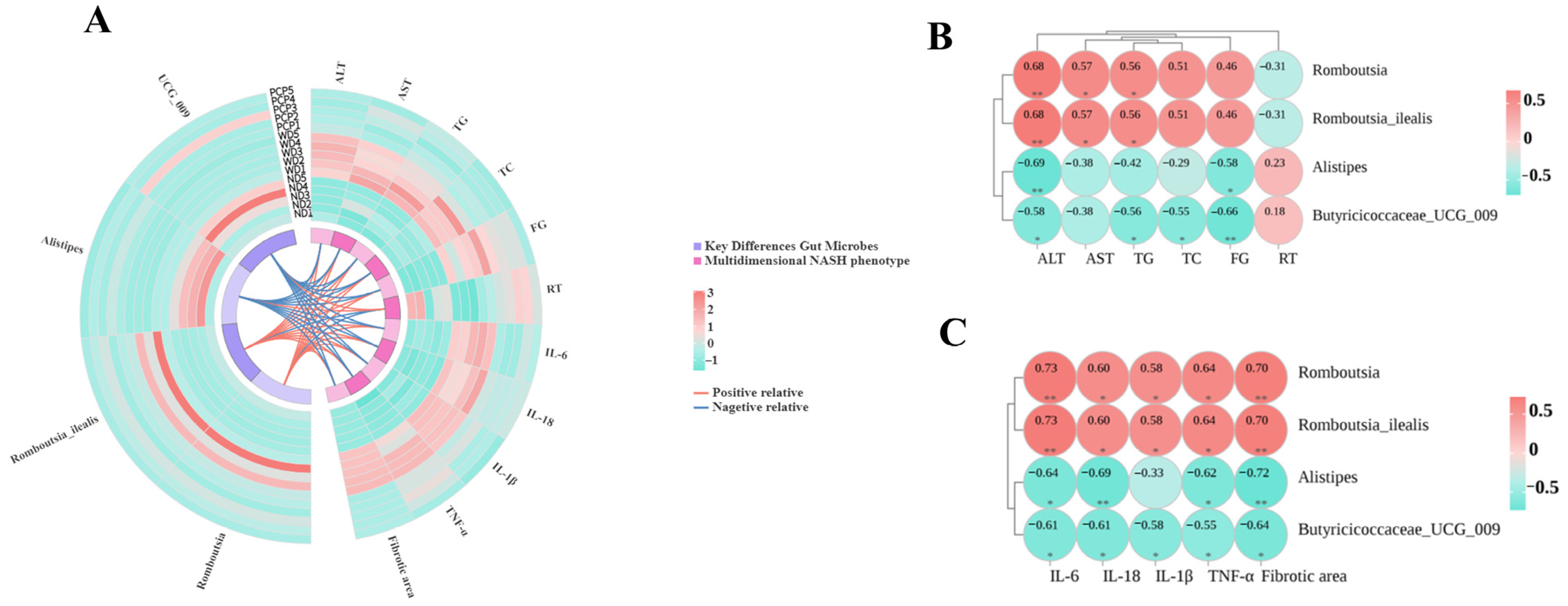
3.9. Association Between Key Differential Gut Microbes and Multidimensional Disease Phenotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Wade, H.; Pan, K.; Zhang, B.; Zheng, W.; Su, Q. Mechanistic role of long non-coding RNAs in the pathogenesis of metabolic dysfunction-associated steatotic liver disease and fibrosis. Egastroenterology 2024, 2, e100115. [Google Scholar] [CrossRef]
- Golabi, P.; Owrangi, S.; Younossi, Z.M. Global perspective on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis–prevalence, clinical impact, economic implications and management strategies. Aliment. Pharmacol. Ther. 2024, 59 (Suppl. S1), S1–S9. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Wang, W.; Qiu, W.; Liu, L.; Ning, A.; Cao, J.; Huang, M.; Zhong, M. Polysaccharides isolated from cordyceps sinensis contribute to the progression of NASH by modifying the gut microbiota in mice fed a high-fat diet. PLoS ONE 2020, 15, e0232972. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 115, 77–82. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Plaza-Diaz, J. Dietary polysaccharides as modulators of the gut microbiota ecosystem: An update on their impact on health. Nutrients 2022, 14, 4116. [Google Scholar] [CrossRef]
- Tong, A.-J.; Hu, R.-K.; Wu, L.-X.; Lv, X.-C.; Li, X.; Zhao, L.-N.; Liu, B. Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters. J. Food Biochem. 2020, 44, e13109. [Google Scholar] [CrossRef]
- Nie, Y.-M.; Zhou, W.-Q.; Niu, T.; Mao, M.-F.; Zhan, Y.-X.; Li, Y.; Wang, K.-P.; Li, M.-X.; Ding, K. Peptidoglycan isolated from the fruit of lycium barbarum alleviates liver fibrosis in mice by regulating the TGF-β/Smad7 signaling and gut microbiota. Acta Pharmacol. Sin. 2025, 46, 1329–1344. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, L. The effect of Poria cocos ethanol extract on the intestinal barrier function and intestinal microbiota in mice with breast cancer. J. Ethnopharmacol. 2021, 266, 113456. [Google Scholar] [CrossRef]
- Deng, L.; Huang, G. Preparation, structure and application of polysaccharides from Poria cocos. RSC Adv. 2024, 14, 31008–31020. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, L.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Immunomodulatory activity of carboxymethyl pachymaran on immunosuppressed mice induced by cyclophosphamide. Molecules 2021, 26, 5733. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Lee, H.H.; Han, M.H.; Kim, G.Y.; Hong, S.H.; Park, C.; Choi, Y.H. Ethanol extract of Poria cocos reduces the production of inflammatory mediators by suppressing the NF-kappaB signaling pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Complement. Altern. Med. 2014, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, S.; Jiang, Y.; Wu, J.; Chen, L.; Ding, Y.; Zhou, Y.; Deng, L.; Chen, X. Poria cocos polysaccharide ameliorated antibiotic-associated diarrhea in mice via regulating the homeostasis of the gut microbiota and intestinal mucosal barrier. Int. J. Mol. Sci. 2023, 24, 1423. [Google Scholar] [CrossRef]
- Liu, W.; Yu, L.; Chen, Q.; Zhang, C.; Wang, L.; Yu, N.; Peng, D.; Ou, J.; Chen, W.; Zhang, Y.; et al. Poria cocos polysaccharides alleviate obesity-related adipose tissue insulin resistance via gut microbiota-derived short-chain fatty acids activation of FGF21/PI3K/AKT signaling. Food Res. Int. 2025, 215, 116671. [Google Scholar] [CrossRef]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.-I.; Hoshida, Y.; et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 2018, 69, 385–395. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, J.; Hong, Y.; Wu, A.; Li, M.; Lin, G.; Ou, W.; Chen, D.; Wang, Y.; Lin, C. IDDF2025-ABS-0168 Altered gut microbiota contributes to intervention effects of PCP on NASH and related fatigue through liver metabolites. Gut 2025, 74 (Suppl. S3), A51–A52. [Google Scholar]
- Zhu, M.; Huang, R.; Wen, P.; Song, Y.; He, B.; Tan, J.; Hao, H.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef]
- Zou, L.; Tian, Y.; Wang, Y.; Chen, D.; Lu, X.; Zeng, Z.; Chen, Z.; Lin, C.; Liang, Y. High-cholesterol diet promotes depression- and anxiety-like behaviors in mice by impact gut microbe and neuroinflammation. J. Affect. Disord. 2023, 327, 425–438. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Liu, T.-H.; He, Y.; Pan, H.-Q.; Zhang, W.-H.; Yin, X.-P.; Tian, X.-L.; Li, B.-M.; Wang, X.-D.; Holmes, A.; et al. Chronic stress remodels synapses in an amygdala circuit-specific manner. Biol. Psychiatry 2019, 85, 189–201. [Google Scholar] [CrossRef]
- Shi, Z.-M.; Jing, J.-J.; Xue, Z.-J.; Chen, W.-J.; Tang, Y.-B.; Chen, D.-J.; Qi, X.-Y.; Huang, L.; Zou, Y.-Q.; Wu, X.-Z.; et al. Stellate ganglion block ameliorated central post-stroke pain with comorbid anxiety and depression through inhibiting HIF-1α/NLRP3 signaling following thalamic hemorrhagic stroke. J. Neuroinflamm. 2023, 20, 82. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Liu, D.; Li, X.; He, L.; Pan, J.; Shen, Q.; Peng, Y. CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice. Brain. Behav. Immun. 2023, 110, 60–79. [Google Scholar] [CrossRef]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflamm. 2022, 19, 41. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Osmon, K.J.; Vyas, M.; Woodley, E.; Thompson, P.; Walia, J.S. Battery of behavioral tests assessing general locomotion, muscular strength, and coordination in mice. J. Vis. Exp. JoVE 2018, 131, 55491. [Google Scholar] [CrossRef]
- Zhang, X.; Jing, S.; Lin, H.; Sun, W.; Jiang, W.; Yu, C.; Sun, J.; Wang, C.; Chen, J.; Li, H. Anti-fatigue effect of anwulignan via the NRF2 and PGC-1α signaling pathway in mice. Food Funct. 2019, 10, 7755–7766. [Google Scholar] [CrossRef]
- Hershey, J.D.; Gifford, J.J.; Zizza, L.J.; Pavlenko, D.A.; Wagner, G.C.; Miller, S. Effects of various cleaning agents on the performance of mice in behavioral assays of anxiety. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2018, 57, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Wu, Y.; Lu, Q. Statistical methods for gene-environment interaction analysis. Wiley Interdiscip. Rev. Comput. Stat. 2024, 16, e1635. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Kim, Y.J.; Lee, W.K.; Choi, B.R.; Oh, S.M.; Lee, Y.S.; Kim, J.K.; Lee, D.Y. Metabolic changes in serum metabolome of beagle dogs fed black ginseng. Metabolites 2020, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014, 1, 19–25. [Google Scholar]
- Zhang, H.; Li, C.; Lai, P.F.H.; Chen, J.; Xie, F.; Xia, Y.; Ai, L. Fractionation, chemical characterization and immunostimulatory activity of β-glucan and galactoglucan from Russula vinosa lindblad. Carbohydr. Polym. 2021, 256, 117559. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef]
- Ni, Y.; Qian, L.; Siliceo, S.L.; Long, X.; Nychas, E.; Liu, Y.; Ismaiah, M.J.; Leung, H.; Zhang, L.; Gao, Q.; et al. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 2023, 35, 1530–1547.e8. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Wang, H.; Wang, D.; Zhu, Y.; Wang, J.; He, Y.; Zheng, Q.; Zhan, X. Microplastic particles alter wheat rhizosphere soil microbial community composition and function. J. Hazard. Mater. 2022, 436, 129176. [Google Scholar] [CrossRef]
- Jin, C.; Wu, S.; Liang, Z.; Zhang, J.; Lei, X.; Bai, H.; Liang, G.; Su, X.; Chen, X.; Wang, P.; et al. Multi-omics reveal mechanisms of high enteral starch diet mediated colonic dysbiosis via microbiome-host interactions in young ruminant. Microbiome 2024, 12, 38. [Google Scholar] [CrossRef]
- Jinato, T.; Chayanupatkul, M.; Dissayabutra, T.; Chutaputti, A.; Tangkijvanich, P.; Chuaypen, N. Litchi-derived polyphenol alleviates liver steatosis and gut dysbiosis in patients with non-alcoholic fatty liver disease: A randomized double-blinded, placebo-controlled study. Nutrients 2022, 14, 2921. [Google Scholar] [CrossRef]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Tacke, F.; Puengel, T.; Loomba, R.; Friedman, S.L. An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J. Hepatol. 2023, 79, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell. Mol. Life Sci. CMLS 2019, 76, 1541–1558. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhang, S.; Li, Y.; Chen, X.; Du, Z.; Shao, C.; Ding, K. The structure elucidation of novel arabinogalactan LRP1-S2 against pancreatic cancer cells growth In Vitro and In Vivo. Carbohydr. Polym. 2021, 267, 118172. [Google Scholar] [CrossRef]
- Dai, X.; Du, Z.; Jin, C.; Tang, B.; Chen, X.; Jing, X.; Shen, Y.; He, F.; Wang, S.; Li, J.; et al. Inulin-like polysaccharide ABWW may impede CCl4 induced hepatic stellate cell activation through mediating the FAK/PI3K/AKT signaling pathway In Vitro & In Vivo. Carbohydr. Polym. 2024, 326, 121637. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Gurung, M.; Li, Z.; García-Jaramillo, M.; Greer, R.; Gaulke, C.; Bauchinger, F.; You, H.; Pederson, J.W.; Vasquez-Perez, S.; et al. Transkingdom interactions between lactobacilli and hepatic mitochondria attenuate western diet-induced diabetes. Nat. Commun. 2021, 12, 101. [Google Scholar] [CrossRef]
- Morissette, A.; André, D.M.; Agrinier, A.-L.; Varin, T.V.; Pilon, G.; Flamand, N.; Houde, V.P.; Marette, A. The metabolic benefits of substituting sucrose for maple syrup are associated with a shift in carbohydrate digestion and gut microbiota composition in high-fat high-sucrose diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E661–E671. [Google Scholar] [CrossRef]
- Tan, Y.-Y.; Yue, S.-R.; Lu, A.-P.; Zhang, L.; Ji, G.; Liu, B.-C.; Wang, R.-R. The improvement of nonalcoholic steatohepatitis by Poria cocos polysaccharides associated with gut microbiota and NF-κB/CCL3/CCR1 axis. Phytomed. Int. J. Phytother. Phytopharm. 2022, 103, 154208. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, Y.; Ge, J.-C.; Wang, L.; Peng, D.-Y.; Yu, N.-J.; Zhang, Y.; Jiang, Y.-H.; Luo, J.-P.; Chen, W.-D. Structural characterization and hepatoprotective activity of a galactoglucan from Poria cocos. Carbohydr. Polym. 2021, 263, 117979. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W.; et al. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice. Int. J. Biol. Macromol. 2023, 249, 125953. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, J.; Zhao, J.; Xiao, X.; Li, W.; Zang, L.; Yu, J.; Liu, H.; Niu, X. Poria cocos polysaccharides reduces high-fat diet-induced arteriosclerosis in ApoE-/- mice by inhibiting inflammation. Phytother. Res. PTR 2021, 35, 2220–2229. [Google Scholar] [CrossRef]
- Wu, K.; Fan, J.; Huang, X.; Wu, X.; Guo, C. Hepatoprotective effects exerted by Poria cocos polysaccharides against acetaminophen-induced liver injury in mice. Int. J. Biol. Macromol. 2018, 114, 137–142. [Google Scholar] [CrossRef]
- Han, H.; Jiang, Y.; Wang, M.; Melaku, M.; Liu, L.; Zhao, Y.; Everaert, N.; Yi, B.; Zhang, H. Intestinal dysbiosis in nonalcoholic fatty liver disease (NAFLD): Focusing on the gut-liver axis. Crit. Rev. Food Sci. Nutr. 2023, 63, 1689–1706. [Google Scholar] [CrossRef]
- Lan, K.; Yang, H.; Zheng, J.; Hu, H.; Zhu, T.; Zou, X.; Hu, B.; Liu, H. Poria cocos oligosaccharides ameliorate dextran sodium sulfate-induced colitis mice by regulating gut microbiota dysbiosis. Food Funct. 2023, 14, 857–873. [Google Scholar] [CrossRef]
- Wei, W.; Wong, C.C.; Jia, Z.; Liu, W.; Liu, C.; Ji, F.; Pan, Y.; Wang, F.; Wang, G.; Zhao, L.; et al. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat. Microbiol. 2023, 8, 1534–1548. [Google Scholar] [CrossRef]
- Gerritsen, J.; Hornung, B.; Renckens, B.; van Hijum, S.A.F.T.; Martins Dos Santos, V.A.P.; Rijkers, G.T.; Schaap, P.J.; de Vos, W.M.; Smidt, H. Genomic and functional analysis of romboutsia ilealis CRIBT reveals adaptation to the small intestine. PeerJ 2017, 5, e3698. [Google Scholar] [CrossRef]
- Zhu, C.-H.; Li, Y.-X.; Xu, Y.-C.; Wang, N.-N.; Yan, Q.-J.; Jiang, Z.-Q. Tamarind xyloglucan oligosaccharides attenuate metabolic disorders via the gut-liver axis in mice with high-fat-diet-induced obesity. Foods 2023, 12, 1382. [Google Scholar] [CrossRef]
- Testerman, T.; Li, Z.; Galuppo, B.; Graf, J.; Santoro, N. Insights from shotgun metagenomics into bacterial species and metabolic pathways associated with NAFLD in obese youth. Hepatol. Commun. 2022, 6, 1962–1974. [Google Scholar] [CrossRef]
- Huang, S.; Pang, D.; Li, X.; You, L.; Zhao, Z.; Cheung, P.C.-K.; Zhang, M.; Liu, D. A sulfated polysaccharide from gracilaria lemaneiformis regulates cholesterol and bile acid metabolism in high-fat diet mice. Food Funct. 2019, 10, 3224–3236. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, F.; Huang, Y.; Liu, B. The positive effects of grifola frondosa heteropolysaccharide on NAFLD and regulation of the gut microbiota. Int. J. Mol. Sci. 2019, 20, 5302. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Ravikrishnan, A.; Wijaya, I.; Png, E.; Chng, K.R.; Ho, E.X.P.; Ng, A.H.Q.; Mohamed Naim, A.N.; Gounot, J.-S.; Guan, S.P.; Hanqing, J.L.; et al. Gut metagenomes of asian octogenarians reveal metabolic potential expansion and distinct microbial species associated with aging phenotypes. Nat. Commun. 2024, 15, 7751. [Google Scholar] [CrossRef]
- Thomann, A.K.; Wüstenberg, T.; Wirbel, J.; Knoedler, L.-L.; Thomann, P.A.; Zeller, G.; Ebert, M.P.; Lis, S.; Reindl, W. Depression and fatigue in active IBD from a microbiome perspective-a bayesian approach to faecal metagenomics. BMC Med. 2022, 20, 366. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Li, M.; Mu, H.; Tan, C.; Wang, M.; Zhang, F.; Sheng, J.; Tian, Y.; Zhao, C. Identification and anti-fatigue activity of walnut protein hydrolysate. Nutrients 2025, 17, 1002. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, X.; Liu, Z.; Zhang, L.; Fang, X.; Sun, J.; Zhang, Z.; Sun, Y. Sodium alginate prevents non-alcoholic fatty liver disease by modulating the gut-liver axis in high-fat diet-fed rats. Nutrients 2022, 14, 4846. [Google Scholar] [CrossRef]
- Pessoa, J.; Belew, G.D.; Barroso, C.; Egas, C.; Jones, J.G. The gut microbiome responds progressively to fat and/or sugar-rich diets and is differentially modified by dietary fat and sugar. Nutrients 2023, 15, 2097. [Google Scholar] [CrossRef]
- Duszka, K. Versatile triad alliance: Bile acid, taurine and microbiota. Cells 2022, 11, 2337. [Google Scholar] [CrossRef]
- Burwen, S.J.; Schmucker, D.L.; Jones, A.L. Subcellular and molecular mechanisms of bile secretion. Int. Rev. Cytol. 1992, 135, 269–313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-L.; Niu, Y.-C.; Deng, H.; Luo, D.-Q. Characterization and phytotoxicity of ophiobolins produced by bipolaris setariae. Mycoscience 2021, 62, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mai, W.; Li, R.; Deng, S.; Li, L.; Zhou, Y.; Qin, Q.; Zhang, Y.; Zhou, X.; Han, M.; et al. Macrophages evoke autophagy of hepatic stellate cells to promote liver fibrosis in NAFLD mice via the PGE2/EP4 pathway. Cell. Mol. Life Sci. CMLS 2022, 79, 303. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]
- Gonzalez, G. Determining the stage of the estrous cycle in female mice by vaginal smear. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot094474. [Google Scholar] [CrossRef]
- Baars, A.; Oosting, A.; Lohuis, M.; Koehorst, M.; El Aidy, S.; Hugenholtz, F.; Smidt, H.; Mischke, M.; Boekschoten, M.V.; Verkade, H.J.; et al. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Sci. Rep. 2018, 8, 13426. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 61–71.e15. [Google Scholar] [CrossRef]
- Klair, J.S.; Yang, J.D.; Abdelmalek, M.F.; Guy, C.D.; Gill, R.M.; Yates, K.; Unalp-Arida, A.; Lavine, J.E.; Clark, J.M.; Diehl, A.M.; et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016, 64, 85–91. [Google Scholar] [CrossRef]
- Park, S.-H.; Oh, M.-R.; Lee, J.; Choi, H.-K.; Hwang, J.-T. Effects of plant-derived dietary supplements on lipid profiles in menopausal women: An updated systematic review and meta-analysis of randomized placebo-controlled trials. Phytother. Res. PTR 2025, 39, 3486–3507. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Haro, C.; Vega-Rojas, A.; Alcala-Diaz, J.F.; Molina-Abril, H.; Leon-Acuña, A.; Lopez-Moreno, J.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; et al. Sex differences in the gut microbiota as potential determinants of gender predisposition to disease. Mol. Nutr. Food Res. 2019, 63, e1800870. [Google Scholar] [CrossRef]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Yang, J.; Wang, Y.; Chen, D.; Wu, A.; Li, M.; Ou, W.; Lin, G.; Lin, C.; Liang, Y. Modulation of Gut Microbes and Hepatic Metabolites by PCP Ameliorates NASH and Fatigue-like Performance in Mice. Nutrients 2025, 17, 3797. https://doi.org/10.3390/nu17233797
Hong Y, Yang J, Wang Y, Chen D, Wu A, Li M, Ou W, Lin G, Lin C, Liang Y. Modulation of Gut Microbes and Hepatic Metabolites by PCP Ameliorates NASH and Fatigue-like Performance in Mice. Nutrients. 2025; 17(23):3797. https://doi.org/10.3390/nu17233797
Chicago/Turabian StyleHong, Yanyan, Jianmei Yang, Yuanfei Wang, Dongliang Chen, Aiping Wu, Minhui Li, Wanyi Ou, Guiru Lin, Chenli Lin, and Yinji Liang. 2025. "Modulation of Gut Microbes and Hepatic Metabolites by PCP Ameliorates NASH and Fatigue-like Performance in Mice" Nutrients 17, no. 23: 3797. https://doi.org/10.3390/nu17233797
APA StyleHong, Y., Yang, J., Wang, Y., Chen, D., Wu, A., Li, M., Ou, W., Lin, G., Lin, C., & Liang, Y. (2025). Modulation of Gut Microbes and Hepatic Metabolites by PCP Ameliorates NASH and Fatigue-like Performance in Mice. Nutrients, 17(23), 3797. https://doi.org/10.3390/nu17233797





