Macronutrient, Micronutrient Supplementation and Monitoring for Patients on GLP-1 Agonists: Can We Learn from Metabolic and Bariatric Surgery?
Abstract
1. Introduction
1.1. Expanding Use of GLP-1 Receptor Agonists in Obesity Management
1.2. Emerging Nutritional Concerns and Gaps in Clinical Guidance
1.3. Physiological Comparisons Between GLP-1 RA and Metabolic and Bariatric Surgery (MBS)
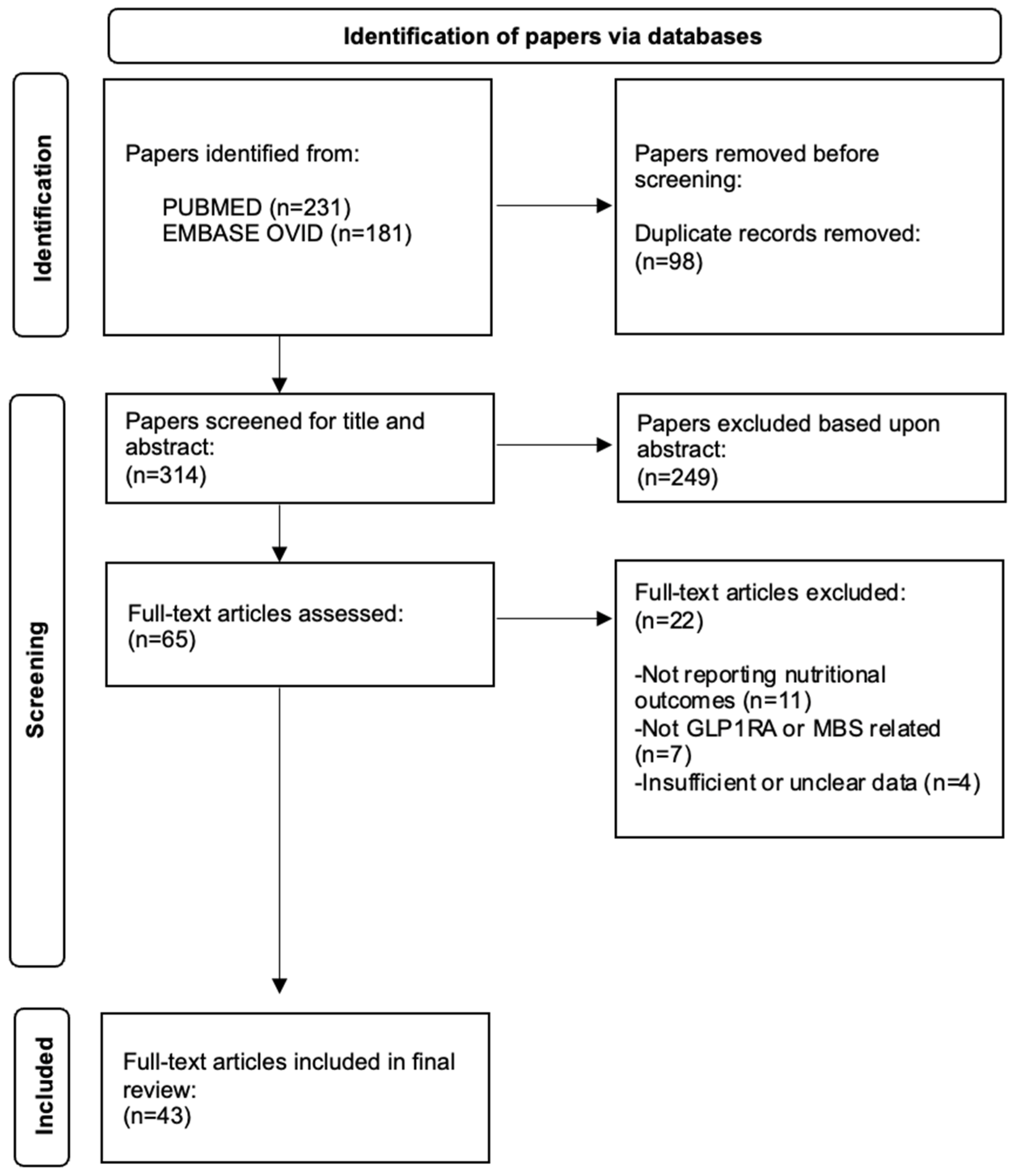
2. Materials and Methods
3. Results
3.1. Impact of GLP-1 Therapy on Energy and Protein Intake
3.2. Vitamin Deficiencies Observed in GLP-1 Therapy
3.3. Mineral Deficiencies Observed in GLP-1 Therapy
3.4. Micronutrient Monitoring Protocols from MBS Guidelines
3.5. Comparative Analysis of Nutritional Risks Between GLP-1 Therapy and MBS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASMBS | American Society for Metabolic and Bariatric Surgery |
| BMI | Body Mass Index |
| BOMSS | British Obesity and Metabolic Specialist Society |
| CVD | Cardiovascular Disease |
| DRI | Dietary Reference Intake |
| EAES | European Association for Endoscopic Surgery |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| GLP-1 | Glucagon-Like Peptide-1 |
| GLP-1RA | Glucagon-Like Peptide-1 Receptor Agonist |
| GIP | Glucose-Dependent Insulinotropic Polypeptide |
| MBS | Metabolic and Bariatric Surgery |
| NV | Nutritional Vigilance |
| RCT | Randomised Controlled Trial |
| RYGB | Roux-en-Y Gastric Bypass |
| T2DM | Type 2 Diabetes Mellitus |
| TDEE | Total Daily Energy Expenditure |
References
- Zinman, B.; Nauck, M.A.; Bosch-Traberg, H.; Frimer-Larsen, H.; Ørsted, D.D.; Buse, J.B.; LEADER Publication Committee on Behalf of the LEADER Trial Investigators. Liraglutide and glycaemic outcomes in the LEADER trial. Diabetes Ther. 2018, 9, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Almandoz, J.P.; Wadden, T.A.; Tewksbury, C.; Apovian, C.M.; Fitch, A.; Ard, J.D.; Li, Z.; Richards, J.; Butsch, W.S.; Jouravskaya, I.; et al. Nutritional considerations with anti-obesity medications. Obesity 2024, 32, 1613–1631. [Google Scholar] [CrossRef]
- Kobylinska, M.; Antosik, K.; Decyk, A.; Kurowska, K. Malnutrition in obesity: Is it possible? Obes. Facts 2022, 15, 19–25. [Google Scholar] [CrossRef]
- O’Kane, M.; Parretti, H.M.; Pinkney, J.; Welbourn, R.; Hughes, C.A.; Mok, J.; Walker, N.; Thomas, D.; Devin, J.; Coulman, K.D.; et al. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery-2020 update. Obes. Rev. 2020, 21, e13087. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient—2016 Update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Busetto, L.; Dicker, D.; Azran, C.; Batterham, R.L.; Farpour-Lambert, N.; Fried, M.; Hjelmesæth, J.; Kinzl, J.; Leitner, D.R.; Makaronidis, J.M.; et al. Practical recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the post-bariatric surgery medical management. Obes. Facts 2017, 10, 597–632. [Google Scholar] [CrossRef] [PubMed]
- Thorell, A.; MacCormick, A.D.; Awad, S.; Reynolds, N.; Roulin, D.; Demartines, N.; Vignaud, M.; Alvarez, A.; Singh, P.M.; Lobo, D.N.; et al. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J. Surg. 2016, 40, 2065–2083. [Google Scholar] [CrossRef]
- Barrett, T.S.; Hafermann, J.O.; Richards, S.; LeJeune, K.; Eid, G.M. Obesity treatment with bariatric surgery versus GLP-1 receptor agonists. JAMA Surg. 2025, 160, e253590. [Google Scholar] [CrossRef]
- Ard, J.; Fitch, A.; Fruh, S.; Herman, L. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide-1 receptor agonists. Adv. Ther. 2021, 38, 2821–2839. [Google Scholar] [CrossRef]
- Evenepoel, C.; Vandermeulen, G.; Luypaerts, A.; Vermeulen, D.; Lannoo, M.; Van der Schueren, B.; Buyse, J.; Verbeke, K. The impact of bariatric surgery on macronutrient malabsorption depends on the type of procedure. Front. Nutr. 2023, 9, 1028881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Felsenreich, D.M.; Artemiou, E.; Steinlechner, K.; Vock, N.; Jedamzik, J.; Eichelter, J.; Gensthaler, L.; Bichler, C.; Sperker, C.; Beckerhinn, P.; et al. Fifteen Years After Sleeve Gastrectomy: Weight Loss, Remission of Associated Medical Problems, Quality of Life, and Conversions to Roux-en-Y Gastric Bypass-Long-Term Follow-Up in a Multicenter Study. Obes. Surg. 2021, 31, 3453–3461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Christensen, S.; Robinson, K.; Thomas, S.; Williams, D.R. Dietary intake by patients taking GLP-1 and dual GIP/GLP-1 receptor agonists: A narrative review and discussion of research needs. Obes. Pillars 2024, 11, 100121, Erratum in Obes. Pillars 2024, 12, 100136. https://doi.org/10.1016/j.obpill.2024.100136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, B.; Milstead, M.; Thomas, O.; McGlasson, T.; Green, L.; Kreider, R.; Jones, R. Investigating nutrient intake during use of glucagon-like peptide-1 receptor agonist: A cross-sectional study. Front. Nutr. 2025, 12, 1566498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silver, H.J.; Olson, D.; Mayfield, D.; Wright, P.; Nian, H.; Mashayekhi, M.; Koethe, J.R.; Niswender, K.D.; Luther, J.M.; Brown, N.J. Effect of the glucagon-like peptide-1 receptor agonist liraglutide, compared to caloric restriction, on appetite, dietary intake, body fat distribution and cardiometabolic biomarkers: A randomized trial in adults with obesity and prediabetes. Diabetes Obes. Metab. 2023, 25, 2340–2350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richardson, K.M.; Schembre, S.M.; Jospe, M.R.; Widmer, A.; Silver, H.J. The influence of the glucagon-like peptide-1 receptor agonist, liraglutide, on dietary patterns and nutrient intakes in patients with obesity and prediabetes: A secondary analysis of a randomized controlled trial. Diabetes Obes. Metab. 2025, 27, 3725–3735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Butsch, W.S.; Sulo, S.; Chang, A.T.; Kim, J.A.; Kerr, K.W.; Williams, D.R.; Hegazi, R.; Panchalingam, T.; Goates, S.; Heymsfield, S.B. Nutritional deficiencies and muscle loss in adults with type 2 diabetes using GLP-1 receptor agonists: A retrospective observational study. Obes. Pillars 2025, 15, 100186. [Google Scholar] [CrossRef]
- Bergmann, N.C.; Lund, A.; Gasbjerg, L.S.; Meessen, E.C.E.; Andersen, M.M.; Bergmann, S.; Hartmann, B.; Holst, J.J.; Jessen, L.; Christensen, M.B.; et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: A randomised, crossover study. Diabetologia 2019, 62, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Al-Najim, W.; Raposo, A.; BinMowyna, M.N.; le Roux, C.W. Unintended Consequences of Obesity Pharmacotherapy: A Nutritional Approach to Ensuring Better Patient Outcomes. Nutrients 2025, 17, 1934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flint, A.; Kapitza, C.; Zdravkovic, M. The once-daily human GLP-1 analogue liraglutide impacts appetite and energy intake in patients with type 2 diabetes after short-term treatment. Diabetes Obes. Metab. 2013, 15, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.; Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation. Am. J. Med. 2025, 138, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Bügel, S. Overfed but undernourished: Recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. Int. J. Obes. 2019, 43, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-Term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pascual, R.W.; Phelan, S.; La Frano, M.R.; Pilolla, K.D.; Griffiths, Z.; Foster, G.D. Diet Quality and Micronutrient Intake Among Long-Term Weight Loss Maintainers. Nutrients 2019, 11, 3046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Zhang, T. Advances in Perioperative Nutritional Management in Metabolic and Bariatric Surgery. Diabetes Metab. Syndr. Obes. 2025, 18, 2191–2202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. 2020, 16, 175–247. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, N.; Antoniou, S.A.; Batterham, R.L.; Busetto, L.; Godoroja, D.; Iossa, A.; Carrano, F.M.; Agresta, F.; Alarçon, I.; Azran, C.; et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: Update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020, 34, 2332–2358. [Google Scholar] [CrossRef]
- Abeles, A.; Kwasnicki, R.M.; Darzi, A. Enhanced recovery after surgery: Current research insights and future direction. World J. Gastrointest. Surg. 2017, 9, 37–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stenberg, E.; Dos Reis Falcão, L.F.; O’Kane, M.; Liem, R.; Pournaras, D.J.; Salminen, P.; Urman, R.D.; Wadhwa, A.; Gustafsson, U.O.; Thorell, A. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: A 2021 Update. World J. Surg. 2022, 46, 729–751, Erratum in World J. Surg. 2022, 46, 752. https://doi.org/10.1007/s00268-022-06459-3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Humięcka, M.; Sawicka, A.; Kędzierska, K.; Binda, A.; Jaworski, P.; Tarnowski, W.; Jankowski, P. Prevalence of Nutrient Deficiencies Following Bariatric Surgery-Long-Term, Prospective Observation. Nutrients 2025, 17, 2599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; Chen, Y.; Yu, X.; Liang, S.; Guan, Y.; Yang, J.; Guan, B. Long-term prevalence of vitamin deficiencies after bariatric surgery: A meta-analysis. Langenbeck’s Arch. Surg. 2024, 409, 226. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Lembo, E.; Saldalamacchia, G.; Avola, C.K.; Angrisani, L.; Capaldo, B. Bariatric surgery and long-term nutritional issues. World J. Diabetes 2017, 8, 464–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bertoni, L.; Valentini, R.; Zattarin, A.; Belligoli, A.; Bettini, S.; Vettor, R.; Foletto, M.; Spinella, P.; Busetto, L. Assessment of Protein Intake in the First Three Months after Sleeve Gastrectomy in Patients with Severe Obesity. Nutrients 2021, 13, 771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guillet, C.; Masgrau, A.; Mishellany-Dutour, A.; Blot, A.; Caille, A.; Lyon, N.; Pereira, B.; Slim, K.; Robert, M.; Disse, E.; et al. 3Bariatric surgery affects obesity-related protein requirements. Clin. Nutr. ESPEN 2020, 40, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, A.; Yang, Y.; Zhao, D.; Ke, J. Effect of Dietary Patterns on Long-Term Weight Maintenance of Patients After Sleeve Gastrectomy. Food Sci. Nutr. 2025, 13, e70834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mozaffarian, D.; Agarwal, M.; Aggarwal, M.; Alexander, L.; Apovian, C.M.; Bindlish, S.; Bonnet, J.; Butsch, W.S.; Christensen, S.; Gianos, E.; et al. Nutritional Priorities to Support GLP-1 Therapy for Obesity: A Joint Advisory from the American College of Lifestyle Medicine, the American Society for Nutrition, the Obesity Medicine Association, and the Obesity Society. Am. J. Lifestyle Med. 2025; epub ahead of print. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fitch, A.; Gigliotti, L.; Bays, H.E. Application of nutrition interventions with GLP-1 based therapies: A narrative review of the challenges and solutions. Obes. Pillars 2025, 16, 100205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Guideline | Micronutrients Monitored | Frequency of Testing | Supplementation Recommendations |
|---|---|---|---|
| ASMBS (USA, 2017) [9] | Iron, B12, folate, vitamin D, calcium | Baseline and annually | Multivitamin ± procedure-specific additions |
| BOMSS (UK, 2020) [8] | Ferritin, folate, B12, 25-hydroxyvitamin D, calcium, zinc, copper | Pre-op; 3, 6, 12 months; annually thereafter | Daily multivitamin + targeted repletion based on procedure |
| ESPEN (2023) [10] | Full micronutrient panel (no fixed list) | Annual | Case-by-case supplementation per deficiency |
| ERAS (2021) [11] | Emphasises early post-operative nutritional recovery | Variable | Encourages multidisciplinary dietetic follow-up rather than fixed micronutrient schedules |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibal, R.; Balamurugan, G.; Langley, J.; Graham, Y.; Mahawar, K. Macronutrient, Micronutrient Supplementation and Monitoring for Patients on GLP-1 Agonists: Can We Learn from Metabolic and Bariatric Surgery? Nutrients 2025, 17, 3659. https://doi.org/10.3390/nu17233659
Sibal R, Balamurugan G, Langley J, Graham Y, Mahawar K. Macronutrient, Micronutrient Supplementation and Monitoring for Patients on GLP-1 Agonists: Can We Learn from Metabolic and Bariatric Surgery? Nutrients. 2025; 17(23):3659. https://doi.org/10.3390/nu17233659
Chicago/Turabian StyleSibal, Rhea, G. Balamurugan, Jasmine Langley, Yitka Graham, and Kamal Mahawar. 2025. "Macronutrient, Micronutrient Supplementation and Monitoring for Patients on GLP-1 Agonists: Can We Learn from Metabolic and Bariatric Surgery?" Nutrients 17, no. 23: 3659. https://doi.org/10.3390/nu17233659
APA StyleSibal, R., Balamurugan, G., Langley, J., Graham, Y., & Mahawar, K. (2025). Macronutrient, Micronutrient Supplementation and Monitoring for Patients on GLP-1 Agonists: Can We Learn from Metabolic and Bariatric Surgery? Nutrients, 17(23), 3659. https://doi.org/10.3390/nu17233659





