Clinical Significance of Phase Angle for Assessing Quality of Life and Prognosis in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Body Composition and Phase Angle
2.2. Statistical Analysis
2.3. Survival Analysis
3. Results
3.1. Baseline Characteristics and Clinical Parameters
3.2. Body Composition Parameters by PA Quartiles
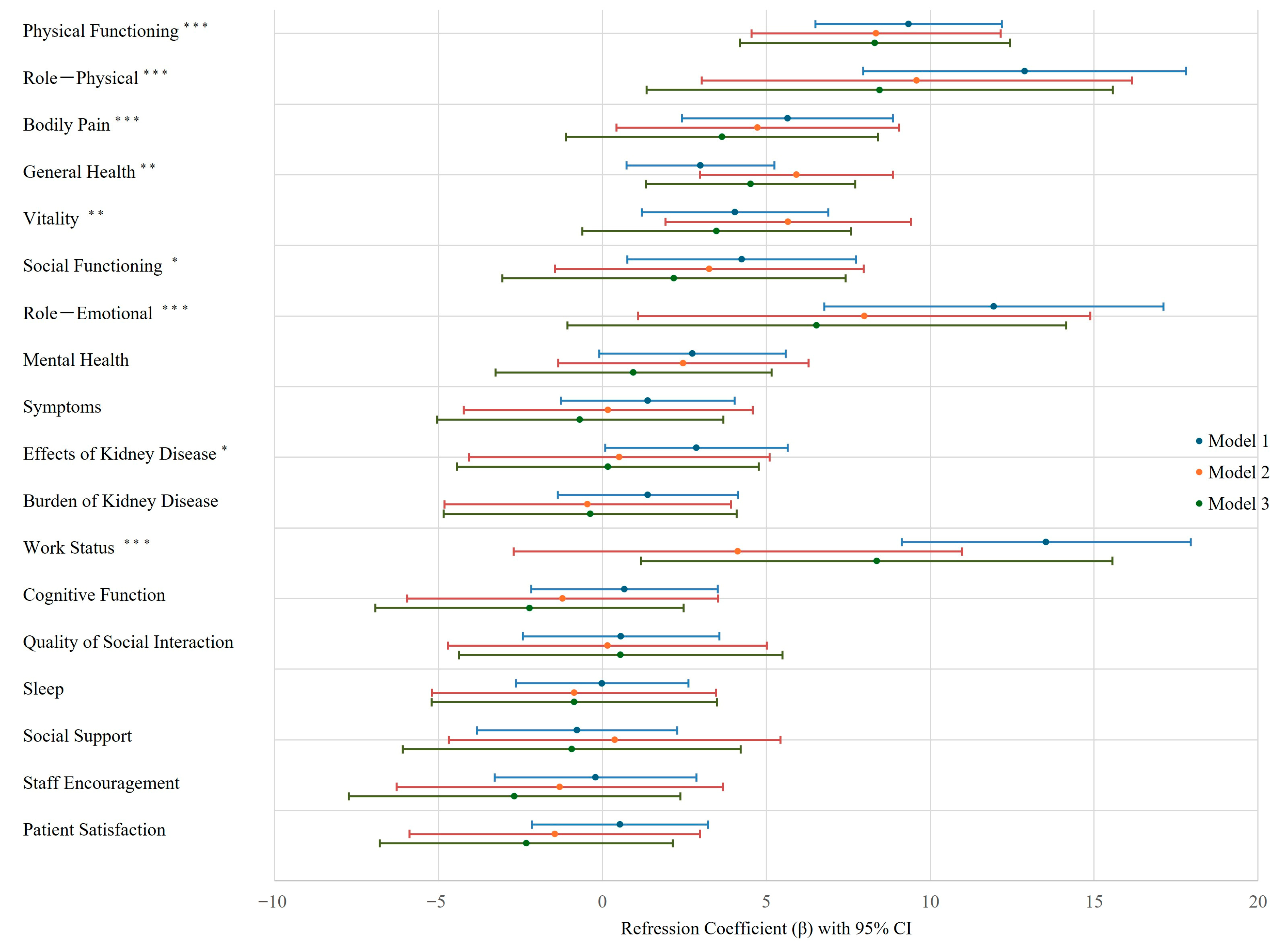
3.3. PA and Health-Related QOL Relationship with Multivariate Analysis
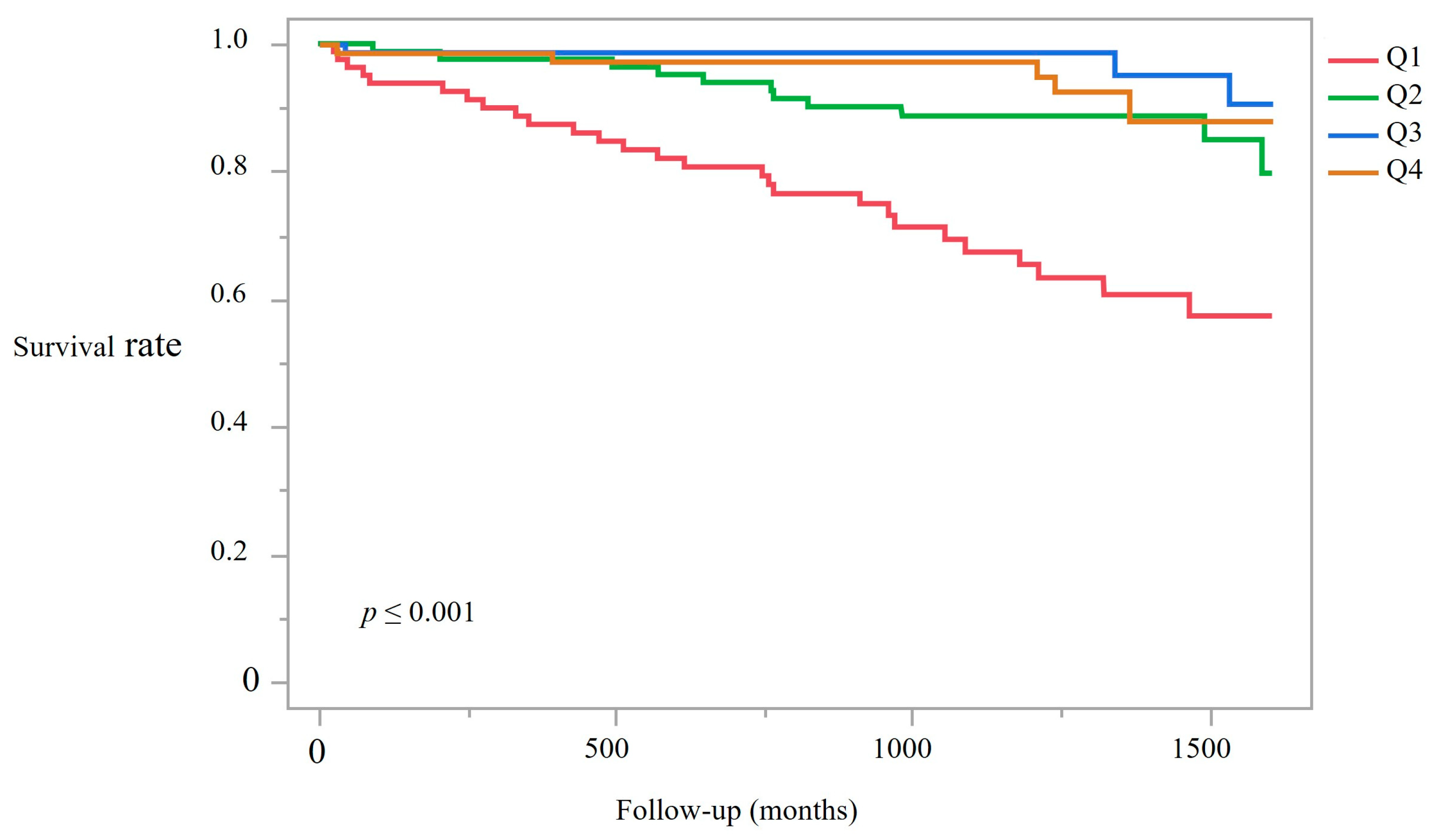
3.4. Kaplan–Meier Survival Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alb | Albumin |
| AUC | Area Under the Curve |
| BIA | Bioelectrical Impedance Analysis |
| BUN | Blood Urea Nitrogen |
| Cl | Chloride |
| CKD | Chronic Kidney Disease |
| Cr | Creatinine |
| CRP | C-Reactive Protein |
| CVD | Cardiovascular Disease |
| DM | Diabetes Mellitus |
| ECW/ICW | Extracellular water to intracellular water ratio |
| GNRI | Geriatric Nutritional Risk Index |
| Hb | Hemoglobin |
| HD | Hemodialysis |
| HDL-C | High-density Lipoprotein Cholesterol |
| iPTH | Intact Parathyroid Hormone |
| Kt/V | Urea Clearance Normalized by Distribution Volume |
| Na | Sodium |
| NT-proBNP | N-terminal Pro-Brain Natriuretic Peptide |
| PA | Phase Angle |
| PEW | Protein-Energy Wasting |
| QOL | Quality of Life |
| ROC | Receiver Operating Characteristic |
| SF-36 | 36-Item Short Form Health Survey |
| UA | Uric Acid |
| β2MG | Beta-2 Microglobulin |
References
- Sharma, S.; Kalra, D.; Rashid, I.; Mehta, S.; Maity, M.K.; Wazir, K.; Gupta, S.; Ansari, S.A.; Alruqi, O.S.; Khan, R.; et al. Assessment of Health-Related Quality of Life in Chronic Kidney Disease Patients: A Hospital-Based Cross-Sectional Study. Medicina 2023, 59, 1788. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T. 2023 Annual Dialysis Data Report, JSDT Renal Data Registry. Nihon Toseki Igakkai Zasshi 2024, 57, 543–620. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Kelly, M.; O’Herlihy, E.; O’Toole, P.W.; Kearney, P.M.; Timmons, S.; O’Shea, E.; Stanton, C.; Hickson, M.; Rolland, Y.; et al. Potentially modifiable determinants of malnutrition in older adults: A systematic review. Clin. Nutr. 2019, 38, 2477–2498. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.; Barros, A.J.; Wang, J.; Heymsfield, S.B.; Pierson, R.N., Jr. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G.; Dahlk, S.L.; King, J.; Vashi, P.G.; Grutsch, J.F.; Lammersfeld, C.A. The relationship between bioelectrical impedance phase angle and subjective global assessment in advanced colorectal cancer. Nutr. J. 2008, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Shinzato, T.; Nakai, S.; Fujita, Y.; Takai, I.; Morita, E.; Nakane, K.; Maeda, K. Determination of Kt/V and protein catabolic rate using pre- and postdialysis blood urea nitrogen concentrations. Nephron 1994, 67, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.; Zhang, Q.; Zhang, H.; Shen, Y.; Jian, G.; Cheng, D.; Wang, N. Association between disability in activities of daily living and phase angle in hemodialysis patients. BMC Nephrol. 2023, 24, 350. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef]
- Piccoli, A. Identification of operational clues to dry weight prescription in hemodialysis using bioimpedance vector analysis. The Italian Hemodialysis-Bioelectrical Impedance Analysis (HD-BIA) Study Group. Kidney Int. 1998, 53, 1036–1043. [Google Scholar] [CrossRef]
- Matias, C.N.; Nunes, C.L.; Francisco, S.; Tomeleri, C.M.; Cyrino, E.S.; Sardinha, L.B.; Silva, A.M. Phase angle predicts physical function in older adults. Arch. Gerontol. Geriatr. 2020, 90, 104151. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Do, J.Y.; Kim, J.C. Impedance-derived phase angle is associated with muscle mass, strength, quality of life, and clinical outcomes in maintenance hemodialysis patients. PLoS ONE 2022, 17, e0261070. [Google Scholar] [CrossRef] [PubMed]
- Beberashvili, I.; Azar, A.; Sinuani, I.; Shapiro, G.; Feldman, L.; Stav, K.; Sandbank, J.; Averbukh, Z. Bioimpedance phase angle predicts muscle function, quality of life and clinical outcome in maintenance hemodialysis patients. Eur. J. Clin. Nutr. 2014, 68, 683–689. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, C.R.; Park, K.H.; Hwang, J.H.; Kim, S.H. Predicting clinical outcomes using phase angle as assessed by bioelectrical impedance analysis in maintenance hemodialysis patients. Nutrition 2017, 41, 7–13. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, W.; Zeng, Y.; Chen, Y.; Yuan, H. Bioelectrical impedance phase angle combined with physical function predicts pre-frailty in maintenance hemodialysis patients: A prospective study. BMC Nephrol. 2024, 25, 243. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-G.; Lee, J.Y.; Kim, J.-S.; Yang, J.-W. Clinical Significance of Phase Angle in Non-Dialysis CKD Stage 5 and Peritoneal Dialysis Patients. Nutrients 2018, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Harun, H.; Pradana, G. The Role of Nutritional Therapy in Inhibiting the Progression of Chronic Kidney Disease: A Narrative Literature Review. Biosci. Med. J. Biomed. Transl. Res. 2023, 7, 3178–3184. [Google Scholar] [CrossRef]
- Avesani, C.M.; Trolonge, S.; Deleaval, P.; Baria, F.; Mafra, D.; Faxen-Irving, G.; Chauveau, P.; Teta, D.; Kamimura, M.A.; Cuppari, L.; et al. Physical activity and energy expenditure in haemodialysis patients: An international survey. Nephrol. Dial. Transplant. 2012, 27, 2430–2434. [Google Scholar] [CrossRef]
- Picciotto, D.; Macciò, L.; Verzola, D.; Baciga, F.; Momentè, C.; Russo, E.; Viazzi, F.; Battaglia, Y.; Esposito, P. Pathophysiology of Physical Exercise in Kidney Patients: Unveiling New Players—The Role of Myokines. Kidney Blood Press. Res. 2024, 49, 457–471. [Google Scholar] [CrossRef]
- Landi, F.; Camprubi-Robles, M.; Bear, D.E.; Cederholm, T.; Malafarina, V.; Welch, A.A.; Cruz-Jentoft, A.J. Muscle loss: The new malnutrition challenge in clinical practice. Clin. Nutr. 2019, 38, 2113–2120. [Google Scholar] [CrossRef]
- Tsujimoto, N.; Matsuzawa, R.; Imai, H.; Kakita, D.; Shimokado, K.; Tamaki, A. #5269 The Nutritional Assessment Tools to Identify Patients at Increased Sarcopenia Risk. Nephrol. Dial. Transplant. 2023, 38, gfad063c_5269. [Google Scholar] [CrossRef]
- Bhandari, S.; Parfrey, P.; White, C.; Anker, S.D.; Farrington, K.; Ford, I.; Kalra, P.A.; McMurray, J.J.V.; Robertson, M.; Tomson, C.R.V.; et al. Predictors of quality of life in patients within the first year of commencing haemodialysis based on baseline data from the PIVOTAL trial and associations with the study outcomes. J. Nephrol. 2023, 36, 1651–1662. [Google Scholar] [CrossRef]
- Yamada, Y.; Schoeller, D.A.; Nakamura, E.; Morimoto, T.; Kimura, M.; Oda, S. Extracellular Water May Mask Actual Muscle Atrophy During Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65A, 510–516. [Google Scholar] [CrossRef]
- Ohashi, Y.; Joki, N.; Yamazaki, K.; Kawamura, T.; Tai, R.; Oguchi, H.; Yuasa, R.; Sakai, K. Changes in the fluid volume balance between intra- and extracellular water in a sample of Japanese adults aged 15–88 yr old: A cross-sectional study. Am. J. Physiol. Ren. Physiol. 2018, 314, F614–F622. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Jo, I.Y.; Lee, S.; Kim, W.; Choi, H.Y.; Ha, S.K.; Kim, H.J.; Park, H.C. Comparison of hydration and nutritional status between young and elderly hemodialysis patients through bioimpedance analysis. Clin. Interv. Aging 2015, 10, 1327. [Google Scholar] [CrossRef]
- Li, C.-I.; Liu, C.-S.; Lin, C.-H.; Yang, S.-Y.; Li, T.-C.; Lin, C.-C. Independent and joint associations of skeletal muscle mass and physical performance with all-cause mortality among older adults: A 12-year prospective cohort study. BMC Geriatr. 2022, 22, 597. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Di Vincenzo, O.; Sammarco, R.; Morlino, D.; Scalfi, L. Bioimpedance phase angle in elite male athletes: A segmental approach. Physiol. Meas. 2021, 41, 125007. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, G.; Valerio, G.; Alicante, P.; Di Vincenzo, O.; Scalfi, L. Bioelectrical Impedance Analysis (BIA)- Derived Phase Angle in Children and Adolescents: A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, O.; Marra, M.; Scalfi, L. Bioelectrical impedance phase angle in sport: A systematic review. J. Int. Soc. Sports Nutr. 2019, 16, 49. [Google Scholar] [CrossRef]
| Q1 (n = 70) | Q2 (n = 75) | Q3 (n = 78) | Q4 (n = 72) | p-Value | |
|---|---|---|---|---|---|
| PA in males PA in females | ≤4.5° ≤4.0° | 4.6–5.3° 4.1–4.8° | 5.4–6.2° 4.9–5.2° | ≥6.3° ≥5.3° | |
| Age (years) | 73.5 (69.0–79.0) | 70.0 (59.0–76.0) | 66.5 (54.8–72.3) | 55.0 (48.0–63.0) | <0.001 |
| Male (%) | 34 (48.6%) | 41 (54.7%) | 40 (51.3%) | 43 (59.7%) | 0.68 |
| HD duration (months) | 82.5 (42.8–221.3) | 68.0 (37.0–158.0) | 68.5 (35.8–112.5) | 60.0 (26.3–118.5) | 0.05 |
| History of CVD (%) | 28 (40.0%) | 23 (30.7%) | 35 (44.9%) | 23 (31.9%) | 0.32 |
| History of DM (%) | 31 (44.3%) | 36 (48.0%) | 37 (47.4%) | 27 (37.5%) | 0.55 |
| Smoking (%) | 18 (25.7%) | 21 (28.0%) | 29 (37.2%) | 16 (22.2%) | 0.21 |
| BMI (kg/m2) | 20.1 (18.4–22.2) | 21.6 (19.9–23.6) | 22.7 (20.6–25.5) | 24.5 (21.9–27.4) | <0.001 |
| % of fluid removal | 4.9 (4.0–6.0) | 5.1 (4.2–5.7) | 5.3 (4.0–6.0) | 5.4 (4.4–6.2) | 0.32 |
| Hb (g/dL) | 11.1 (10.5–11.5) | 11.2 (10.6–11.7) | 11.4 (10.8–12.0) | 11.7 (10.9–12.3) | <0.001 |
| Alb (g/dL) | 3.5 (3.3–3.7) | 3.6 (3.3–3.7) | 3.6 (3.5–3.8) | 3.7 (3.5–3.9) | <0.001 |
| BUN (mg/dL) | 53.9 (46.5–60.8) | 57.0 (46.0–72.9) | 56.1 (46.8–65.9) | 65.9 (55.7–73.9) | <0.001 |
| Cr (mg/dL) | 8.70 (7.51–9.89) | 9.57 (8.62–10.92) | 10.75 (9.42–11.92) | 12.69 (10.54–13.73) | <0.001 |
| Uric acid (mg/dL) | 6.9 (6.4–7.8) | 7.3 (6.6–8.2) | 7.8 (7.2–8.4) | 8.5 (7.6–9.4) | <0.001 |
| Na (mEq/L) | 139 (138–140) | 139 (138–141) | 139 (138–140) | 139 (137–141) | 0.86 |
| K (mEq/L) | 4.6 (4.2–5.1) | 4.8 (4.5–5.3) | 4.8 (4.4–5.3) | 4.9 (4.4–5.4) | 0.045 |
| Cl (mEq/L) | 104 (102–106) | 104 (102–106) | 103 (102–105) | 103 (100–104) | 0.003 |
| HDL-C (mg/dL) | 50.0 (43.0–61.3) | 55.0 (43.0–65.0) | 46.0 (38.0–61.0) | 45.0 (37.0–55.8) | 0.011 |
| iPTH (pg/mL) | 149 (94–219) | 172 (100–221) | 151 (113–230) | 170 (107–229) | 0.56 |
| β2MG (mg/L) | 26.0 (23.0–29.5) | 25.5 (22.4–29.7) | 26.3 (23.8–28.5) | 27.0 (24.0–30.5) | 0.40 |
| CRP (mg/dL) | 0.15 (0.07–0.36) | 0.10 (0.04–0.28) | 0.07 (0.04–0.23) | 0.14 (0.06–0.31) | 0.06 |
| NT-proBNP (pg/mL) | 8825 (4928–19,625) | 3690 (1850–7650) | 2930 (1813–5763) | 2000 (1133–3760) | <0.001 |
| Kt/V urea | 1.80 (1.69–2.07) | 1.92 (1.72–2.17) | 1.87 (1.64–2.06) | 1.72 (1.59–1.93) | 0.003 |
| GNRI | 90.3 (84.8–97.0) | 92.0 (87.7–98.1) | 96.9 (92.0–104.0) | 101.7 (96.0–107.0) | <0.001 |
| Q1 (n = 70) | Q2 (n = 75) | Q3 (n = 78) | Q4 (n = 72) | p-Value | |
|---|---|---|---|---|---|
| PA in males PA in females | ≤4.5° ≤4.0° | 4.6–5.3° 4.1–4.8° | 5.4–6.2° 4.9–5.2° | ≥6.3° ≥5.3° | |
| TBW (L) | 29.9 (24.3–33.7) | 31.5 (26.4–35.4) | 34.9 (28.3–40.5) | 36.2 (29.7–41.0) | <0.001 |
| ECW (L) | 12.2 (9.9–13.7) | 12.4 (10.5–13.7) | 13.5 (11.1–15.5) | 13.5 (11.5–15.3) | 0.002 |
| ICW (L) | 17.8 (14.4–20.1) | 19.1 (15.9–21.4) | 21.5 (17.4–25.0) | 22.7 (18.4–26.1) | <0.001 |
| Muscle mass (kg) | 8.4 (6.7–9.0) | 8.9 (7.4–9.9) | 10.0 (7.8–11.3) | 10.4 (8.5–11.9) | <0.001 |
| Fat-free mass (kg) | 44.0 (35.6–48.3) | 46.6 (39.5–51.3) | 51.5 (40.9–58.4) | 53.7 (43.9–61.0) | <0.001 |
| Fat mass (kg) | 12.2 (8.1–16.9) | 12.5 (9.3–20.3) | 14.6 (10.9–21.2) | 17.8 (12.8–24.2) | < 0.001 |
| ECW/ICW ratio | 0.704 (0.694–0.721) | 0.677 (0.667–0.693) | 0.659 (0.646–0.672) | 0.633 (0.619–0.651) | <0.001 |
| Q1 (n = 70) | Q2 (n = 75) | Q3 (n = 78) | Q4 (n = 72) | p-Value | |
|---|---|---|---|---|---|
| PA in Males PA in Females | ≤4.5° ≤4.0° | 4.6–5.3° 4.1–4.8° | 5.4–6.2° 4.9–5.2° | ≥6.3° ≥5.3° | |
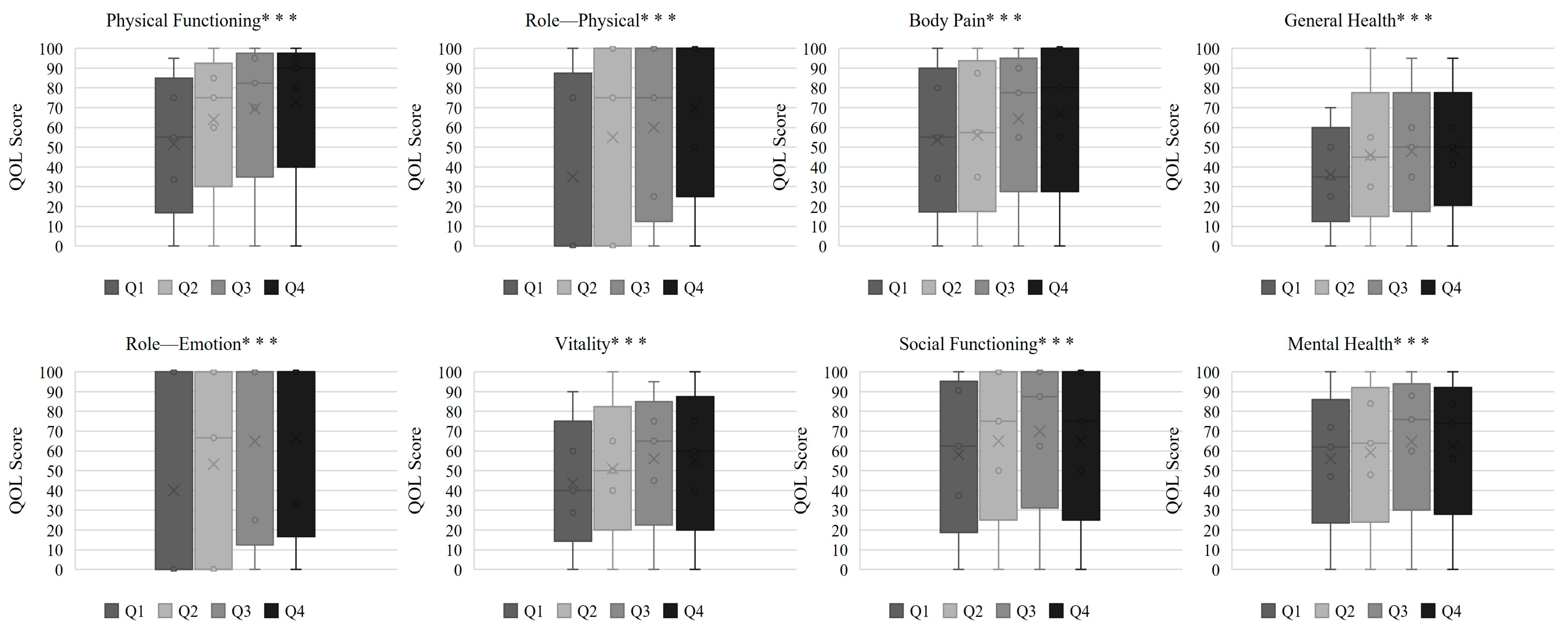
| Physical Functioning | 55.0 (33.8–75.0) | 75.0 (60.0–85.0) | 82.5 (70.0–95.0) | 90.0 (80.0–95.0) | <0.001 |
| Role—Physical | 0.0 (0.0–75.0) | 75.0 (0.0–100.0) | 75.0 (25.0–100.0) | 100.0 (50.0–100.0) | <0.001 |
| Bodily Pain | 55.0 (34.4–80.0) | 57.5 (35.0–87.5) | 77.5 (55.0–90.0) | 80.0 (55.0–100.0) | <0.001 |
| General Health | 35.0 (25.0–50.0) | 45.0 (30.0–55.0) | 50.0 (35.0–60.0) | 50.0 (41.3–60.0) | <0.001 |
| Vitality | 40.0 (28.8–60.0) | 50.0 (40.0–65.0) | 65.0 (45.0–75.0) | 60.0 (40.0–75.0) | <0.001 |
| Social Functioning | 62.5 (37.5–90.6) | 75.0 (50.0–100.0) | 87.5 (62.5–100.0) | 75.0 (50.0–100.0) | 0.002 |
| Role—Emotional | 0.0 (0.0–100.0) | 66.7 (0.0–100.0) | 100.0 (25.0–100.0) | 100.0 (33.3–100.0) | <0.001 |
| Mental Health | 62.0 (47.0–72.0) | 64.0 (48.0–84.0) | 76.0 (60.0–88.0) | 74.0 (56.0–84.0) | 0.002 |
| Q1 (n = 70) | Q2 (n = 75) | Q3 (n = 78) | Q4 (n = 72) | p-Value | |
|---|---|---|---|---|---|
| PA in Males PA in Females | ≤4.5° ≤4.0° | 4.6–5.3° 4.1–4.8° | 5.4–6.2° 4.9–5.2° | ≥6.3° ≥5.3° | |
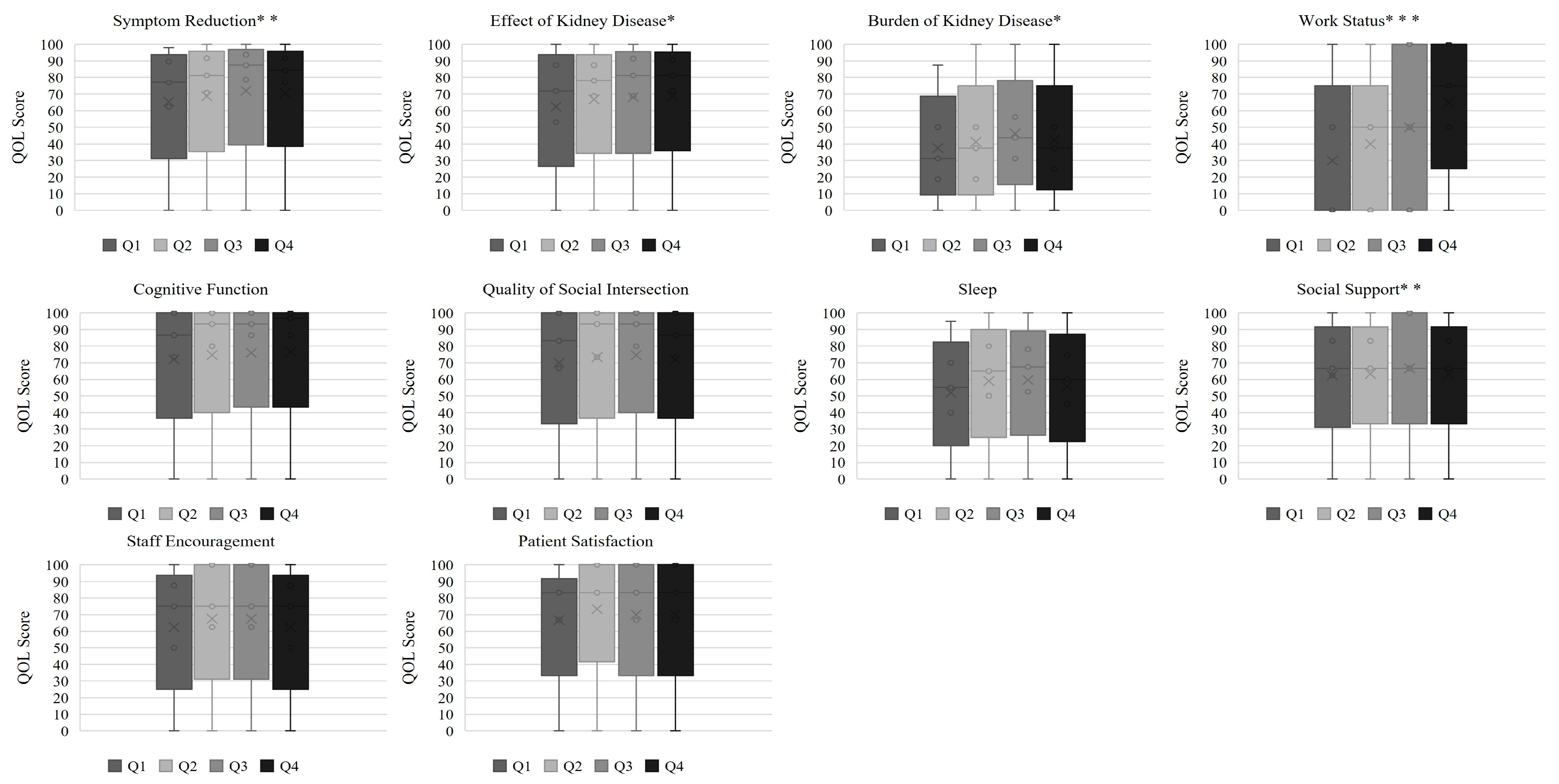
| Symptom Reduction | 77.1 (62.5–89.6) | 81.3 (70.8–91.7) | 87.5 (78.6–93.8) | 84.4 (77.1–91.7) | 0.007 |
| Effects of Kidney Disease | 71.9 (53.1–87.5) | 78.1 (68.8–87.5) | 81.3 (68.8–91.4) | 81.3 (71.9–90.6) | 0.015 |
| Burden of Kidney Disease | 31.3 (18.8–50.0) | 37.5 (18.8–50.0) | 43.8 (31.3–56.3) | 37.5 (25.0–50.0) | 0.030 |
| Work Status | 0.0 (0.0–50.0) | 50.0 (0.0–50.0) | 50.0 (0.0–100.0) | 75.0 (50.0–100.0) | <0.001 |
| Cognitive Function | 86.7 (73.3–100.0) | 93.3 (80.0–100.0) | 93.3 (86.7–100.0) | 96.7 (86.7–100.0) | 0.180 |
| Quality of Social Interaction | 83.3 (66.7–100.0) | 93.3 (73.3–100.0) | 93.3 (80.0–100.0) | 86.7 (73.3–100.0) | 0.060 |
| Sleep | 55.0 (40.0–70.0) | 65.0 (50.0–80.0) | 67.5 (52.5–78.1) | 60.0 (45.0–74.4) | 0.060 |
| Social Support | 66.7 (62.5–83.3) | 66.7 (66.7–83.3) | 66.7 (66.7–100.0) | 66.7 (66.7–83.3) | 0.046 |
| Staff Encouragement | 75.0 (50.0–100.0) | 75.0 (62.5–100.0) | 75.0 (62.5–100.0) | 75.0 (50.0–100.0) | 0.070 |
| Patient Satisfaction | 83.3 (66.7–100.0) | 83.3 (83.3–100.0) | 83.3 (66.7–100.0) | 83.3 (66.7–100.0) | 0.190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, N.; Tanaka, T.; Suzuki, Y.; Takahashi, S.; Hitaka, M.; Ishii, S.; Yamazaki, K.; Ohashi, Y. Clinical Significance of Phase Angle for Assessing Quality of Life and Prognosis in Hemodialysis Patients. Nutrients 2025, 17, 3631. https://doi.org/10.3390/nu17233631
Yoshida N, Tanaka T, Suzuki Y, Takahashi S, Hitaka M, Ishii S, Yamazaki K, Ohashi Y. Clinical Significance of Phase Angle for Assessing Quality of Life and Prognosis in Hemodialysis Patients. Nutrients. 2025; 17(23):3631. https://doi.org/10.3390/nu17233631
Chicago/Turabian StyleYoshida, Norihito, Tatsuki Tanaka, Yusuke Suzuki, Sadamu Takahashi, Mai Hitaka, Shingo Ishii, Keisuke Yamazaki, and Yasushi Ohashi. 2025. "Clinical Significance of Phase Angle for Assessing Quality of Life and Prognosis in Hemodialysis Patients" Nutrients 17, no. 23: 3631. https://doi.org/10.3390/nu17233631
APA StyleYoshida, N., Tanaka, T., Suzuki, Y., Takahashi, S., Hitaka, M., Ishii, S., Yamazaki, K., & Ohashi, Y. (2025). Clinical Significance of Phase Angle for Assessing Quality of Life and Prognosis in Hemodialysis Patients. Nutrients, 17(23), 3631. https://doi.org/10.3390/nu17233631






