Synergistic Effects of Microencapsulated Polyphenols and Concurrent Training on Metabolic Health and Fitness in Overweight/Obese Adults with Prediabetes
Abstract
1. Introduction
2. Materials and Methods
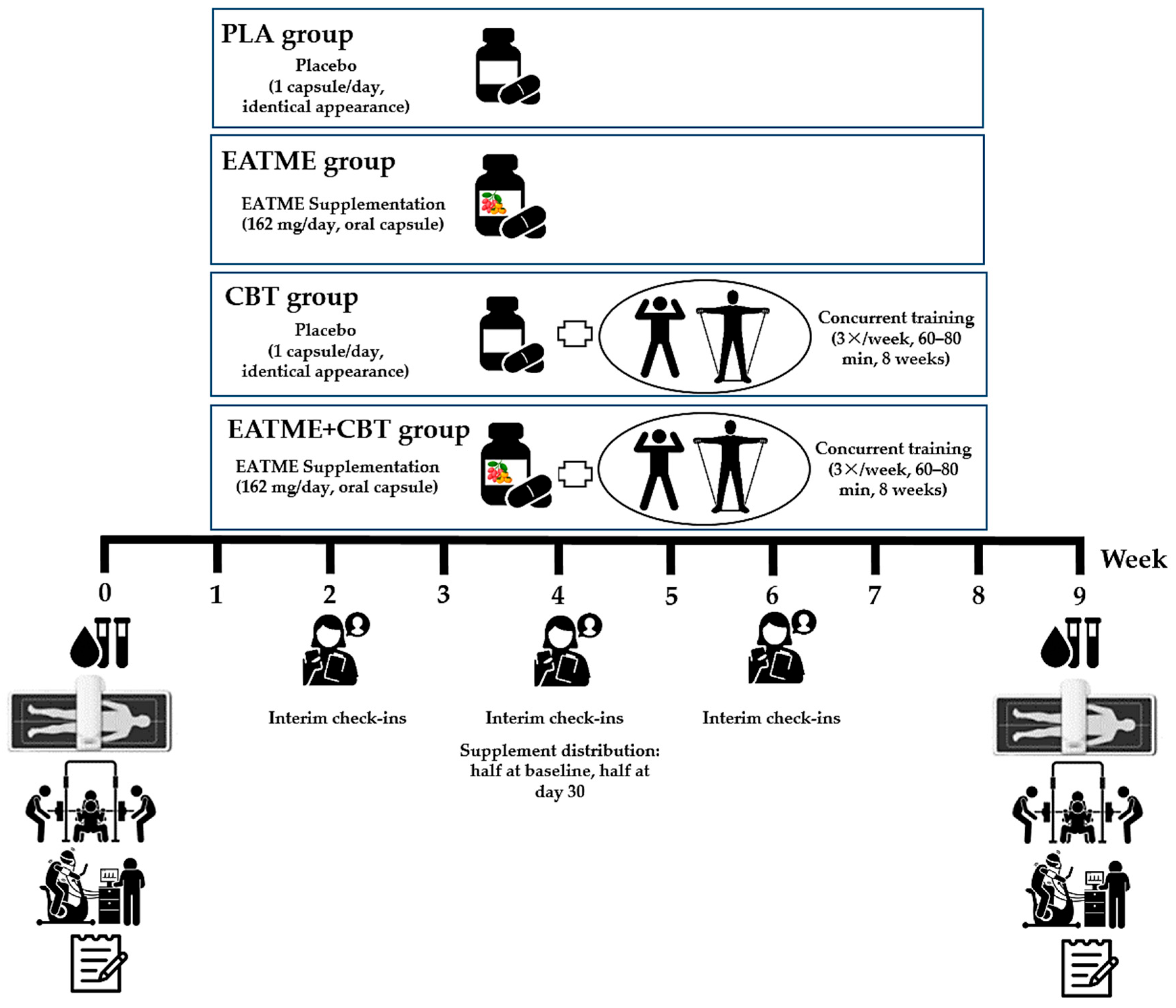
2.1. Study Design
2.2. Participants
2.2.1. Sample Size Calculation
2.2.2. Inclusion and Exclusion Criteria
2.2.3. Randomization, Allocation, and Blinding
2.3. Experimental Procedures
2.4. Preparation of Supplement Extracts and Encapsulation
2.5. Exercise Training Protocol
2.6. Outcomes Measurement
2.6.1. Anthropometry and Body Composition
2.6.2. Blood Samples and Analysis
Blood Sampling
Analysis of Glycemic Control Markers
Analysis of Lipid Profiles and Atherogenic Indices
Analysis of Inflammatory and Adipokine Markers
Analysis of Markers of Renal and Liver Functions
2.6.3. Upper and Lower Muscular Strength
2.6.4. Cardiorespiratory Fitness
2.6.5. Quality of Life
2.6.6. Dietary Intake
2.7. Statistical Analysis
3. Results
3.1. Metabolic Biomarkers
3.1.1. Glycemic Control
3.1.2. Lipid Profiles and Atherogenic Indices
3.1.3. Renal and Liver Functions
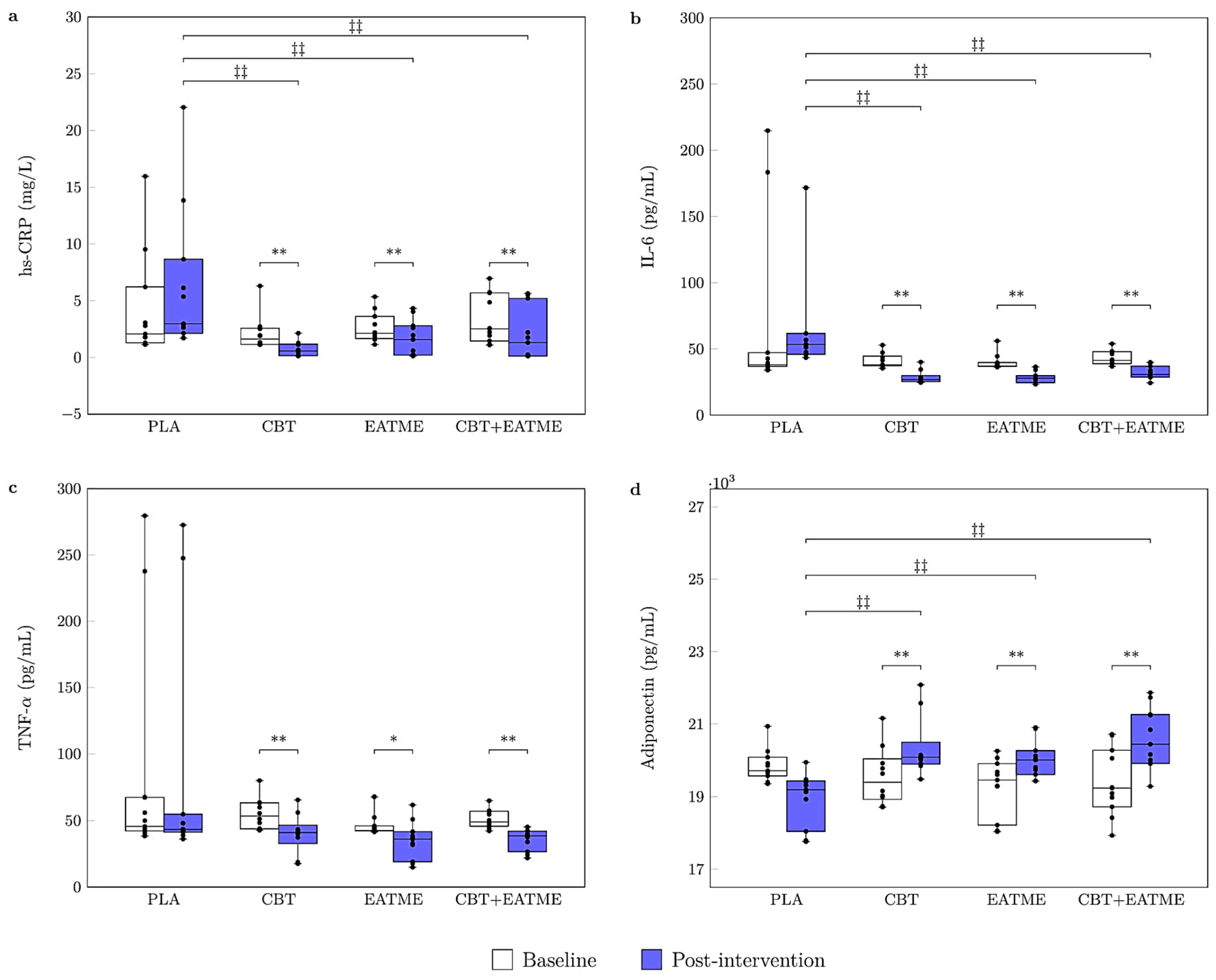
3.2. Inflammatory and Adipokine Markers
3.3. Physical Fitness
3.4. Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 diabetes mellitus |
| CBT | Concurrent training group |
| EATME | Microencapsulated polyphenol compounds supplementation group |
| CBT + EATME | Combined concurrent training with microencapsulated polyphenol compounds supplementation group |
| PLA | Placebo group |
| FBG | Fasting blood glucose |
| HbA1c | Glycated hemoglobin |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| hs-CRP | High-sensitive C-reactive protein |
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Iinterleukin-6 |
| CRP | C-reactive protein |
| QoL | Quality of life |
| MetS | Metabolic syndrome |
| V̇O2max | Maximum oxygen consumption |
| HR | Heart rate |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| MAP | Mean arterial pressure |
| 1RM | One-repetition maximum |
| CVD | Cardiovascular disease |
| AT | Aerobic training |
| RT | Resistance training |
| %BF | Body fat percentage |
| FM | Fat mass |
| FFM | Fat-free mass |
| LM | Lean mass |
| A/G ratio | Android and gynoid fat ratio |
| CV | Coefficients of variation |
| TC | Total cholesterol |
| TG | Triglycerides |
| HDL-c | High-density lipoprotein cholesterol |
| LDL-c | Low-density lipoprotein cholesterol |
| AIP | Atherogenic index of plasma |
| CRI-I | Castelli Risk Index I |
| CRI-II | Castelli Risk Index II |
| AC | Atherogenic coefficient |
| BUN | Blood urea nitrogen |
| eGFR | Estimated glomerular filtration rate |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| ALP | Alkaline phosphatase |
| NSCA | National Strength and Conditioning Association |
| WHOQOL-BREF | World Health Organization quality of life brief questionnaire |
References
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab. Syndr. Obes. 2021, 14, 3567–3602. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Caselli, C. Role of adiponectin system in insulin resistance. Mol. Genet. Metab. 2014, 113, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Shimomura, I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord. 2014, 15, 1–10. [Google Scholar] [CrossRef]
- Bartel, I.; Koszarska, M.; Strzałkowska, N.; Tzvetkov, N.T.; Wang, D.; Horbańczuk, J.O.; Wierzbicka, A.; Atanasov, A.G.; Jóźwik, A. Cyanidin-3-O-glucoside as a nutrigenomic factor in type 2 diabetes and its prominent impact on health. Int. J. Mol. Sci. 2023, 24, 9765. [Google Scholar] [CrossRef]
- Özdemir, K.; Demir, Y. Phenolic compounds in exercise physiology: Dual role in oxidative stress and recovery adaptation. Food Sci. Nutr. 2025, 13, e70714. [Google Scholar] [CrossRef]
- Jain, S.; Ranganna, G.; Mohapatra, S.; Rathod, M.; Homeshvari, D.; Kore, D.; Saxena, S.; Anushi, N. A comprehensive review on Persimmon (Diospyros kaki): Botanical, horticultural, and varietal perspectives. Int. J. Environ. Clim. Chang. 2023, 13, 4437–4457. [Google Scholar] [CrossRef]
- Mishra, B.; Tomer, V.; Kumar, A. Karonda (Carissa carandas L.): A miracle fruit with multifaceted potential. J. Agric. Food Res. 2024, 18, 101417. [Google Scholar] [CrossRef]
- Kang, B.B.; Chiang, B.H. Amelioration of insulin resistance using the additive effect of ferulic acid and resveratrol on vesicle trafficking for skeletal muscle glucose metabolism. Phytother. Res. 2020, 34, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of quercetin supplementation on glycemic control among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, L.Y.; Aceves-de la Mora, M.C.A.-d.; Gonzalez-Ortiz, M.; Ramos-Nunez, J.L.; Martinez-Abundis, E. Effect of chlorogenic acid administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance. J. Med. Food 2018, 21, 469–473. [Google Scholar] [CrossRef]
- Li, R.; Du, S.; Ye, Z.; Yang, W.; Ding, Z.; Liu, Y. Protective effect of cyanidin-3-O-glucoside from blueberry anthocyanin extracts against hyperglycemia-induced outer BRB damage by suppressing REDD1-mediated VEGFA upregulation. Food Biosci. 2024, 61, 104695. [Google Scholar] [CrossRef]
- Sivasinprasasn, S.; Pantan, R.; Thummayot, S.; Tocharus, J.; Suksamrarn, A.; Tocharus, C. Cyanidin-3-glucoside attenuates angiotensin II-induced oxidative stress and inflammation in vascular endothelial cells. Chem. Biol. Interact. 2016, 260, 67–74. [Google Scholar] [CrossRef]
- Qazi, H.J.; Ye, A.; Acevedo-Fani, A.; Singh, H. Delivery of encapsulated bioactive compounds within food matrices to the digestive tract: Recent trends and future perspectives. Crit. Rev. Food Sci. Nutr. 2025, 65, 2921–2942. [Google Scholar] [CrossRef]
- Bińkowska, W.; Szpicer, A.; Stelmasiak, A.; Wojtasik-Kalinowska, I.; Półtorak, A. Microencapsulation of polyphenols and their application in food technology. Appl. Sci. 2024, 14, 11954. [Google Scholar] [CrossRef]
- González, E.; Gómez-Caravaca, A.M.; Giménez, B.; Cebrian, R.; Maqueda, M.; Parada, J.; Martinez-Ferez, A.; Segura-Carretero, A.; Robert, P. Role of maltodextrin and inulin as encapsulating agents on the protection of oleuropein during in vitro gastrointestinal digestion. Food Chem. 2020, 310, 125976. [Google Scholar] [CrossRef]
- Vonghirundecha, P.; Chusri, S.; Meunprasertdee, P.; Kaewmanee, T. Microencapsulated functional ingredients from a Moringa oleifera leaf polyphenol-rich extract: Characterization, antioxidant properties, in vitro simulated digestion, and storage stability. LWT Food Sci. Technol. 2022, 154, 112820. [Google Scholar] [CrossRef]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Grubbs, B.; Motevalli, M.S.; Baker, J.S.; Laher, I.; Zouhal, H. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 915–923. [Google Scholar] [CrossRef]
- Qiu, L.; Gao, C.; Wang, H.; Ren, Y.; Li, J.; Li, M.; Du, X.; Li, W.; Zhang, J. Effects of dietary polyphenol curcumin supplementation on metabolic, inflammatory, and oxidative stress indices in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 2023, 14, 1216708. [Google Scholar] [CrossRef]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Mateos, D.; Ugarriza, L.; Gómez, C.; Sureda, A.; Tur, J.A. Long-term impact of nutritional intervention with increased polyphenol intake and physical activity promotion on oxidative and inflammatory profiles in patients with metabolic syndrome. Nutrients 2024, 16, 2121. [Google Scholar] [CrossRef]
- Oliveira, C.; Simões, M.; Carvalho, J.; Ribeiro, J. Combined exercise for people with type 2 diabetes mellitus: A systematic review. Diabetes Res. Clin. Pract. 2012, 98, 187–198. [Google Scholar] [CrossRef]
- Zhao, X.; He, Q.; Zeng, Y.; Cheng, L. Effectiveness of combined exercise in people with type 2 diabetes and concurrent overweight/obesity: A systematic review and meta-analysis. BMJ Open 2021, 11, e046252. [Google Scholar] [CrossRef]
- Al-Mhanna, S.B.; Batrakoulis, A.; Ghazali, W.S.W.; Mohamed, M.; Aldayel, A.; Alhussain, M.H.; Afolabi, H.A.; Wada, Y.; Gülü, M.; Elkholi, S. Effects of combined aerobic and resistance training on glycemic control, blood pressure, inflammation, cardiorespiratory fitness and quality of life in patients with type 2 diabetes and overweight/obesity: A systematic review and meta-analysis. PeerJ 2024, 12, e17525. [Google Scholar] [CrossRef]
- Silva, F.M.; Duarte-Mendes, P.; Teixeira, A.M.; Soares, C.M.; Ferreira, J.P. The effects of combined exercise training on glucose metabolism and inflammatory markers in sedentary adults: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 1936. [Google Scholar] [CrossRef]
- Wang, Y. The interplay of exercise and polyphenols in cancer treatment: A focus on oxidative stress and antioxidant mechanisms. Phytother. Res. 2024, 38, 3459–3488. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, S.; Chan, A.-W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A. CONSORT 2025 statement: Updated guideline for reporting randomised trials. Lancet 2025, 405, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Bateni, Z.; Behrouz, V.; Rahimi, H.R.; Hedayati, M.; Afsharian, S.; Sohrab, G. Effects of nano-curcumin supplementation on oxidative stress, systemic inflammation, adiponectin, and NF-κB in patients with metabolic syndrome: A randomized, double-blind clinical trial. J. Herb. Med. 2022, 31, 100531. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011, 34, S11–S61. [Google Scholar] [CrossRef]
- Morishita, S.; Tsubaki, A.; Takabayashi, T.; Fu, J.B. Relationship between the rating of perceived exertion scale and the load intensity of resistance training. Strength. Cond. J. 2018, 40, 94–109. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.; Treacher, D.F.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M. New creatinine-and cystatin C–based equations to estimate GFR without race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int. J. Ment. Health 1994, 23, 24–56. [Google Scholar] [CrossRef]
- Banjong, O.; Wanijjakul, C.; Peemanee, K.; Ananthasuk, S. Application Manual: INMUCAL-Nutrients V.3 Institute of Nutrition; Mahidol University: Nakhon Pathom, Thailand, 2016. [Google Scholar]
- Consitt, L.A.; Dudley, C.; Saxena, G. Impact of endurance and resistance training on skeletal muscle glucose metabolism in older adults. Nutrients 2019, 11, 2636. [Google Scholar] [CrossRef]
- Prior, S.J.; Goldberg, A.P.; Ortmeyer, H.K.; Chin, E.R.; Chen, D.; Blumenthal, J.B.; Ryan, A.S. Increased skeletal muscle capillarization independently enhances insulin sensitivity in older adults after exercise training and detraining. Diabetes 2015, 64, 3386–3395. [Google Scholar] [CrossRef]
- Stuart, C.A.; Lee, M.L.; South, M.A.; Howell, M.E.; Stone, M.H. Muscle hypertrophy in prediabetic men after 16 wk of resistance training. J. Appl. Physiol. 2017, 123, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Page, R.A.; Sukala, W.R.; Giri, M.; Ghimbovschi, S.D.; Hayat, I.; Cheema, B.S.; Lys, I.; Leikis, M.; Sheard, P.W. Multi-omic integrated networks connect DNA methylation and miRNA with skeletal muscle plasticity to chronic exercise in Type 2 diabetic obesity. Physiol. Genomics 2014, 46, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Elmageed, G.M.A.; El-Qazaz, I.G.; El-Sayed, D.S.; El-Samad, L.M.; Abdou, H.M. The synergistic influence of polyflavonoids from Citrus aurantifolia on diabetes treatment and their modulation of the PI3K/AKT/FOXO1 signaling pathways: Molecular docking analyses and in vivo investigations. Pharmaceutics 2023, 15, 2306. [Google Scholar] [CrossRef]
- Saikia, L.; Talukdar, N.C.; Dutta, P.P. Exploring the therapeutic role of flavonoids through AMPK activation in metabolic syndrome: A narrative review. Phytother. Res. 2025, 39, 1403–1421. [Google Scholar]
- Singh, S.S.; Eligar, S.M.; Patil, K.N. Ferulic acid from Beta vulgaris subsp. vulgaris Altissima Group pulp targets the CaMKKβ/SIRT-1/AMPK pathway To combat hyperglycemia. J. Agric. Food Chem. 2025, 73, 22347–22361. [Google Scholar]
- Edirisinghe, I.; Burton-Freeman, B. Anti-diabetic actions of Berry polyphenols–Review on proposed mechanisms of action. J. Berry Res. 2016, 6, 237–250. [Google Scholar]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus—Systematic review and meta-analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Bateni, Z.; Rahimi, H.R.; Hedayati, M.; Afsharian, S.; Goudarzi, R.; Sohrab, G. The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with the metabolic syndrome: A randomized, double-blind clinical trial. Phytother. Res. 2021, 35, 3945–3953. [Google Scholar] [CrossRef] [PubMed]
- Sataei-Mokhtari, F.; Fanaei, H.; Saravani, M.; Mortazavi, Z.; Payandeh, A.; Salajeghe, S. Comparative effects of nano-curcumin versus curcumin on plasma levels of spexin and its gene expression in liver tissue, insulin resistance, lipid profile, and fasting blood glucose in type 2 diabetic rats. J. Diabetes Metab. Disord. 2025, 24, 182. [Google Scholar] [CrossRef] [PubMed]
- Shamsi-Goushki, A.; Mortazavi, Z.; Mirshekar, M.A.; Mohammadi, M.; Moradi-Kor, N.; Jafari-Maskouni, S.; Shahraki, M. Comparative effects of curcumin versus nano-curcumin on insulin resistance, serum levels of apelin and lipid profile in type 2 diabetic rats. Diabetes Metab. Syndr. Obes. 2020, 13, 2337–2346. [Google Scholar]
- Luc, K.; Schramm-Luc, A.; Guzik, T.; Mikolajczyk, T. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Kowalczyk, T.; Muskała, M.; Merecz-Sadowska, A.; Sikora, J.; Picot, L.; Sitarek, P. Anti-inflammatory and anticancer effects of anthocyanins in in vitro and in vivo studies. Antioxidants 2024, 13, 1143. [Google Scholar] [CrossRef]
- Nuszkiewicz, J.; Wróblewska, J.; Wróblewski, M.; Woźniak, A. Anthocyanin-rich purple plant foods: Bioavailability, antioxidant aechanisms, and functional roles in redox regulation and exercise recovery. Nutrients 2025, 17, 2453. [Google Scholar] [CrossRef]
- Abdalla, M.M.I. Therapeutic potential of adiponectin in prediabetes: Strategies, challenges, and future directions. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222371. [Google Scholar] [CrossRef]
- Martemucci, G.; Khalil, M.; Di Luca, A.; Abdallah, H.; D’Alessandro, A.G. Comprehensive strategies for metabolic syndrome: How nutrition, dietary polyphenols, physical activity, and lifestyle modifications address diabesity, cardiovascular diseases, and neurodegenerative conditions. Metabolites 2024, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhong, Q.; Zhao, L.; An, Z.; Li, S. Combined effect of triglyceride-glucose index and atherogenic index of plasma on cardiovascular disease: A national cohort study. Sci. Rep. 2024, 14, 31092. [Google Scholar] [CrossRef]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Hu, P.; Feng, L.-P.; Huang, L.-L.; Wang, Y.; Yan, X.; Xiong, J.; Xia, H.-L. Protective effects of ferulic acid on metabolic syndrome: A comprehensive review. Molecules 2022, 28, 281. [Google Scholar] [CrossRef]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Mobarhan, M.G.; Oskuee, R.K. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed 2016, 6, 567. [Google Scholar]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport. Exerc. 2017, 2, e000143. [Google Scholar] [CrossRef] [PubMed]
- Farhat, G.; Drummond, S.; Al-Dujaili, E.A. Polyphenols and their role in obesity management: A systematic review of randomized clinical trials. Phytother. Res. 2017, 31, 1005–1018. [Google Scholar] [CrossRef]
- Abrego-Guandique, D.M.; Aguilera Rojas, N.M.; Chiari, A.; Luciani, F.; Cione, E.; Cannataro, R. The impact of exercise on mitochondrial biogenesis in skeletal muscle: A systematic review and meta-analysis of randomized trials. Biomol. Concepts 2025, 16, 20250055. [Google Scholar] [CrossRef]
- Mesquita, P.H.; Vann, C.G.; Phillips, S.M.; McKendry, J.; Young, K.C.; Kavazis, A.N.; Roberts, M.D. Skeletal muscle ribosome and mitochondrial biogenesis in response to different exercise training modalities. Front. Physiol. 2021, 12, 725866. [Google Scholar] [CrossRef]
- Wei, J.; Fan, L.; Xia, F.; Zhu, X.; Chen, L.; Wang, T. Association of aerobic and muscle-strengthening physical activity with all-cause and cardiovascular disease mortality among adults with type 2 diabetes: A prospective cohort of US adults. Diabetes Metab. Syndr. Clin. Res. Rev. 2024, 18, 102975. [Google Scholar] [CrossRef]
- Gale, C.R.; Cooper, C.; Deary, I.J.; Sayer, A.A. Psychological well-being and incident frailty in men and women: The English Longitudinal Study of Ageing. Psychol. Med. 2014, 44, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Molinero, O.; Salguero, A.; Márquez, S. Perceived health, depression and psychological well-being in older adults: Physical activity and osteoarticular disease. Sustainability 2021, 13, 8157. [Google Scholar] [CrossRef]
- Higham, S.M.; Mendham, A.E.; Rosenbaum, S.; Allen, N.G.; Smith, G.; Duffield, R. Effect of concurrent exercise training on stress, depression and anxiety in inactive academics: Secondary analysis of a randomized controlled trial. Res. Q. Exerc. Sport. 2025, 96, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Terracina, S.; Ferraguti, G. Brain neurotrophins and plant polyphenols: A powerful connection. Molecules 2025, 30, 2657. [Google Scholar] [CrossRef]


| Total (n = 43) | PLA (n = 11) | CBT (n = 10) | EATME (n = 11) | CBT + EATME (n = 11) | F | p-Value | |
|---|---|---|---|---|---|---|---|
| Age (yr) | 46.3 ± 9.0 | 44.4 ± 6.4 | 48.7 ± 11.1 | 47.9 ± 7.3 | 44.3 ± 11.0 | 0.689 | 0.564 |
| Gender (F/M) | 38/5 | 9/2 | 9/1 | 10/1 | 10/1 | 0.891 | |
| Height (cm) | 159.0 ± 8.2 | 160.3 ± 9.3 | 158.0 ± 8.5 | 159.5 ± 7.9 | 158.3 ± 8.2 | 0.172 | 0.914 |
| Weight (kg) | 66.0 ± 11.3 | 68.5 ± 14.2 | 64.9 ± 10.3 | 67.0 ± 8.4 | 63.4 ± 12.1 | 0.417 | 0.742 |
| BMI (kg/m2) | 26.1 ± 4.0 | 26.5 ± 4.1 | 26.0 ± 3.3 | 26.5 ± 3.8 | 25.4 ± 5.0 | 0.178 | 0.911 |
| FBG (mg/dL) | 111.7 ± 17.2 | 117.4 ± 28.3 | 110.1 ± 7.8 | 111.1 ± 17.9 | 107.9 ± 2.8 | 0.594 | 0.622 |
| HbA1c (%) | 6.1 ± 0.7 | 6.2 ± 0.8 | 6.0 ± 0.5 | 6.3 ± 1.0 | 5.9 ± 0.1 | 0.953 | 0.425 |
| Resting SBP (mmHg) | 124.2 ± 11.3 | 127.4 ± 17.6 | 123.8 ± 11.7 | 123.9 ± 9.0 | 121.7 ± 4.5 | 0.450 | 0.719 |
| Resting DBP (mmHg) | 80.0 ± 8.5 | 79.6 ± 10.8 | 77.5 ± 6.3 | 82.6 ± 8.0 | 80.1 ± 8.5 | 0.636 | 0.596 |
| Resting MAP (mmHg) | 94.7 ± 8.4 | 95.3 ± 12.1 | 92.8 ± 7.5 | 96.5 ± 7.2 | 93.9 ± 6.6 | 0.360 | 0.782 |
| Resting HR (beats/min) | 71.4 ± 10.8 | 67.6 ± 7.5 | 69.7 ± 11.6 | 72.6 ± 11.4 | 75.6 ± 11.9 | 1.149 | 0.341 |
| Physical activity levels | |||||||
| Sitting (h/day) | 10.5 ± 0.5 | 10.4 ± 0.5 | 10.5 ± 0.6 | 10.6 ± 0.5 | 10.4 ± 0.6 | 0.357 | 0.785 |
| Walking (h/day) | 5.5 ± 0.6 | 5.6 ± 0.5 | 5.4 ± 0.6 | 5.4 ± 0.6 | 5.6 ± 0.6 | 0.530 | 0.664 |
| Moderate activity (h/day) | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.0 | 0.591 | 0.625 |
| Vigorous activity (h/day) | 0.3 ± 0.3 | 0.3 ± 0.3 | 0.3 ± 0.2 | 0.2 ± 0.3 | 0.4 ± 0.2 | 0.347 | 0.791 |
| PLA (n = 11) | CBT (n = 10) | EATME (n = 11) | CBT + EATME (n = 11) | Time Effect η2 (p-Value) | Group × Time Interaction η2 (p-Value) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Test | Baseline | Post-Test | Baseline | Post-Test | Baseline | Post-Test | |||
| TC (mg/dL) | 212.4 ± 42.3 | 211.9 ± 43.6 | 207.5 ± 33.5 | 200.1 ± 32.6 | 207.2 ± 19.8 | 211.6 ± 21.4 | 205.6 ± 28.8 | 208.3 ± 33.3 | 0.000 (0.957) | 0.049 (0.573) |
| TG (mg/dL) | 131.6 ± 40.2 | 167.8 ± 83.8 a** | 116.0 ± 30.2 | 97.7 ± 22.9 a*,b* | 145.4 ± 51.9 | 106.2 ± 40.7 a**,b** | 114.5 ± 36.4 | 109.8 ± 39.5 a* | 0.366 (0.001) †† | |
| HDL-c (mg/dL) | 55.4 ± 12.7 | 52.1 ± 11.5 | 59.2 ± 12.5 | 61.3 ± 10.0 | 51.5 ± 11.9 | 55.5 ± 11.6 | 62.5 ± 10.1 | 62.8 ± 12.9 | 0.014 (0.466) | 0.134 (0.129) |
| LDL-c (mg/dL) | 146.4 ± 37.1 | 136.6 ± 32.8 | 138.5 ± 30.4 | 128.2 ± 23.9 | 138.5 ± 20.5 | 137.1 ± 20.2 | 138.3 ± 21.4 | 126.4 ± 27.0 a* | 0.144 (0.014) † | 0.040 (0.657) |
| AIP | 0.4 ± 0.2 | 0.5 ± 0.3 a* | 0.3 ± 0.1 | 0.2 ± 0.2 a*,b* | 0.4 ± 0.3 | 0.3 ± 0.2 a** | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.097 (0.048) † | 0.360 (0.001) †† |
| CRI-I | 4.0 ± 1.1 | 4.2 ± 1.0 | 3.6 ± 0.8 | 3.3 ± 0.6 | 4.2 ± 0.9 | 4.0 ± 0.9 | 3.4 ± 0.7 | 3.4 ± 0.7 | 0.020 (0.372) | 0.136 (0.124) |
| CRI-II | 2.8 ± 0.9 | 2.7 ± 0.8 | 2.4 ± 0.7 | 2.1 ± 0.5 a* | 2.8 ± 0.8 | 2.6 ± 0.7 | 2.3 ± 0.5 | 2.1 ± 0.6 | 0.157 (0.010) †† | 0.042 (0.636) |
| AC | 3.0 ± 1.1 | 3.2 ± 1.2 | 2.6 ± 0.8 | 2.5 ± 0.8 | 3.2 ± 0.9 | 2.9 ± 0.7 a*,b* | 2.4 ± 0.7 | 2.4 ± 0.8 | 0.204 (0.032) †† | |
| PLA (n = 11) | CBT (n = 10) | EATME (n = 11) | CBT + EATME (n = 11) | Time Effect η2 (p-Value) | Group × Time Interaction η2 (p-Value) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Test | Baseline | Post-Test | Baseline | Post-Test | Baseline | Post-Test | |||
| Absolute bench press 1RM (kg) | 34.4 ± 14.8 | 34.6 ± 13.6 | 33.5 ± 9.5 | 37.0 ± 10.5 a** | 35.8 ± 10.8 | 38.6 ± 12.2 a** | 31.1 ± 7.1 | 35.3 ± 5.6 a**,b* | 0.426 (0.000) †† | 0.200 (0.032) †† |
| Relative bench press 1RM (kg/BW) | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ±0.2 a** | 0.5 ± 0.2 | 0.6 ± 0.2 a* | 0.5 ± 0.1 | 0.6 ± 0.1 a**,b* | 0.435 (0.000) †† | 0.232 (0.015) †† |
| Absolute leg press 1RM (kg) | 226.6 ± 76.6 | 223.6 ± 79.5 | 201.2 ± 43.8 | 220.1 ± 55.9 a** | 212.5 ± 56.4 | 219.6 ± 66.2 | 198.2 ± 59.4 | 210.0 ± 61.7 a* | 0.192 (0.004) †† | 0.163 (0.072) |
| Relative leg press 1RM (kg/BW) | 3.3 ± 0.7 | 3.2 ± 0.7 | 3.1 ± 0.4 | 3.5 ± 0.5 a**,b* | 3.2 ± 0.9 | 3.4 ± 1.0 | 3.1 ± 0.5 | 3.4 ± 0.3 a**,b* | 0.316 (0.000) †† | 0.251 (0.010) †† |
| V̇O2max (mL/kg/min) | 36.7 ± 4.7 | 36.7 ± 4.6 | 35.4 ± 3.9 | 39.0 ± 4.3 a**,b* | 35.9 ± 4.5 | 36.0 ± 5.0 | 35.7 ± 5.5 | 38.4 ± 3.8 a**,b* | 0.228 (0.002) †† | 0.219 (0.021) †† |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukatta, U.; Rugthaworn, P.; Klinsukhon, K.; Tumnark, P.; Songcharern, N.; Teethaisong, Y.; Kanpetta, Y.; Phoemsapthawee, J. Synergistic Effects of Microencapsulated Polyphenols and Concurrent Training on Metabolic Health and Fitness in Overweight/Obese Adults with Prediabetes. Nutrients 2025, 17, 3358. https://doi.org/10.3390/nu17213358
Sukatta U, Rugthaworn P, Klinsukhon K, Tumnark P, Songcharern N, Teethaisong Y, Kanpetta Y, Phoemsapthawee J. Synergistic Effects of Microencapsulated Polyphenols and Concurrent Training on Metabolic Health and Fitness in Overweight/Obese Adults with Prediabetes. Nutrients. 2025; 17(21):3358. https://doi.org/10.3390/nu17213358
Chicago/Turabian StyleSukatta, Udomlak, Prapassorn Rugthaworn, Ketsaree Klinsukhon, Piyaporn Tumnark, Nattawut Songcharern, Yothin Teethaisong, Yupaporn Kanpetta, and Jatuporn Phoemsapthawee. 2025. "Synergistic Effects of Microencapsulated Polyphenols and Concurrent Training on Metabolic Health and Fitness in Overweight/Obese Adults with Prediabetes" Nutrients 17, no. 21: 3358. https://doi.org/10.3390/nu17213358
APA StyleSukatta, U., Rugthaworn, P., Klinsukhon, K., Tumnark, P., Songcharern, N., Teethaisong, Y., Kanpetta, Y., & Phoemsapthawee, J. (2025). Synergistic Effects of Microencapsulated Polyphenols and Concurrent Training on Metabolic Health and Fitness in Overweight/Obese Adults with Prediabetes. Nutrients, 17(21), 3358. https://doi.org/10.3390/nu17213358







