Maternal and Infant Characteristics and Pumping Profiles of Women That Predominantly Pump Milk for Their Infants
Abstract
1. Introduction
2. Materials and Methods
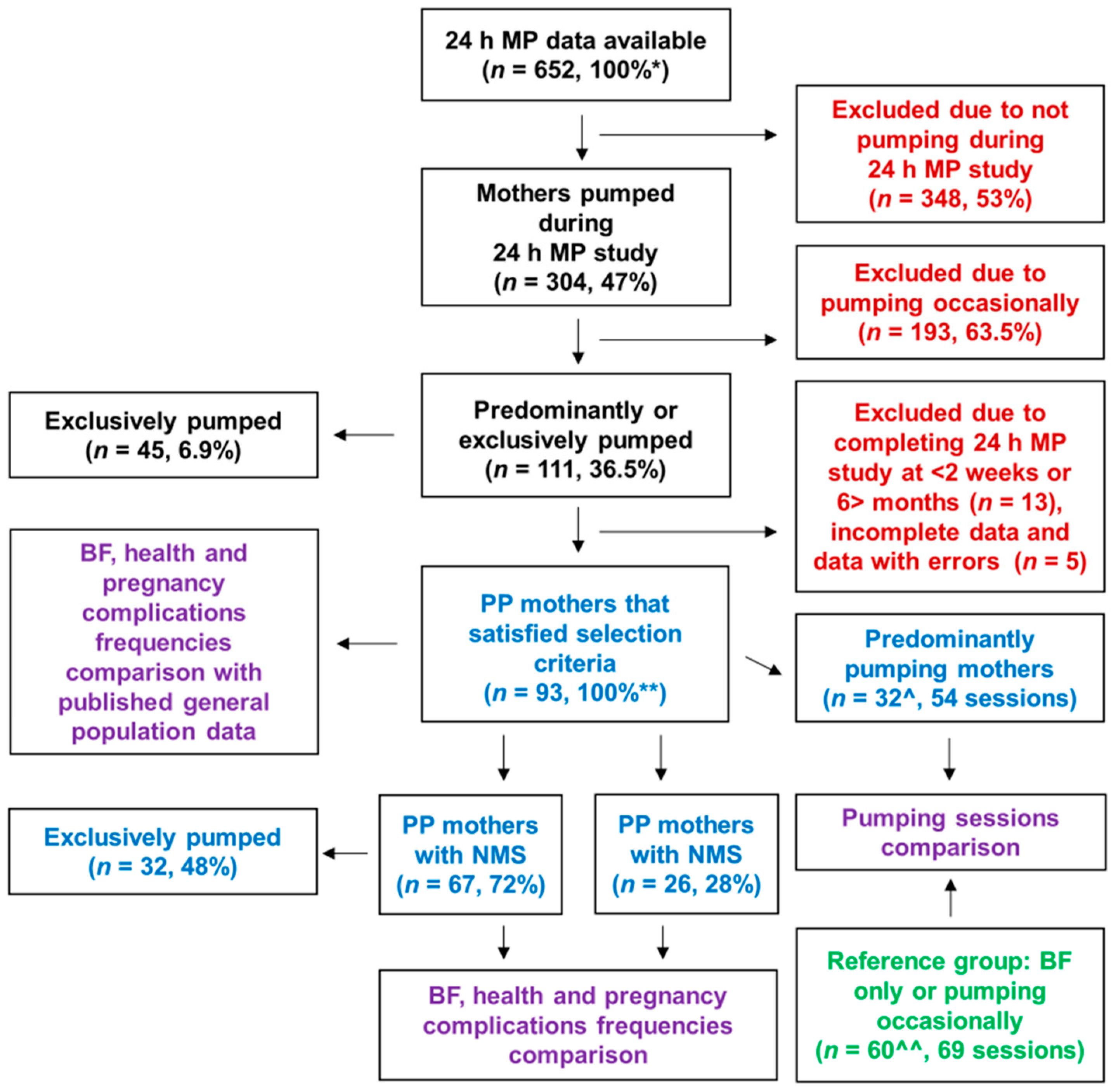
2.1. Study Design and Participants
2.2. Twenty-Four-Hour Milk Profile Data Collection
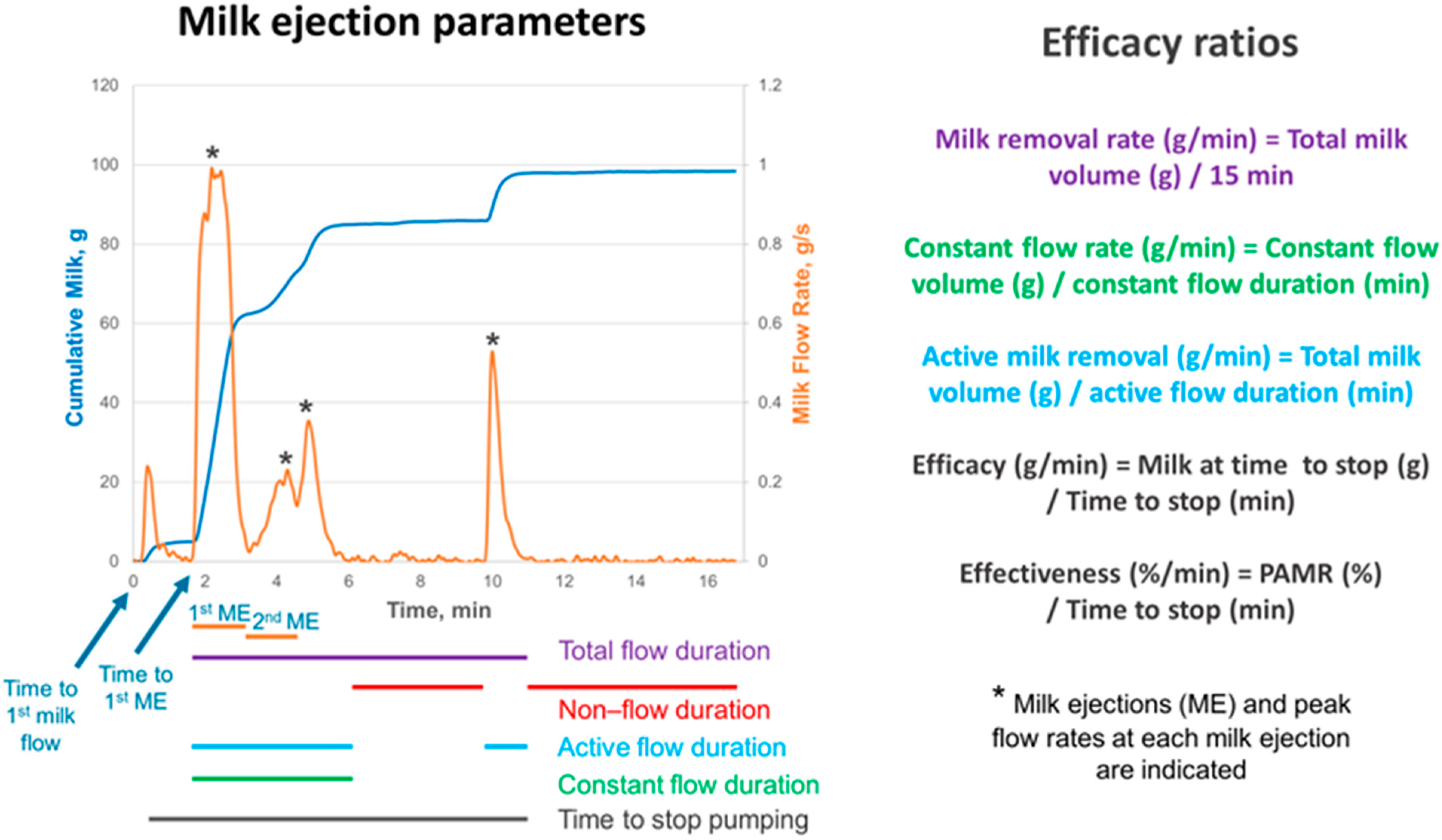
2.3. Milk Removal Data Collection
2.4. Statistical Analyses
3. Results
3.1. Participants’ Demographic, Perinatal and Breastfeeding Characteristics
3.2. Twenty-Four-Hour Pumping and Breastfeeding Characteristics
3.3. Milk Removal Parameters During Pumping Session
4. Discussion
4.1. Breastfeeding Difficulties
4.2. Pregnancy Complications and Mental Health
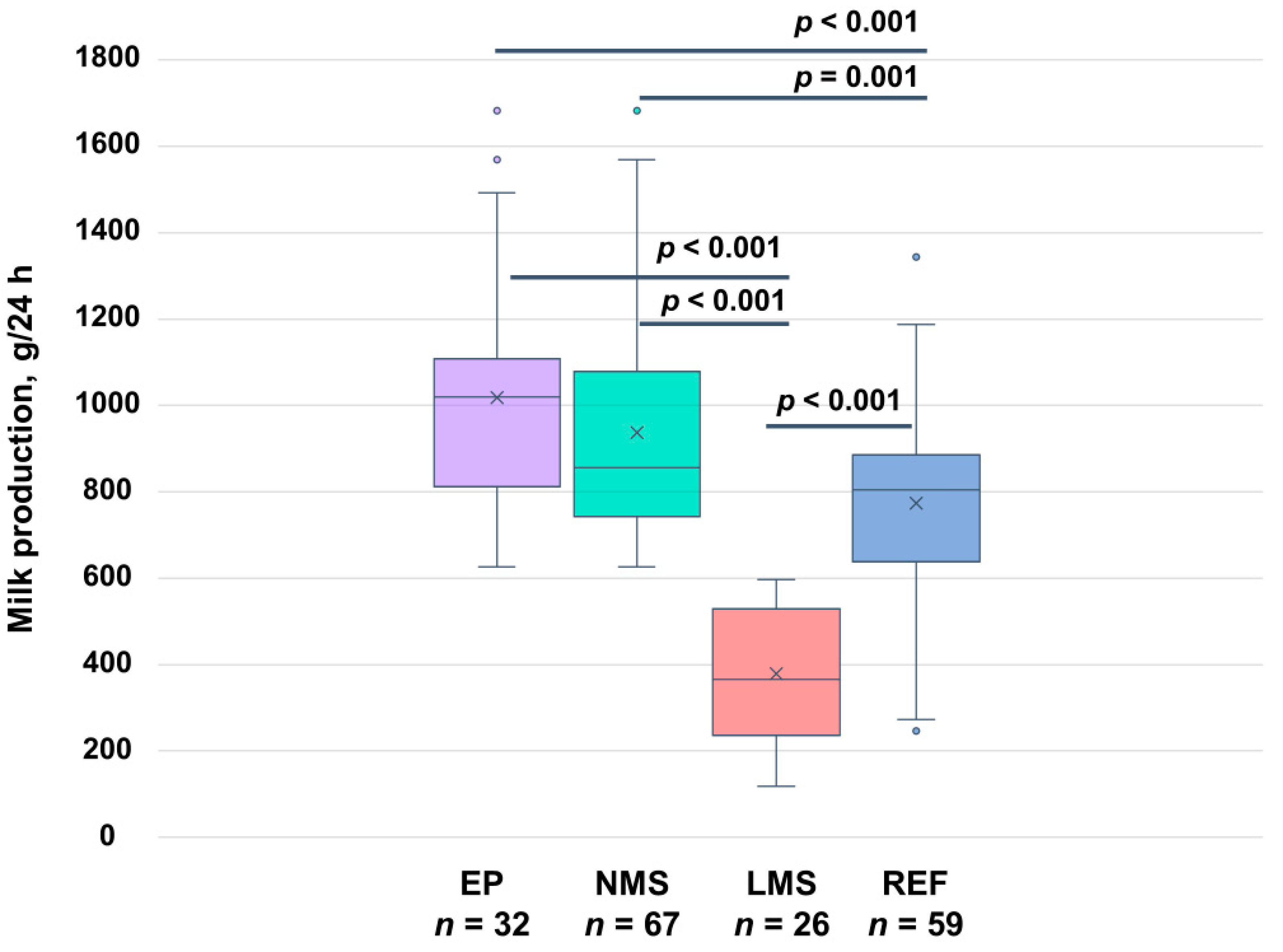
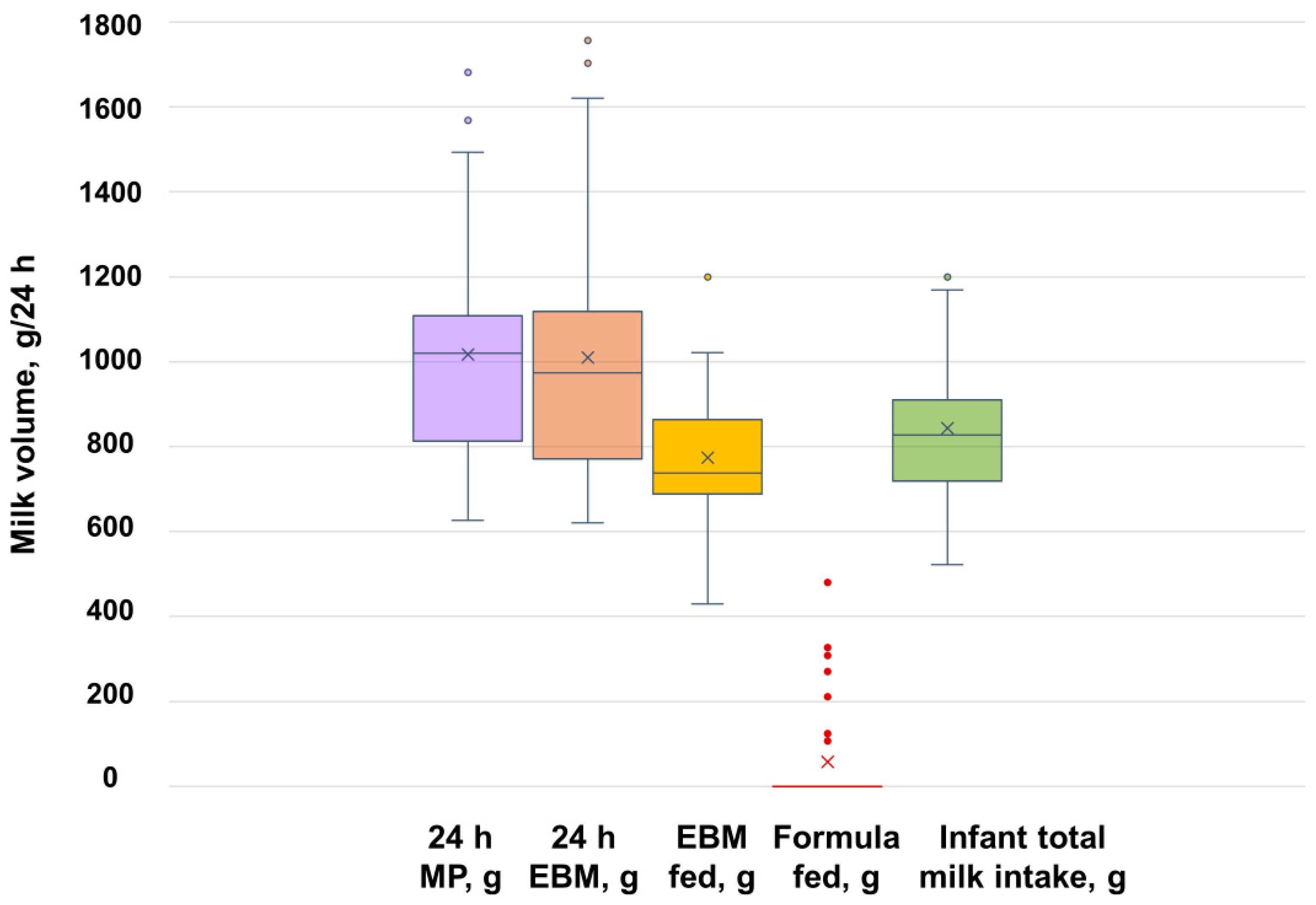
4.3. Pumping vs. Breastfeeding—24 h Milk Production and Infant Milk Intake
4.4. Pumping vs. Breastfeeding—More Information and Support Is Needed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dieterich, C.M.; Felice, J.P.; O’Sullivan, E.; Rasmussen, K.M. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr. Clin. N. Am. 2013, 60, 31–48. [Google Scholar] [CrossRef]
- Hojsak, I.; Chourdakis, M.; Gerasimidis, K.; Hulst, J.; Huysentruyt, K.; Moreno-Villares, J.M.; Joosten, K. What are the new guidelines and position papers in pediatric nutrition: A 2015–2020 overview. Clin. Nutr. ESPEN 2021, 43, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Colbenson, G.A.; Hoff, O.; Olson, E.M.; Ducharme-Smith, A. The impact of wearable breast pumps on physicians’ breastfeeding experience and success. Breastfeed. Med. 2022, 17, 537–543. [Google Scholar] [CrossRef]
- Hubbard, L.; Reis, P.; Neil, J. Beliefs and decision-making of first-time mothers planning to use a breast pump. J. Perinat. Educ. 2023, 32, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Loewenberg Weisband, Y.; Keim, S.A.; Keder, L.M.; Geraghty, S.R.; Gallo, M.F. Early breast milk pumping intentions among postpartum women. Breastfeed. Med. 2017, 12, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Felice, J.P.; Geraghty, S.R.; Quaglieri, C.W.; Yamada, R.; Wong, A.J.; Rasmussen, K.M. “Breastfeeding” without baby: A longitudinal, qualitative investigation of how mothers perceive, feel about, and practice human milk expression. Matern. Child. Nutr. 2017, 13, e12426. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.P.; Patel, A.L.; Hoban, R.; Engstrom, J.L. Which breast pump for which mother: An evidenced-based approach to individualizing breast pump technology. J. Perinatol. 2016, 36, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, K.A. Exclusive breastmilk pumping: A concept analysis. Nurs. Forum 2022, 57, 946–953. [Google Scholar] [CrossRef]
- Crossland, N.; Thomson, G.; Morgan, H.; MacLennan, G.; Campbell, M.; Dykes, F.; Hoddinott, P. Breast pumps as an incentive for breastfeeding: A mixed methods study of acceptability. Matern. Child. Nutr. 2016, 12, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Keim, S.A.; Boone, K.M.; Oza-Frank, R.; Geraghty, S.R. Pumping milk without ever feeding at the breast in the Moms2Moms Study. Breastfeed. Med. 2017, 12, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Strauch, L.; Sweet, L.; Scott, H.; Müller, A. Mechanisms of support for exclusive breastmilk expressers in the community: A scoping review. BMC Pregnancy Childbirth 2019, 19, 511. [Google Scholar] [CrossRef]
- Slater, C.N.; Juntereal, N.A.; Kral, T.V.E.; Spatz, D.L.; Chao, A.M. Comparison of the effect of direct breastfeeding, expressed human milk, and infant formula feeding on infant weight trajectories: A systematic review. Breastfeed. Med. 2024, 19, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Liberty, A.; Rubin, E.S.; Bullard, K.A.; Au, K. Human milk–expression technologies: A primer for obstetricians. Obstet. Gynecol. 2022, 139, 1180–1188. [Google Scholar] [CrossRef]
- Yamada, R.; Rasmussen, K.M.; Felice, J.P. “What is ‘enough’, and how do I make it?”: A qualitative examination of questions mothers ask on social media about pumping and providing an adequate amount of milk for their infants. Breastfeed. Med. 2019, 14, 17–21. [Google Scholar] [CrossRef]
- Jardine, F.M. Breastfeeding without nursing: “If only I’d known more about exclusively pumping before giving birth”. J. Hum. Lact. 2019, 35, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Jardine, F.M. When online support groups prevail: The information experience of chest/breastfeeders who only express their milk. In Proceedings of the ISIC: The Information Behaviour Conference, Pretoria, South Africa, 28 September–1 October 2020. [Google Scholar]
- Nommsen-Rivers, L.A.; Wagner, E.A.; Roznowski, D.M.; Riddle, S.W.; Ward, L.P.; Thompson, A. Measures of maternal metabolic health as predictors of severely low milk production. Breastfeed. Med. 2022, 17, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P.E. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef] [PubMed]
- Hoban, R.; Bigger, H.; Schoeny, M.; Engstrom, J.; Meier, P.; Patel, A.L. Milk volume at 2 weeks predicts mother’s own milk feeding at Neonatal Intensive Care Unit discharge for very low birthweight Infants. Breastfeed. Med. 2018, 13, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.; Hepworth, A.; Sherriff, J.; Cox, D.; Mitoulas, L.; Hartmann, P. Longitudinal changes in breastfeeding patterns from 1 to 6 months of lactation. Breastfeed. Med. 2013, 8, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.C.; Keller, R.; Seacat, J.; Lutes, V.; Neifert, M.; Casey, C.; Allen, J.; Archer, P. Studies in human lactation: Milk volumes in lactating women during the onset of lactation and full lactation. Am. J. Clin. Nutr. 1988, 48, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Geddes, D.T.; Larsson, M.; Doherty, D.A.; Hartmann, P.E. Importance of vacuum for breastmilk expression. Breastfeed. Med. 2008, 3, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Prime, D.K.; Kent, J.C.; Hepworth, A.R.; Trengove, N.J.; Hartmann, P.E. Dynamics of milk removal during simultaneous breast expression in women. Breastfeed. Med. 2012, 7, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.; Lai, C.T.; Ward, L.C.; Geddes, D.T. Thermal physiology of the lactating nipple influences the removal of human milk. Sci. Rep. 2019, 9, 11854. [Google Scholar] [CrossRef] [PubMed]
- Tie, W.J.; Kent, J.C.; Lai, C.T.; Rea, A.; Hepworth, A.R.; Murray, K.; Geddes, D.T. Reproducibility of the creamatocrit technique for the measurement of fat content in human milk. Food Chem. 2021, 356, 129708. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Ramsay, D.T.; Doherty, D.; Larsson, M.; Hartmann, P.E. Response of breasts to different stimulation patterns of an electric breast pump. J. Hum. Lact. 2003, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Australia’s Mothers and Babies; AIHW: Canberra, ACT, Australia, 2024.
- Australian Institute of Health and Welfare. Australia’s Mothers and Babies 2017—In Brief; AIHW: Canberra, ACT, Australia, 2019.
- Australian Institute of Health and Welfare. Diabetes: Australian Facts; AIHW: Canberra, ACT, Australia, 2024.
- Australian Bureau of Statistics. Mental Health; ABS: Canberra, ACT, Australia, 2018.
- Varanasi, L.C.; Subasinghe, A.; Jayasinghe, Y.L.; Callegari, E.T.; Garland, S.M.; Gorelik, A.; Wark, J.D. Polycystic ovarian syndrome: Prevalence and impact on the wellbeing of Australian women aged 16–29 years. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 222–233. [Google Scholar] [CrossRef]
- Kent, J.C.; Ashton, E.; Hardwick, C.M.; Rowan, M.K.; Chia, E.S.; Fairclough, K.A.; Menon, L.L.; Scott, C.; Mather-McCaw, G.; Navarro, K.; et al. Nipple pain in breastfeeding mothers: Incidence, causes and treatments. Int. J. Environ. Res. Public Health 2015, 12, 12247–12263. [Google Scholar] [CrossRef] [PubMed]
- Pincombe, J.; Baghurst, P.; Antoniou, G.; Peat, B.; Henderson, A.; Reddin, E. Baby Friendly Hospital Initiative practices and breast feeding duration in a cohort of first-time mothers in Adelaide, Australia. Midwifery 2008, 24, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.M.F.; Flor, T.B.M.; Araújo, M.G.G.; Xavier, A.M.S.F.; Mata, A.M.B.D.; Pires, V.C.D.C.; Oliveira, L.I.C.; Andrade, F.B. Feeding practices and early weaning in the neonatal period: A cohort study. Rev. Saude Publica 2021, 55, 63. [Google Scholar] [CrossRef] [PubMed]
- Gianni, M.L.; Bettinelli, M.E.; Manfra, P.; Sorrentino, G.; Bezze, E.; Plevani, L.; Cavallaro, G.; Raffaeli, G.; Crippa, B.L.; Colombo, L.; et al. Breastfeeding difficulties and risk for early breastfeeding cessation. Nutrients 2019, 11, 2266. [Google Scholar] [CrossRef]
- Yakov, Y.Y. Perceived insufficient milk supply (pims) in lactating mothers. In Multiple Pregnancy; Hassan, S.A., Ed.; IntechOpen: London, UK, 2022; pp. 1–8. [Google Scholar]
- Law, A.; McCoy, M.; Lynen, R.; Curkendall, S.M.; Gatwood, J.; Juneau, P.L.; Landsman-Blumberg, P. The prevalence of complications and healthcare costs during pregnancy. J. Med. Econ. 2015, 18, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; D’Arcy, H.; Gillespie, B.; Bobo, J.K.; Schwartz, K. Lactation mastitis: Occurrence and medical management among 946 breastfeeding women in the United States. Am. J. Epidemiol. 2002, 155, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian, M.; Mata, M. 2—Advances in Analysis of Mean and Covariance Structure when Data are Incomplete. In Handbook of Computing and Statistics with Applications; Lee, S.-Y., Ed.; Handbook of Latent Variable and Related Models; North-Holland: Amsterdam, The Netherlands, 2007; pp. 21–44. [Google Scholar]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; Franca, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Nardella, D. Pumps: A possible tool to promote more equitable lactation outcomes. Yale J. Biol. Med. 2024, 97, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Johns, H.; Amir, L.; McLachlan, H.; Forster, D. Breast pump use amongst mothers of healthy term infants in Melbourne, Australia: A prospective cohort study. Midwifery 2016, 33, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.A.R.; Vaz, J.S.; Maia, F.S.; Baker, P.; Gatica-Domínguez, G.; Piwoz, E.; Rollins, N.; Victora, C.G. Rates and time trends in the consumption of breastmilk, formula, and animal milk by children younger than 2 years from 2000 to 2019: Analysis of 113 countries. Lancet Child Adolesc. Health 2021, 5, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L. Maternal perceptions of insufficient milk supply in breastfeeding. J. Nurs. Scholarsh. 2008, 40, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Ashton, E.; Hardwick, C.M.; Rea, A.; Murray, K.; Geddes, D.T. Causes of perception of insufficient milk supply in Western Australian mothers. Matern. Child. Nutr. 2021, 17, e13080. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Yu, X.Y.; Zeng, T.Y. The rates and factors of perceived insufficient milk supply: A systematic review. Matern. Child. Nutr. 2022, 18, e13255. [Google Scholar] [CrossRef]
- Jin, X.; Perrella, S.L.; Lai, C.T.; Taylor, N.L.; Geddes, D.T. Causes of low milk supply: The roles of estrogens, progesterone, and related external factors. Adv. Nutr. 2023, 15, 100129. [Google Scholar] [CrossRef] [PubMed]
- Farah, E.; Barger, M.K.; Klima, C.; Rossman, B.; Hershberger, P. Impaired Lactation: Review of Delayed Lactogenesis and Insufficient Lactation. J. Midwifery Womens Health 2021, 66, 631–640. [Google Scholar] [CrossRef]
- Peacock-Chambers, E.; Dicks, K.; Sarathy, L.; Brown, A.A.; Boynton-Jarrett, R. Perceived maternal behavioral control, infant behavior, and milk supply: A qualitative study. J. Dev. Behav. Pediatr. 2017, 38, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Quinn, E.A.; Sobonya, S.; Palmquist, A.E.L. Maternal perceptions of human milk expression output: An experimental design using photographs of milk. Soc. Sci. Med. 2023, 324, 115871. [Google Scholar] [CrossRef] [PubMed]
- Suwaydi, M.A.; Wlodek, M.E.; Lai, C.T.; Prosser, S.A.; Geddes, D.T.; Perrella, S.L. Delayed secretory activation and low milk production in women with gestational diabetes: A case series. BMC Pregnancy Childbirth 2022, 22, 350. [Google Scholar] [CrossRef] [PubMed]
- Jirakittidul, P.; Panichyawat, N.; Chotrungrote, B.; Mala, A. Prevalence and associated factors of breastfeeding in women with gestational diabetes in a University Hospital in Thailand. Int. Breastfeed. J. 2019, 14, 34. [Google Scholar] [CrossRef]
- Soltani, H.; Arden, M. Factors associated with breastfeeding up to 6 months postpartum in mothers with diabetes. J. Obstet. Gynecol. Neonatal Nurs. 2009, 38, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Sarki, M.; Parlesak, A.; Robertson, A. Comparison of national cross-sectional breast-feeding surveys by maternal education in Europe (2006–2016). Public Health Nutr. 2019, 22, 848–861. [Google Scholar] [CrossRef]
- Daly, S.E.J.; Kent, J.C.; Owens, R.A.; Hartmann, P.E. Frequency and degree of milk removal and the short-term control of human milk synthesis. Exp. Physiol. 1996, 81, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Perrella, S.L.; Abelha, S.G.; Vlaskovsky, P.; McEachran, J.L.; Prosser, S.A.; Geddes, D.T. Australian women’s experiences of establishing breastfeeding after Caesarean birth. Int. J. Environ. Res. Public. Health 2024, 21, 296. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wan, W.; Zhu, C. Breastfeeding after a cesarean section: A literature review. Midwifery 2021, 103, 103117. [Google Scholar] [CrossRef] [PubMed]
- Mallick, L.M.; Shenassa, E.D. Variation in breastfeeding initiation and duration by mode of childbirth: A prospective, population-based study. Breastfeed. Med. 2024, 19, 262–274. [Google Scholar] [CrossRef]
- Geddes, D.T.; Prosser, S.A.; Gridneva, Z.; Perrella, S.L. Risk factors for low milk production. Proceedings 2023, 93, 17. [Google Scholar] [CrossRef]
- Chen, D.C.; Nommsen-Rivers, L.; Dewey, K.G.; Lönnerdal, B. Stress during labor and delivery and early lactation performance. Am. J. Clin. Nutr. 1998, 68, 335–344. [Google Scholar] [CrossRef]
- Brown, A.; Rance, J.; Bennett, P. Understanding the relationship between breastfeeding and postnatal depression: The role of pain and physical difficulties. J. Adv. Nurs. 2016, 72, 273–282. [Google Scholar] [CrossRef]
- Rollins, N.C.; Bhandari, N.; Hajeebhoy, N.; Horton, S.; Lutter, C.K.; Martines, J.C.; Piwoz, E.G.; Richter, L.M.; Victoria, C.G. The Lancet Breastfeeding Series Group. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016, 387, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.; Kent, J.C.; Lai, C.T.; Geddes, D.T. Comparison of maternal milk ejection characteristics during pumping using infant-derived and 2-phase vacuum patterns. Int. Breastfeed. J. 2019, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.; Kent, J.C.; Lai, C.T.; Mitoulas, L.R.; Cregan, M.D.; Hartmann, P.E.; Geddes, D.T. Milk ejection patterns: An intra- individual comparison of breastfeeding and pumping. BMC Pregnancy Childbirth 2015, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Warden, A.H.; McEachran, J.L.; Lai, C.T.; Perrella, S.L.; Geddes, D.T. Human milk expression technologies: An evaluation of efficacy and comfort of hands-free, in-bra, breastmilk collection pump set. Clin. Nutr. Open Sci. 2023, 49, 28–36. [Google Scholar] [CrossRef]
- Meier, P.P.; Engstrom, J.L.; Hurst, N.M.; Ackerman, B.; Allen, M.; Motykowski, J.E.; Zuleger, J.L.; Jegier, B.J. A comparison of the efficiency, efficacy, comfort, and convenience of two hospital-grade electric breast pumps for mothers of very low birthweight infants. Breastfeed. Med. 2008, 3, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.T.; Gridneva, Z.; Perrella, S.L.; Mitoulas, L.R.; Kent, J.C.; Stinson, L.F.; Lai, C.T.; Sakalidis, V.; Twigger, A.J.; Hartmann, P.E. 25 years of research in human lactation: From discovery to translation. Nutrients 2021, 13, 3071. [Google Scholar] [CrossRef]
- Dewey, K.G.; Lönnerdal, B. Infant self-regulation of breast milk intake. Acta Paediatr. 1986, 75, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.; Oliva-Rasbach, J. Is maternal milk production limiting for infant growth during the first year of life in breastfed infants. In Human Lactation 3: The Effects of Human Milk on the Recipient Infant; Goldman, A.S., Atkinson, S.A., Hanson, L.A., Eds.; Plenum Press: New York, NY, USA, 1987; pp. 123–133. [Google Scholar]
- Win, N.N.; Binns, C.W.; Zhao, Y.; Scott, J.A.; Oddy, W.H. Breastfeeding duration in mothers who express breast milk: A cohort study. Int. Breastfeed. J. 2006, 1, 28. [Google Scholar] [CrossRef]
- Lee, S.; Kelleher, S.L. Biological underpinnings of breastfeeding challenges: The role of genetics, diet, and environment on lactation physiology. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E405–E422. [Google Scholar] [CrossRef]
- Becker, G.E.; Smith, H.A.; Cooney, F. Methods of milk expression for lactating women. Cochrane Database Syst. Rev. 2016, 9, CD006170. [Google Scholar] [CrossRef] [PubMed]
- Jardine, F.M. Breastfeeding Without Nursing: The Lived Experiences of Exclusive Pumpers; University of Maryland: College Park, MD, USA, 2020. [Google Scholar]
- Scanlon, K.S.; Alexander, M.P.; Serdula, M.K.; Davis, M.K.; Bowman, B.A. Assessment of infant feeding: The validity of measuring milk intake. Nutr. Rev. 2002, 60, 235–251. [Google Scholar] [CrossRef]
| Maternal and Infant Characteristcs | PP (n = 93) | EP (n = 32) | NMS (n = 67) | LMS (n = 26) | p-Value 2 |
|---|---|---|---|---|---|
| Maternal age at 24 h MP (years) | 33.9 ± 4.3 1 | 33.9 ± 4.9 | 34.0 ± 4.4 | 33.4 ± 4.2 | 0.56 |
| Parity (primiparous) | 70 (75.3) | 23 (71.9) | 49 (73.1) | 21 (80.8) | 0.59 |
| Body mass index (kg/m2) | 28.5 ± 6.9 3 | 28.7 ± 4.7 4 | 28.4 ± 7.1 5 | 28.7 ± 6.6 6 | 0.88 |
| 24 h MP time (months postpartum) | 2.5 ± 1.6 | 3.4 ± 1.6 | 2.7 ± 1.6 | 1.8 ± 1.4 | 0.008 |
| Highest maternal education level | n = 93 | n = 32 | n = 67 | n = 26 | |
| High school | 7 (7.5) | 3 (9.4) | 7 (10.5) | 0 (0.0) | 0.18 |
| Certificate/apprenticeship | 17 (18.3) | 4 (12.5) | 10 (14.9) | 7 (26.9) | 0.23 |
| Tertiary degree | 69 (74.2) | 25 (78.1) | 50 (74.6) | 19 (73.1) | 1.00 |
| Race | n = 92 | n = 32 | n = 66 | n = 26 | |
| Caucasian | 76 (82.6) | 25 (78.1) | 56 (84.8) | 21 (80.8) | 0.76 |
| Asian | 9 (9.8) | 4 (12.5) | 6 (9.1) | 3 (11.5) | 0.71 |
| Other | 6 (6.5) | 3 (9.4) | 4 (6.1) | 2 (7.5) | 1.00 |
| Infant characteristcs | n = 93 | n = 32 | n = 67 | n = 26 | |
| Sex (Male) | 47 (50.5) | 18 (56.3) | 33 (49.3) | 14 (53.9) | 0.69 |
| Birth gestation (weeks) | 38.7 ± 1.5 7 | 38.7 ± 1.1 | 38.5 ± 1.5 8 | 39.0 ± 1.4 4 | 0.23 |
| Birth weight (g) | 3251 ± 560 | 3412 ± 578 | 3242 ± 579 | 3273 ± 518 | 0.81 |
| Birth mode | n = 91 | n = 30 | n = 65 | n = 26 | |
| Unassisted vaginal | 29 (31.9) | 8 (26.7) | 23 (35.4) | 6 (23.1) | 0.32 |
| Assisted vaginal | 14 (15.4) | 3 (10.0) | 8 (12.3) | 6 (23.1) | 0.21 |
| Elective caesarean | 28 (30.8) | 11 (36.7) | 23 (35.4) | 5 (19.2) | 0.21 |
| Non-elective caesarean | 20 (22.0) | 8 (26.7) | 11 (16.9) | 9 (34.6) | 0.092 |
| PP (n = 93) | EP (n = 32) | NMS (n = 67) | LMS (n = 26) | p-Value 2 | |
|---|---|---|---|---|---|
| Perinatal complications | n = 93 | n = 32 | n = 67 | n = 26 | |
| Overall | 54 (58.1) 1 | 22 (68.8) | 40 (59.7) | 14 (53.9) | 0.61 |
| Gestational diabetes mellitus | 26 (28.0) | 11 (34.4) | 19 (28.4) | 7 (26.9) | 1.00 |
| Type 2 diabetes | 3 (3.2) | 2 (6.3) | 3 (4.5) | 0 (0.0) | 0.56 |
| Preterm birth | 9 (9.7) | 2 (6.3) | 7 (10.6) 3 | 1 (0.4) 4 | 0.44 |
| Gestational hypertension | 7 (7.9) | 5 (16.7) | 7 (10.5) | 0 (0.0) | 0.18 |
| Foetal growth restriction | 6 (6.5) | 1 (3.1) | 4 (6.0) | 2 (7.7) | 0.67 |
| Maternal health conditions | n = 91 | n = 30 | n = 64 | n = 26 | |
| Overall | 50 (54.9) | 18 (60.0) | 37 (57.8) | 13 (50.0) | 0.50 |
| Anxiety/depression | 25 (28.1) 5 | 7 (23.3) | 17 (27.0) 6 | 8 (30.8) | 0.80 |
| Polycystic ovary syndrome | 17 (19.1) 5 | 8 (26.7) | 13 (20.6) 6 | 4 (15.4) | 0.77 |
| Fertility issues | 10 (11.2) 5 | 4 (13.3) | 6 (9.5) 6 | 4 (15.4) | 0.47 |
| Infant health conditions | n = 93 | n = 32 | n = 67 | n = 26 | |
| Overall | 20 (21.5) | 11 (34.4) | 18 (26.9) | 2 (7.7) | 0.051 |
| Infant transfer to NICU | 5 (5.4) | 3 (9.4) | 5 (7.5) | 0 (0.0) | 0.32 |
| Laryngomalacia | 2 (2.2) | 1 (3.1) | 2 (3.0) | 0 (0.0) | 1.00 |
| Breastfeeding difficulties | n = 73 | n = 25 | n = 54 | n = 19 | |
| Attachement difficulties | 36 (49.3) | 11 (44.0) | 26 (48.2) | 10 (52.6) | 0.74 |
| Nipple pain | 30 (41.1) | 10 (40.0) | 24 (44.4) | 6 (31.6) | 0.42 |
| Percieved low milk supply | 29 (39.7) | 5 (20.0) | 13 (24.1) | 16 (84.2) | <0.001 |
| Nipple shield use | 23 (31.5) | 6 (24.0) | 15 (27.8) | 8 (42.1) | 0.27 |
| Blocked milk ducts | 11 (15.1) | 4 (16.0) | 8 (14.8) | 3 (15.8) | 1.00 |
| Breast surgery/nipple piercing | 8 (8.6) | 2 (6.3) | 4 (6.0) | 4 (15.4) | 0.19 |
| Mastitis | 6 (8.2) | 1 (4.0) | 4 (7.4) | 2 (10.5) | 0.65 |
| Oversupply | 5 (6.8) | 4 (16.0) | 5 (9.3) | 0 (0.0) | 0.32 |
| Other breastfeeding difficulties | 4 (5.5) | 2 (8.0) | 3 (5.6) | 1 (5.3) | 1.00 |
| Absence of breastfeeding difficluties | 12 (16.4) | 5 (20.0) | 12 (22.2) | 0 (0.0) | 0.029 |
| Maternal Conditions | PP Frequency | General Population Frequency | Total Sample Size | Chi-Square Statistic Value | p-Value 2 |
|---|---|---|---|---|---|
| Mode of birth | |||||
| Caesarean section | 48 (52.8) 1 | 114,440 (39.0) 3 | 293,526 | 7.23 | 0.007 |
| Pregnancy complications | |||||
| Overall | 54 (58.1) | 151,084 (46.9) 4 | 322,234 | 4.65 | 0.031 |
| Gestational diabetes mellitus | 26 (28.0) | 50,511 (17.9) 5 | 293,528 | 7.53 | 0.006 |
| Gestational hypertension | 7 (7.9) | 9205 (3.1) 5 | 293,528 | 5.89 | 0.015 |
| Health conditions | |||||
| Polycystic ovary syndrome | 17 (19.1) | 31 (12.2) 6 | 343 | 2.60 | 0.11 |
| Anxiety/depression | 25 (28.1) | 1,917,557 (15.7) 7 | 12,213,831 | 9.53 | 0.002 |
| Breastfeeding difficulties | |||||
| Attachment difficulties | 36 (49.3) | 50 (23.5) 8 | 286 | 17.27 | <0.001 |
| Nipple pain | 30 (41.1) | 169 (36.0) 9 | 542 | 0.70 | 0.40 |
| Percieved low milk supply | 29 (39.7) | 2101 (38.8) 10 | 5487 | 0.03 | 0.87 |
| Nipple shield use | 23 (31.5) | 45 (14.2) 11 | 390 | 12.35 | <0.001 |
| Mastitis | 6 (8.2) | 90 (9.5) 12 | 1019 | 1.13 | 0.72 |
| Absence of breastfeeding difficluties | 12 (16.4) | 497 (90.0) 13 | 625 | 231.05 | <0.001 |
| Characteristics | EPM (n = 32) | NMS (n = 67) | LMS (n = 26) | p-Value 2 |
|---|---|---|---|---|
| 24 h milk production (g) | 1018 ± 274 1 | 937 ± 263 | 379 ± 149 | <0.001 |
| Breastfeeding frequency | 0.0 ± 0.0 | 3.6 ± 4.7 | 4.1 ± 4.0 | 0.64 |
| Total amount breastfed (g) | 0.0 ± 0.0 | 110 ± 140 | 73 ± 83 | 0.20 |
| Average amount breastfed (g) | 0.0 ± 0.0 | 19.8 ± 29.6 | 13.7 ± 16.4 | 0.33 |
| Pumping frequency (per breast) | 11.6 ± 2.8 | 11.1 ± 3.6 | 9.8 ± 3.4 | 0.13 |
| Pumping session frequency | 6.2 ± 1.8 | 6.0 ± 2.0 | 5.2 ± 1.7 | 0.058 |
| Total expressed (g) | 1010 ± 307 | 822 ± 333 | 320 ± 137 | <0.001 |
| EBM feed frequency | 7.7 ± 2.6 3 | 7.3 ± 2.7 4 | 4.1 ± 2.6 | <0.001 |
| Total EBM fed (g) | 773 ± 184 3 | 603 ± 235 4 | 244 ± 161 | <0.001 |
| Average EBM per feed (g) | 108.2 ± 35.0 3 | 86.7 ± 35.6 4 | 60.4 ± 35.0 | 0.002 |
| Average amount of breast milk fed (g) | 773 ± 184 3 | 721 ± 172 4 | 317 ± 182 | <0.001 |
| Formula feed frequency | 0.6 ± 1.5 5 | 0.6 ± 1.4 | 4.1 ± 2.8 | <0.001 |
| Total formula fed (g) | 57 ± 123 5 | 49 ± 108 | 331 ± 231 | <0.001 |
| Average formula per feed (g) | 26 ± 51 5 | 24 ± 46 6 | 73 ± 54 | <0.001 |
| Total infant milk intake (g) 7 | 843 ± 170 | 756 ± 178 | 654 ± 160 | 0.014 |
| Maternal and Infant Characteristcs | PP (n = 32) | Reference Group (n = 60) | p-Value 2 |
| Maternal age at 24 h MP (years) | 33.2 ± 4.6 1 | 32.7 ± 4.4 | 0.53 |
| Parity (primiparous) | 24 (75.0) | 38 (63.3) | 0.26 |
| Infant birth gestation (weeks) | 38.9 ± 1.5 | 39.0 ± 1.4 | 0.72 |
| Birth weight (g) | 3389 ± 552 | 3473 ± 527 | 0.39 |
| Session time (months postpartum) | 3.4 ± 1.3 | 4.2 ± 1.5 | 0.003 |
| 24 h milk production (g) | 962 ± 338 3 | 773 ± 233 4 | 0.004 |
| Low milk supply (<600 g/24 h) | 3 (11.5) 3 | 10 (17.0) 4 | 0.75 |
| Milk Removal Parameters | PP (n = 54 Sessions) | Reference Group (n = 69 Sessions) | p-Value |
| Total milk removed (g) | 78 ± 51 | 93 ± 51 | 0.090 |
| PAMR (%) | 64 ± 34 5 | 70 ± 18 | 0.24 |
| Crt pre-expression (%) | 3.9 ± 2.4 | 4.2 ± 2.8 | 0.56 |
| Crt post-expression (%) | 12.6 ± 4.7 | 13.6 ± 4.6 | 0.26 |
| DOF pre-expression (%) | 0.70 ± 0.23 5 | 0.74 ± 0.24 | 0.39 |
| DOF post-expression (%) | 0.08 ± 0.13 5 | 0.12 ± 0.13 | 0.099 |
| Parameters | PP (n = 52 Sessions) | Reference Group (n = 67 Sessions) | p-Value 2 |
|---|---|---|---|
| Milk ejection parameters | n = 46 | n = 64 | |
| Number of ME | 5.0 ± 1.4 1 | 3.7 ± 1.3 | <0.001 |
| Time to 1st milk flow (min) | 0.95 ± 0.88 3 | 1.01 ± 0.68 4 | 0.72 |
| Time to 1st ME (min) | 1.28 ± 0.87 5 | 1.47 ± 1.00 | 0.30 |
| 1st ME duration (min) | 2.02 ± 0.46 | 2.04 ± 0.56 | 0.85 |
| 2nd ME duration (min) | 1.91 ± 0.40 | 2.11 ± 0.61 | 0.050 |
| Peak flow rate 1st ME (g/s) | 0.31 ± 0.15 | 0.51 ± 0.29 | <0.001 |
| Peak flow rate 2nd ME (g/s) | 0.29 ± 0.24 | 0.42 ± 0.26 | 0.011 |
| Time to peak flow rate 1st ME (g/s) | 0.74 ± 0.56 | 0.74 ± 0.42 | 0.96 |
| Time to peak flow rate 2nd ME (g/s) | 0.56 ± 0.38 | 0.62 ± 0.28 | 0.32 |
| Milk during 1st ME (g) | 23 ± 13 | 36 ± 26 | 0.002 |
| Milk during 2nd ME (g) | 21 ± 19 | 29 ± 22 | 0.051 |
| Milk during 1st ME (%) | 33 ± 17 | 40 ± 19 | 0.067 |
| Milk during 2nd ME (%) | 24 ± 8 | 30 ± 15 | 0.014 |
| Milk during first two ME (g) | 43 ± 27 | 63 ± 36 | 0.002 |
| Milk during first two ME (%) | 58 ± 18 | 69 ± 17 | 0.001 |
| Flow duration parameters | n = 44 | n = 36 | |
| Total flow duration (min) | 12.9 ± 2.6 | 13.2 ± 3.3 | 0.69 |
| Active flow duration (min) | 11.6 ± 3.2 | 9.0 ± 3.2 6 | 0.002 |
| Constant flow duration (min) | 8.6 ± 4.0 3 | 7.5 ± 3.7 | 0.22 |
| Non-flow duration (min) | 1.4 ± 1.9 | 3.6 ± 3.2 | 0.009 |
| Time to stop pumping (min) | 11.8 ± 3.0 | 11.4 ± 3.5 | 0.65 |
| Parameters | PP (n = 54 Sessions) | Reference Group (n = 68 Sessions) | p-Value 2 |
|---|---|---|---|
| Efficacy ratios | n = 44 | n = 32 | |
| Milk removal rate (g/min) | 5.2 ± 3.4 1,3 | 6.3 ± 3.4 4 | 0.094 |
| Constant flow rate (g/min) | 8.2 ± 4.8 | 10.2 ± 5.0 | 0.079 |
| Active milk removal (g/min) | 7.0 ± 4.1 | 11.0 ± 6.5 5 | 0.001 |
| Efficacy (g/min) | 7.0 ± 4.9 6 | 7.8 ± 4.0 | 0.45 |
| Effectiveness (%/min) | 5.4 ± 2.2 7 | 6.9 ± 3.6 | 0.044 |
| Milk after time to stop pumping (g) | 2.1 ± 2.4 6 | 3.7 ± 3.1 | 0.011 |
| Comfort parameters | n = 54 | n = 67 | |
| Expression vacuum (mmHg) | −187 ± 56 8 | −221 ± 34 9 | <0.001 |
| Nipple temperature changes (°C) | −0.6 ± 2.5 10 | 0.1 ± 2.8 11 | 0.14 |
| Initial comfort level (1 to 5) 12 | 1.5 ± 0.7 | 1.6 ± 0.7 | 0.72 |
| Final comfort level (1 to 5) 12 | 1.5 ± 0.6 | 1.5 ± 0.6 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gridneva, Z.; Warden, A.H.; McEachran, J.L.; Perrella, S.L.; Lai, C.T.; Geddes, D.T. Maternal and Infant Characteristics and Pumping Profiles of Women That Predominantly Pump Milk for Their Infants. Nutrients 2025, 17, 366. https://doi.org/10.3390/nu17020366
Gridneva Z, Warden AH, McEachran JL, Perrella SL, Lai CT, Geddes DT. Maternal and Infant Characteristics and Pumping Profiles of Women That Predominantly Pump Milk for Their Infants. Nutrients. 2025; 17(2):366. https://doi.org/10.3390/nu17020366
Chicago/Turabian StyleGridneva, Zoya, Ashleigh H. Warden, Jacki L. McEachran, Sharon L. Perrella, Ching Tat Lai, and Donna T. Geddes. 2025. "Maternal and Infant Characteristics and Pumping Profiles of Women That Predominantly Pump Milk for Their Infants" Nutrients 17, no. 2: 366. https://doi.org/10.3390/nu17020366
APA StyleGridneva, Z., Warden, A. H., McEachran, J. L., Perrella, S. L., Lai, C. T., & Geddes, D. T. (2025). Maternal and Infant Characteristics and Pumping Profiles of Women That Predominantly Pump Milk for Their Infants. Nutrients, 17(2), 366. https://doi.org/10.3390/nu17020366







