The Improvement Effects of Weizmannia coagulans BC99 on Liver Function and Gut Microbiota of Long-Term Alcohol Drinkers: A Randomized Double-Blind Clinical Trial
Highlights
- Weizmannia coagulans BC99 intervention can help mitigate liver damage caused by alcohol intake.
- Weizmannia coagulans BC99 intervention can reduce the levels of pro-inflammatory factors and increase the levels of anti-inflammatory factors.
- The increase in abundance of Muribaculaceae induced by Weizmannia coagulans BC99 is a key factor in alleviating alcohol-induced liver damage.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Study Design
2.3. Probiotic
2.4. Collection and Determination of Blood Samples
2.5. Collection and Preparation of Fecal Samples
2.6. Intestinal Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Blood Routine Indicators
3.3. Liver Function Indicators
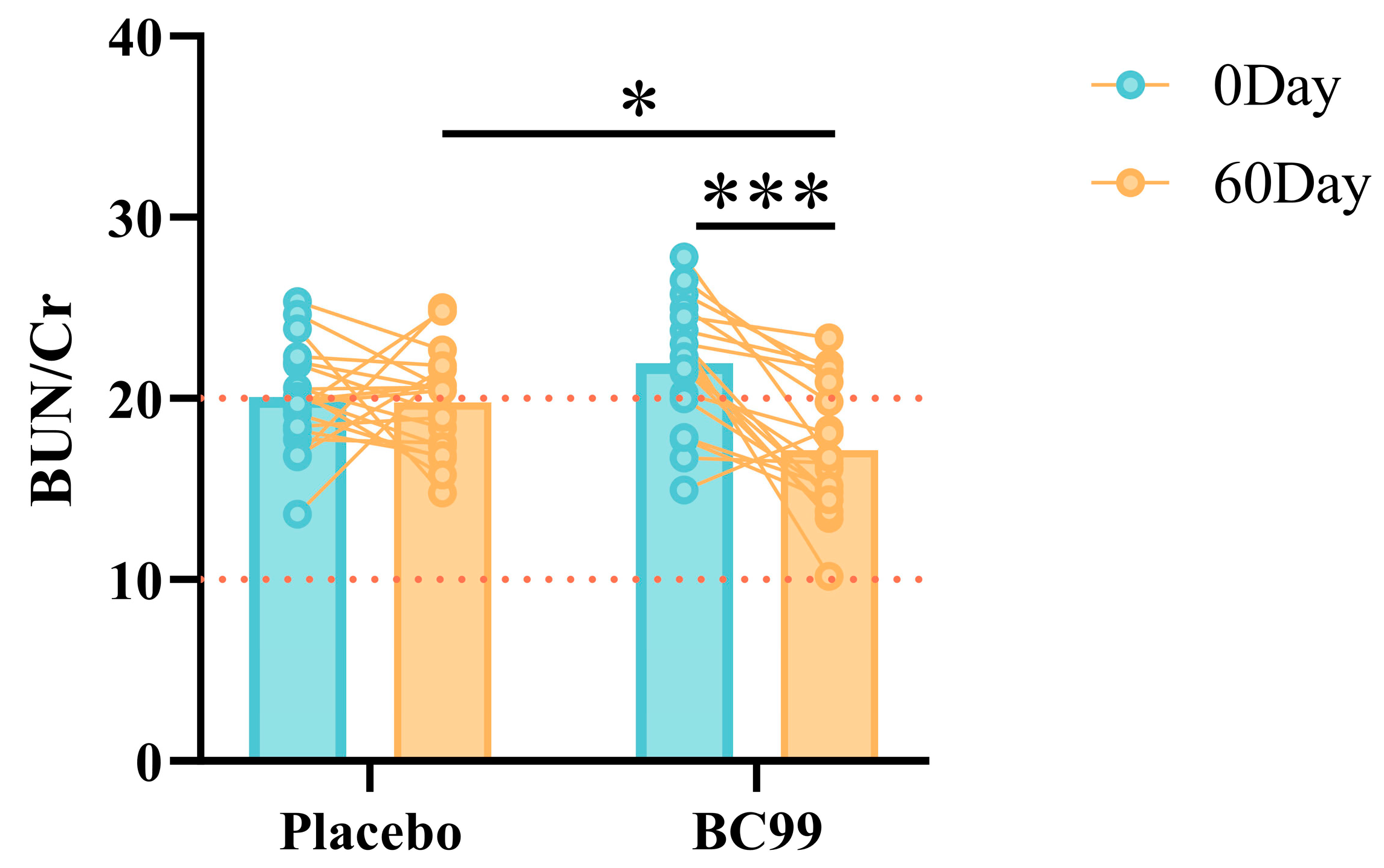
3.4. Renal Function Indicators
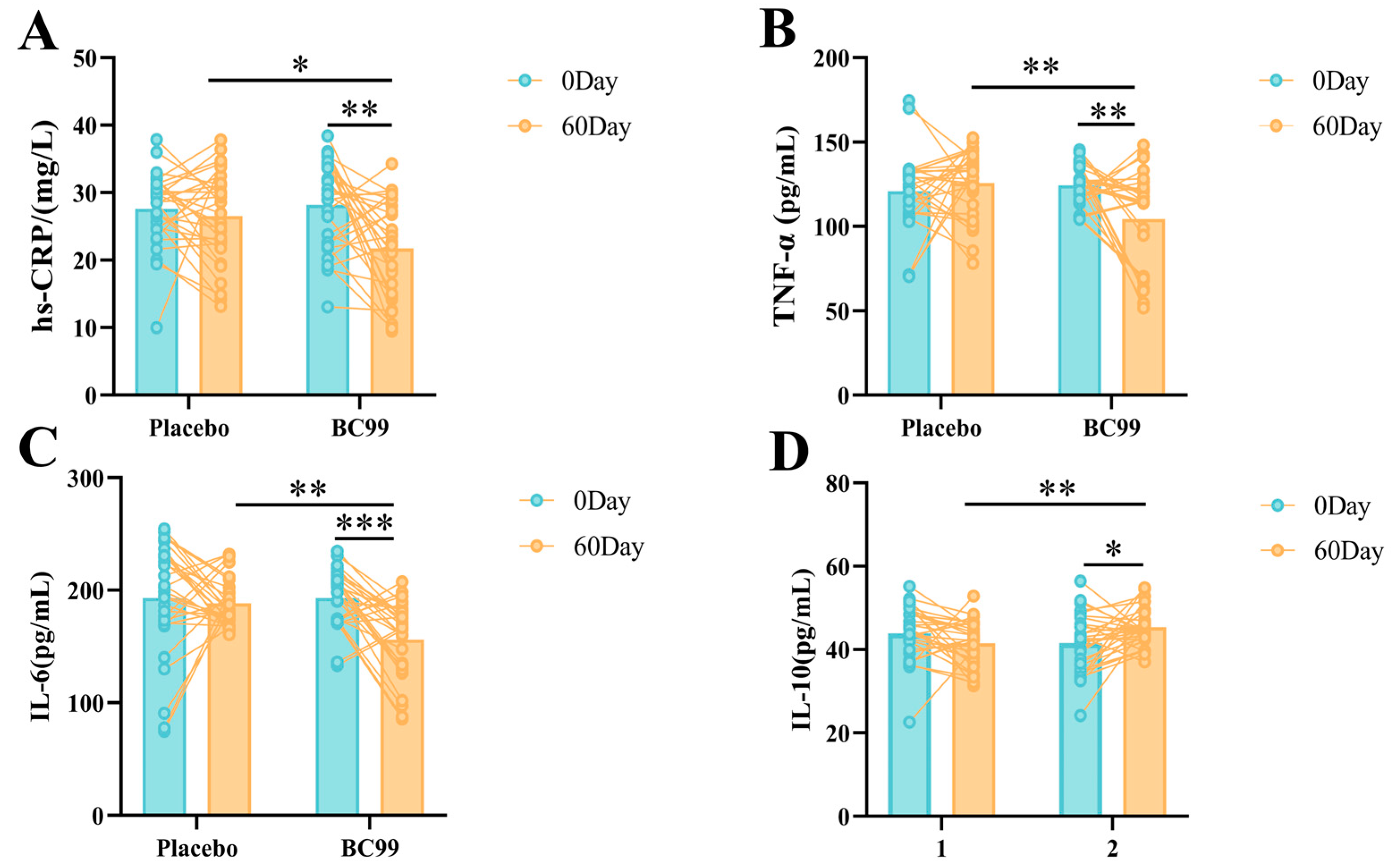
3.5. hs-CRP and Inflammatory Factors
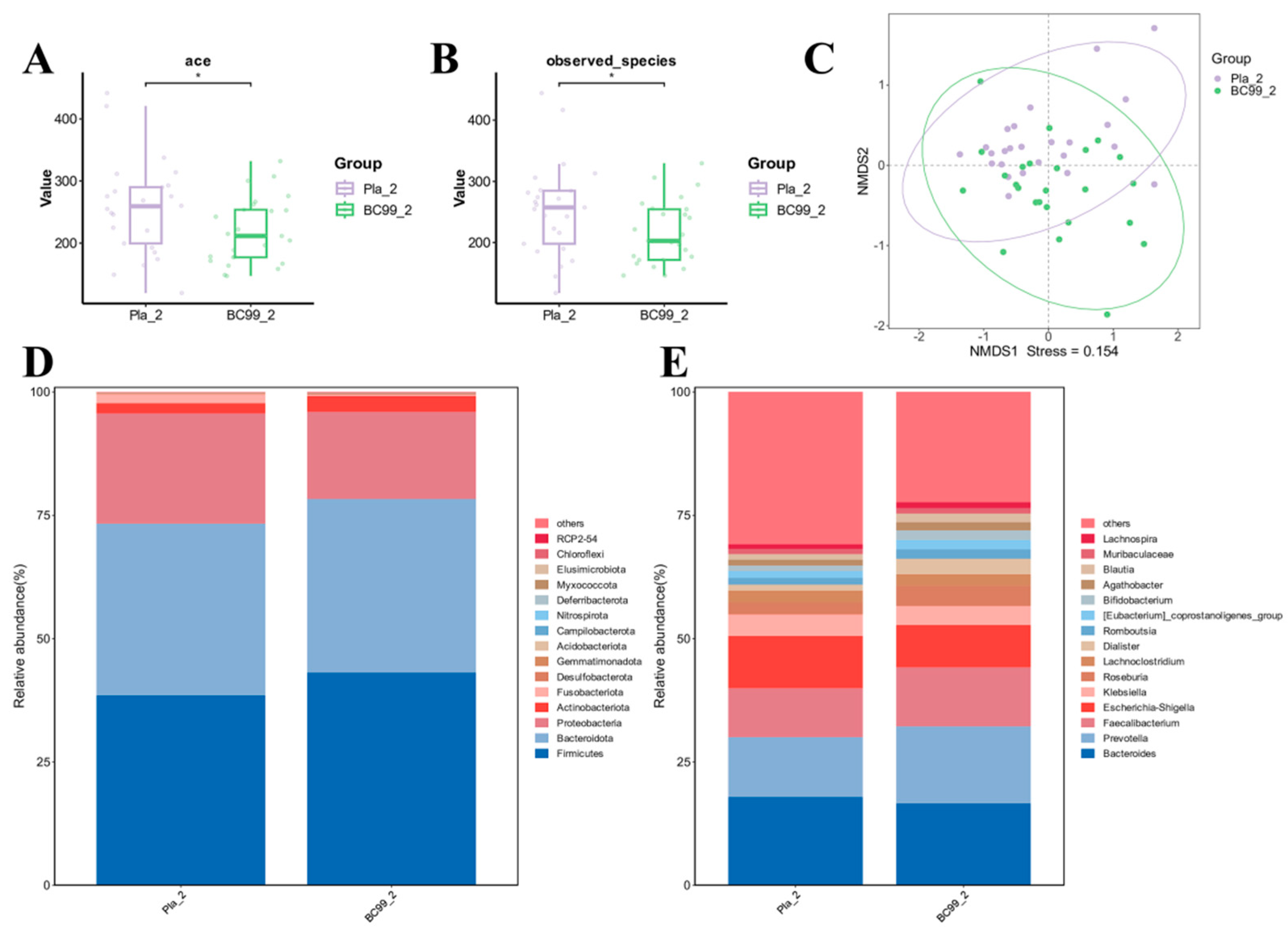
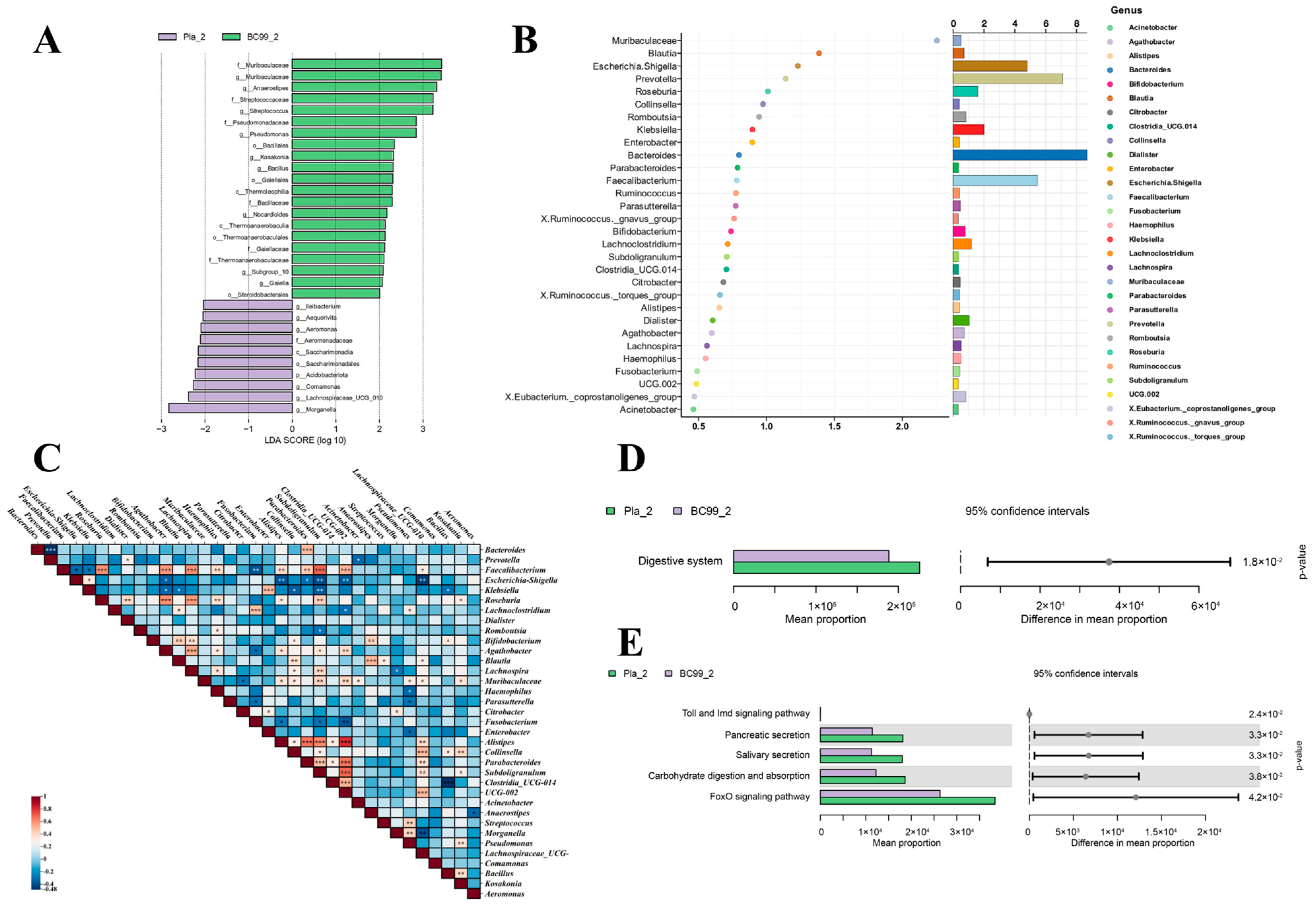
3.6. Efficacy of BC99 on Gut Microbiota
3.7. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, C.; Zhou, F.; Zhao, M.; Su, G.; Sun, B. Chicken breast muscle hydrolysates ameliorate acute alcohol-induced liver injury in mice through alcohol dehydrogenase (ADH) activation and oxidative stress reduction. Food Funct. 2018, 9, 774–784. [Google Scholar] [CrossRef]
- Gowing, L.R.; Ali, R.L.; Allsop, S.; Marsden, J.; Turf, E.E.; West, R.; Witton, J. Global statistics on addictive behaviours: 2014 status report. Addiction 2015, 110, 904–919. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.T.; Wang, Y.Y.; Hu, X.Y.; Wang, S.B. The Protective Effects of Water Extracts of Compound Turmeric Recipe on Acute Alcoholism: An Experimental Research Using a Mouse Model. Evid. Based Complement. Alternat. Med. 2021, 2021, 6641919. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, W.; Dai, J.; Cheng, Y.; Chen, Y.; Liu, W.; Xu, J.; Su, W.; Zhang, X.; Wang, C.; et al. Hydrogen gas alleviates acute ethanol-induced hepatotoxicity in mice via modulating TLR4/9 innate immune signaling and pyroptosis. Int. Immunopharmacol. 2024, 127, 111399. [Google Scholar] [CrossRef]
- Khanam, A.; Kottilil, S. Acute-on-Chronic Liver Failure: Pathophysiological Mechanisms and Management. Front. Med. 2021, 8, 752875. [Google Scholar] [CrossRef]
- Abulikemu, A.; Zhao, X.; Xu, H.; Li, Y.; Ma, R.; Yao, Q.; Wang, J.; Sun, Z.; Li, Y.; Guo, C. Silica nanoparticles aggravated the metabolic associated fatty liver disease through disturbed amino acid and lipid metabolisms-mediated oxidative stress. Redox Biol. 2023, 59, 102569. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Zhao, H.; Kong, L.; Shao, M.; Liu, J.; Sun, C.; Li, C.; Wang, Y.; Chai, X.; Wang, Y.; Zhang, Y.; et al. Protective effect of flavonoids extract of Hippophae rhamnoides L. on alcoholic fatty liver disease through regulating intestinal flora and inhibiting TAK1/p38MAPK/p65NF-κB pathway. J. Ethnopharmacol. 2022, 292, 115225. [Google Scholar] [CrossRef]
- Giuffrè, M.; Campigotto, M.; Campisciano, G.; Comar, M.; Crocè, L.S. A story of liver and gut microbes: How does the intestinal flora affect liver disease? A review of the literature. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G889–G906. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; Jönsson, K.A. Between-subject and within-subject variations in the pharmacokinetics of ethanol. Br. J. Clin. Pharmacol. 1994, 37, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Wang, J.; Zhao, L.; Wang, J.; Wang, P.; Zhang, F.; Wang, R. Bifidobacterium lactis TY-S01 protects against alcoholic liver injury in mice by regulating intestinal barrier function and gut microbiota. Heliyon 2023, 9, e17878. [Google Scholar] [CrossRef]
- Wang, Y.; Kirpich, I.; Liu, Y.; Ma, Z.; Barve, S.; McClain, C.J.; Feng, W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am. J. Pathol. 2011, 179, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhu, X.; Zhang, C.; Lu, F.; Lu, Z.; Lu, Y. Co-expression of alcohol dehydrogenase and aldehyde dehydrogenase in Bacillus subtilis for alcohol detoxification. Food Chem. Toxicol. 2020, 135, 110890. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association (WMA). WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Participants. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki/ (accessed on 31 October 2024).
- IBM Corp. IBM SPSS Statistics for Windows, Version 25.0; Released 2017; IBM Corp.: Armonk, NY, USA, 2017. [Google Scholar]
- Beeler, M.F. SI Units and the AJCP. Am. J. Clin. Pathol. 1987, 87, 140. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Gutiérrez-Reyes, G. The role of oxidative stress in the development of alcoholic liver disease. Rev. Gastroenterol. Mex. 2014, 79, 135–144. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, C.; Zhou, J.; Zhang, Z.; Che, Q.; Cao, H.; Bai, Y.; Guo, J.; Su, Z. Targeted treatment of alcoholic liver disease based on inflammatory signalling pathways. Pharmacol. Ther. 2021, 222, 107752. [Google Scholar] [CrossRef]
- Björnsson, H.K.; Björnsson, E.S. Drug-induced liver injury: Pathogenesis, epidemiology, clinical features, and practical management. Eur. J. Intern. Med. 2022, 97, 26–31. [Google Scholar] [CrossRef]
- Grodin, E.N.; Meredith, L.R.; Burnette, E.M.; Miotto, K.; Irwin, M.R.; Ray, L.A. Baseline C-reactive protein levels are predictive of treatment response to a neuroimmune modulator in individuals with an alcohol use disorder: A preliminary study. Am. J. Drug Alcohol Abus. 2023, 49, 333–344. [Google Scholar] [CrossRef]
- Vachliotis, I.D.; Polyzos, S.A. The Role of Tumor Necrosis Factor-Alpha in the Pathogenesis and Treatment of Nonalcoholic Fatty Liver Disease. Curr. Obes. Rep. 2023, 12, 191–206. [Google Scholar] [CrossRef]
- Obeagu, E.; Muhimbura, E.; Kagenderezo, B.; Nakyeyune, S.; Obeagu, G. An Insight of Interleukin -6 and Fibrinogen: In Regulating the Immune System. J. Biomed. Sci. 2022, 11, 83. [Google Scholar]
- Luo, Y.; Lin, H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun. Inflamm. Dis. 2021, 9, 59–73. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhai, S.; Duan, M.; Cao, L.; Zhang, J.; Wang, Y.; Wu, Y.; Gu, S. Weizmannia coagulans BC99 Enhances Intestinal Barrier Function by Modulating Butyrate Formation to Alleviate Acute Alcohol Intoxication in Rats. Nutrients 2024, 16, 4142. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abo-El-Sooud, K.; Aleya, L.; Bungau, S.G.; Najda, A.; Saluja, R. Alleviation of drugs and chemicals toxicity: Biomedical value of antioxidants. Oxid. Med. Cell. Longev. 2019, 2018, 6276438. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lin, Z.; Zeng, Y.; Lin, X.; Zhang, Y. Probiotic and glutamine treatments attenuate alcoholic liver disease in a rat model. Exp. Ther. Med. 2019, 18, 4733–4739. [Google Scholar] [CrossRef]
- Lang, S.; Schnabl, B. Microbiota and Fatty Liver Disease-the Known, the Unknown, and the Future. Cell Host Microbe 2020, 28, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Baltazar-Díaz, T.A.; González-Hernández, L.A.; Aldana-Ledesma, J.M.; Peña-Rodríguez, M.; Vega-Magaña, A.N.; Zepeda-Morales, A.S.M.; López-Roa, R.I.; Del Toro-Arreola, S.; Martínez-López, E.; Salazar-Montes, A.M.; et al. Escherichia/Shigella, SCFAs, and Metabolic Pathways-The Triad That Orchestrates Intestinal Dysbiosis in Patients with Decompensated Alcoholic Cirrhosis from Western Mexico. Microorganisms 2022, 10, 1231. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lu, M.C.; Lin, C.; Chiang, M.K.; Jan, M.S.; Tang, H.L.; Liu, H.C.; Lin, W.L.; Huang, C.Y.; Chen, C.M.; et al. Activation of IFN-γ/STAT/IRF-1 in hepatic responses to Klebsiella pneumoniae infection. PLoS ONE 2013, 8, e79961. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, C.; Du, H.; Zheng, X.; Zhao, A.; Jia, W.; Xu, Y. Flavor compounds in fermented Chinese alcoholic beverage alter gut microbiota and attenuate ethanol-induced liver damages. bioRxiv 2018. [Google Scholar] [CrossRef]
- Mangalam, A.K.; Murray, J. Microbial monotherapy with Prevotella histicola for patients with multiple sclerosis. Expert Rev. Neurother. 2019, 19, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jinato, T.; Anuntakarun, S.; Satthawiwat, N.; Chuaypen, N.; Tangkijvanich, P. Distinct alterations of gut microbiota between viral- and non-viral-related hepatocellular carcinoma. Appl. Microbiol. Biotechnol. 2024, 108, 34. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, N.; Zhao, L.; Wu, W.; Zhang, L.; Zhou, F.; Li, J. Astragalus Polysaccharides and Saponins Alleviate Liver Injury and Regulate Gut Microbiota in Alcohol Liver Disease Mice. Foods 2021, 10, 2688. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, C.R.; Um, T.H.; Rhu, J.Y.; Kim, E.S.; Jeong, J.W.; Lee, H.R. Morganella morganii sepsis with massive hemolysis. J. Korean Med. Sci. 2007, 22, 1082–1084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef]
- Ning, E.J.; Sun, C.W.; Wang, X.F.; Chen, L.; Li, F.F.; Zhang, L.X.; Wang, L.P.; Ma, Y.N.; Zhu, J.; Li, X.; et al. Artemisia argyi polysaccharide alleviates intestinal inflammation and intestinal flora dysbiosis in lipopolysaccharide-treated mice. Food Med. Homol. 2024, 1, 9420008. [Google Scholar] [CrossRef]
- Liu, Y.F.; Ling, N.; Zhang, B.; Chen, C.; Mo, X.N.; Cai, J.Y.; Tan, X.D.; Yu, Q.M. Flavonoid-rich mulberry leaf extract modulate lipid metabolism, antioxidant capacity, and gut microbiota in high-fat diet-induced obesity: Potential roles of FGF21 and SOCS2. Food Med. Homol. 2024, 1, 9420016. [Google Scholar] [CrossRef]
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Montesano, D. Phytochemicals from Plant Foods as Potential Source of Antiviral Agents: An Overview. Pharmaceuticals 2021, 14, 381. [Google Scholar] [CrossRef]
- Wang, T.; Jia, Z.; An, C.; Ren, P.; Yang, Y.; Wang, W.; Su, L. The Protective Effect of Auricularia cornea var. Li. Polysaccharide on Alcoholic Liver Disease and Its Effect on Intestinal Microbiota. Molecules 2023, 28, 8003. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, Y.; Zhao, H.; Zhao, H.; Wang, Y.; Zhang, X.; Zhang, J.; Wang, P.; Sun, L.; Zhang, H.; et al. Folic acid ameliorates alcohol-induced liver injury via gut-liver axis homeostasis. Front. Nutr. 2022, 9, 989311. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.S.; Seki, E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S1), 38–42. [Google Scholar] [CrossRef] [PubMed]
- Lugea, A.; Gong, J.; Nguyen, J.; Nieto, J.; French, S.W.; Pandol, S.J. Cholinergic mediation of alcohol-induced experimental pancreatitis. Alcohol Clin. Exp. Res. 2010, 34, 1768–1781. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Machida, K.; Dynnyk, A.; Mkrtchyan, H. “Second hit” models of alcoholic liver disease. Semin. Liver Dis. 2009, 29, 178–187. [Google Scholar] [CrossRef]
- Kops, G.J.; Dansen, T.B.; Polderman, P.E.; Saarloos, I.; Wirtz, K.W.; Coffer, P.J.; Huang, T.T.; Bos, J.L.; Medema, R.H.; Burgering, B.M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002, 419, 316–321. [Google Scholar] [CrossRef]



| Projects | Placebo Group (n = 30) | BC99 Group (n = 30) | p Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 43.60 | 11.31 | 42.87 | 11.15 | 0.801 |
| Sex (M/F) | 30/0 | 29/1 | 0.313 | ||
| Metabolic characteristics | |||||
| Height (cm) | 173.4 | 5.02 | 171.03 | 5.38 | 0.084 |
| Weight (kg) | 72.85 | 9.15 | 72.63 | 7.09 | 0.919 |
| BMI (kg/m2) | 24.24 | 2.89 | 24.87 | 2.68 | 0.382 |
| Liver function indicators | |||||
| ALT (U/L) | 40.60 | 22.81 | 40.50 | 23.73 | 0.990 |
| AST (U/L) | 35.82 | 11.13 | 40.17 | 21.38 | 0.392 |
| γ-GT (U/L) | 60.79 | 22.49 | 61.08 | 51.06 | 0.979 |
| TBil (μmol/L) | 12.42 | 3.48 | 14.03 | 3.11 | 0.073 |
| Renal function indicators | |||||
| BUN (mmol/L) | 5.92 | 0.88 | 6.16 | 0.78 | 0.393 |
| Cr (μmol/L) | 73.67 | 10.20 | 70.41 | 10.20 | 0.325 |
| UA (μmol/L) | 393.67 | 75.41 | 406.74 | 71.13 | 0.500 |
| Projects | Placebo Group (n = 30) | BC99 Group (n = 30) | ||||
|---|---|---|---|---|---|---|
| 0 Day | 60 Day | p-Value | 0 Day | 60 Day | p-Value | |
| Neutrophils (×109/L) | 3.08 ± 1.15 | 3.44 ± 1.42 | 0.277 | 3.04 ± 0.77 | 3.2 ± 0.75 | 0.429 |
| Lymphocyte (×109/L) | 1.99 ± 0.51 | 2.08 ± 0.66 | 0.558 | 1.99 ± 0.53 | 3.36 ± 5.94 | 0.213 |
| Red blood cell (×109/L) | 4.73 ± 0.80 | 4.48 ± 0.32 | 0.122 | 4.48 ± 0.42 | 4.36 ± 0.37 | 0.266 |
| Hemoglobin (g/L) | 136.83 ± 13.73 | 130.93 ± 11.06 | 0.072 | 141.67 ± 35.74 | 132.83 ± 10.90 | 0.201 |
| Platelet (×109/L) | 181.33 ± 52.43 | 170.40 ± 46.44 | 0.396 | 179.53 ± 50.24 | 172.16 ± 68.50 | 0.637 |
| Projects | Placebo Group (n = 30) | BC99 Group (n = 30) | ||||
|---|---|---|---|---|---|---|
| 0 Day | 60 Day | p-Value | 0 Day | 60 Day | p-Value | |
| BUN (mmol/L) | 5.92 ± 0.88 | 5.67 ± 0.87 | 0.366 | 6.16 ± 0.78 | 5.00 ± 0.98 | <0.001 |
| UA (μmol/L) | 393.67 ± 75.41 | 375.11 ± 113.17 | 0.466 | 406.74 ± 71.13 | 365.99 ± 61.68 | 0.023 |
| Cr (μmol/L) | 73.67 ± 10.20 | 73.25 ± 11.04 | 0.902 | 70.41 ± 10.20 | 72.91 ± 9.12 | 0.431 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, C.; Duan, M.; Qu, Z.; Wang, Y.; Dong, Y.; Wu, Y.; Fang, S.; Gu, S. The Improvement Effects of Weizmannia coagulans BC99 on Liver Function and Gut Microbiota of Long-Term Alcohol Drinkers: A Randomized Double-Blind Clinical Trial. Nutrients 2025, 17, 320. https://doi.org/10.3390/nu17020320
Zhang J, Li C, Duan M, Qu Z, Wang Y, Dong Y, Wu Y, Fang S, Gu S. The Improvement Effects of Weizmannia coagulans BC99 on Liver Function and Gut Microbiota of Long-Term Alcohol Drinkers: A Randomized Double-Blind Clinical Trial. Nutrients. 2025; 17(2):320. https://doi.org/10.3390/nu17020320
Chicago/Turabian StyleZhang, Jie, Cheng Li, Mengyao Duan, Zhen Qu, Yi Wang, Yao Dong, Ying Wu, Shuguang Fang, and Shaobin Gu. 2025. "The Improvement Effects of Weizmannia coagulans BC99 on Liver Function and Gut Microbiota of Long-Term Alcohol Drinkers: A Randomized Double-Blind Clinical Trial" Nutrients 17, no. 2: 320. https://doi.org/10.3390/nu17020320
APA StyleZhang, J., Li, C., Duan, M., Qu, Z., Wang, Y., Dong, Y., Wu, Y., Fang, S., & Gu, S. (2025). The Improvement Effects of Weizmannia coagulans BC99 on Liver Function and Gut Microbiota of Long-Term Alcohol Drinkers: A Randomized Double-Blind Clinical Trial. Nutrients, 17(2), 320. https://doi.org/10.3390/nu17020320







