Effects of D-Tagatose on Cariogenic Risk: A Systematic Review of Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Eligibility Criteria
- Participants: adults with oral health characterized by the absence of active caries; advanced periodontitis; and significant oral lesions associated with smoking, excessive alcohol consumption, or diets extremely high in simple sugars.
- Intervention: administration of D-tagatose as a standalone intervention.
- Comparison: the use of any non-caloric sweetener other than D-tagatose (e.g., sucrose, stevia, or xylitol).
- Outcome: changes in colony-forming units (CFUs) or salivary pH.
- Study design: randomized controlled trials (RCTs) with variable intervention durations.
2.3. Data Sources and Search
2.4. Study Selection and Data Collection
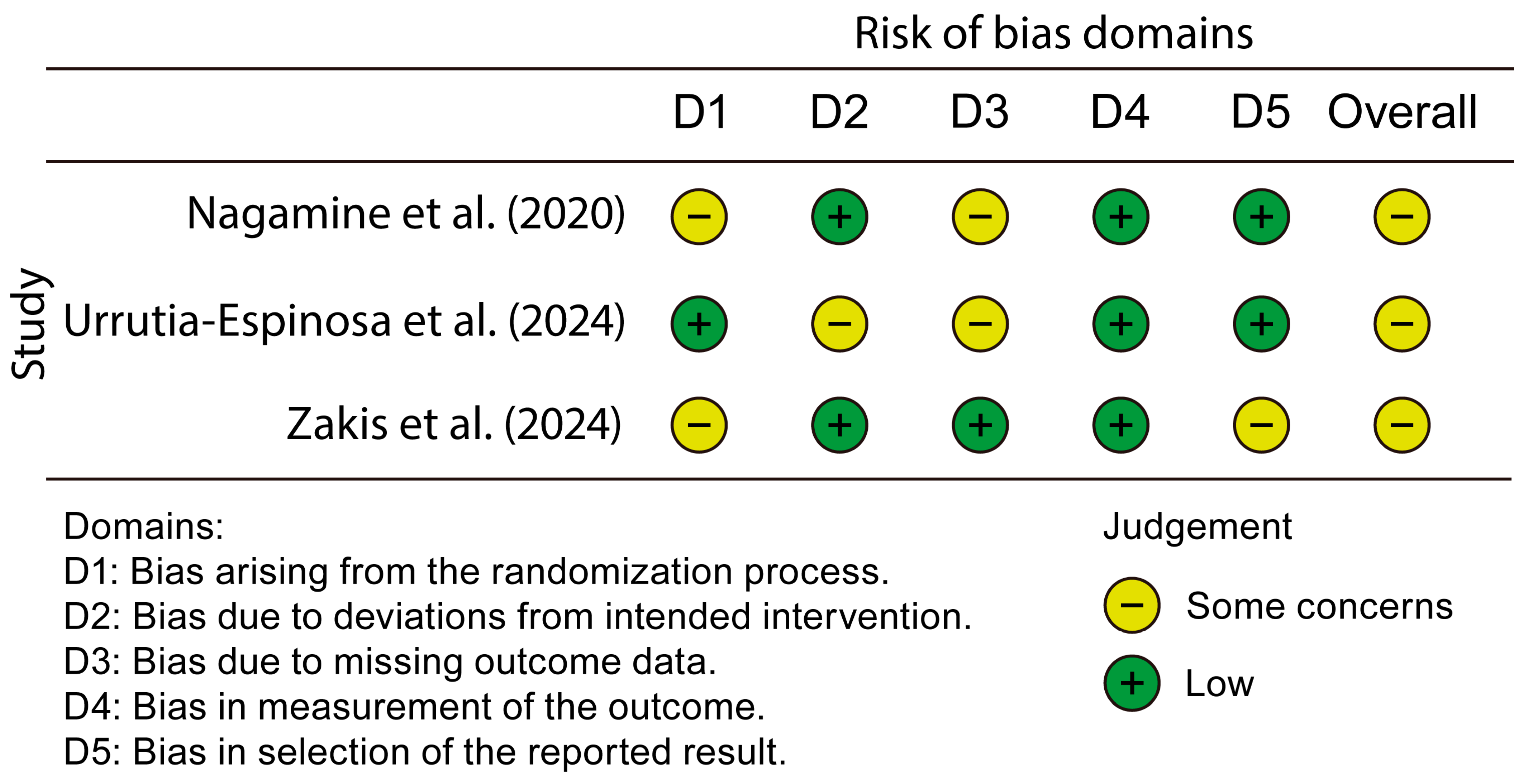
2.5. Risk of Bias
2.6. Strategy for Data Synthesis
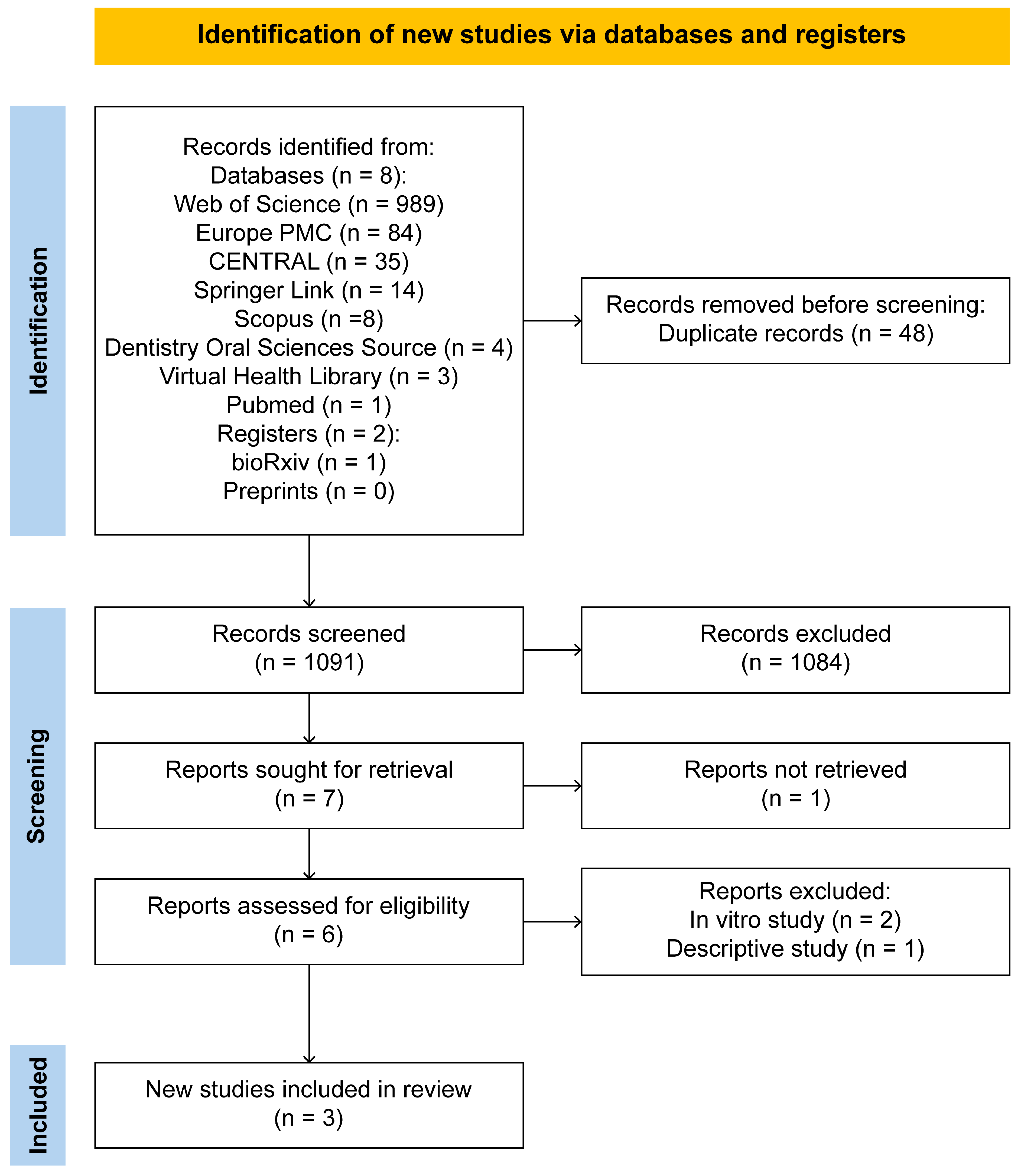
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ealla, K.K.R.; Kumari, N.; Chintalapani, S.; Uppu, S.; Sahu, V.; Veeraraghavan, V.P.; Ramani, P.; Govindool, S.R. Interplay between dental caries pathogens, periodontal pathogens, and sugar molecules: Approaches for prevention and treatment. Arch. Microbiol. 2024, 206, 127. [Google Scholar] [CrossRef] [PubMed]
- Innes, N.P.; Clarkson, J.E.; Douglas, G.V.A.; Ryan, V.; Wilson, N.; Homer, T.; Marshman, Z.; McColl, E.; Vale, L.; Robertson, M.; et al. Child Caries Management: A Randomized Controlled Trial in Dental Practice. J. Dent. Res. 2020, 99, 36–43. [Google Scholar] [CrossRef]
- Martignon, S.; Roncalli, A.G.; Alvarez, E.; Aránguiz, V.; Feldens, C.A.; Buzalaf, M.A.R. Risk factors for dental caries in Latin American and Caribbean countries. Braz. Oral Res. 2021, 35, e053. [Google Scholar] [CrossRef]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Cui, G.; Li, P.; Wu, R.; Lin, H. Streptococcus mutans membrane vesicles inhibit the biofilm formation of Streptococcus gordonii and Streptococcus sanguinis. AMB Express 2022, 12, 154. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Q.; Wang, Y.; Wu, H.; Zou, J. Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Int. J. Oral Sci. 2021, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, Y.; Hasibul, K.; Ogawa, T.; Tada, A.; Kamitori, K.; Hossain, A.; Yamaguchi, F.; Tokuda, M.; Kuwahara, T.; Miyake, M. D-Tagatose Effectively Reduces the Number of Streptococcus mutans and Oral Bacteria in Healthy Adult Subjects: A Chewing Gum Pilot Study and Randomized Clinical Trial. Acta Med. Okayama 2020, 74, 307–317. [Google Scholar] [PubMed]
- Mayumi, S.; Kuboniwa, M.; Sakanaka, A.; Hashino, E.; Ishikawa, A.; Ijima, Y.; Amano, A. Potential of Prebiotic D-Tagatose for Prevention of Oral Disease. Front. Cell Infect. Microbiol. 2021, 11, 767944. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Sun, Q.; Zeng, W.; Li, Y. Inhibition of Biofilm Formation and Virulence Factors of Cariogenic Oral Pathogen Streptococcus mutans by Shikimic Acid. Microbiol. Spectr. 2022, 10, e0119922. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Espinosa, M.; Concha-Fuentealba, F.; Fuentes-Barría, H.; Angarita Dávila, L.C.; Carrasco Hernández, M.E.; Aguilera-Eguía, R.; Alarcón Rivera, M.; López Soto, O.P. Effects of D-tagatose, Stevia and Sucrose on pH and oral bacterial activity in dentistry students. A randomized controlled trial. Nutr. Hosp. 2024, 41, 1091–1097. [Google Scholar] [PubMed]
- Ortiz, A.C.; Fideles, S.O.M.; Reis, C.H.B.; Pagani, B.T.; Bueno, L.M.M.; Moscatel, M.B.M.; Buchaim, R.L.; Buchaim, D.V. D-Tagatose: A Rare Sugar with Functional Properties and Antimicrobial Potential against Oral Species. Nutrients 2024, 16, 1943. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Wyss, M.; Durán Agüero, S.; Angarita Dávila, L. D-Tagatose Is a Promising Sweetener to Control Glycaemia: A New Functional Food. Biomed. Res. Int. 2018, 2018, 8718053. [Google Scholar] [CrossRef] [PubMed]
- Zakis, D.R.; Brandt, B.W.; van der Waal, S.V.; Keijser, B.J.F.; Crielaard, W.; van der Plas, D.W.K.; Volgenant, C.M.C.; Zaura, E. The effect of different sweeteners on the oral microbiome: A randomized clinical exploratory pilot study. J. Oral Microbiol. 2024, 16, 2369350. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Walker, A.R.; Pham, D.N.; Noeparvar, P.; Peterson, A.M.; Lipp, M.K.; Lemos, J.A.; Zeng, L. Fructose activates a stress response shared by methylglyoxal and hydrogen peroxide in Streptococcus mutans. bioRxiv 2024. bioRxiv:2024.10.26.620100. [Google Scholar]
- Hasibul, K.; Nakayama-Imaohji, H.; Hashimoto, M.; Yamasaki, H.; Ogawa, T.; Waki, J.; Tada, A.; Yoneda, S.; Tokuda, M.; Miyake, M.; et al. D-Tagatose Inhibits the Growth and Biofilm Formation of Streptococcus mutans. Mol. Med. Rep. 2018, 17, 843–851. [Google Scholar] [PubMed]
- Sawada, D.; Ogawa, T.; Miyake, M.; Hasui, Y.; Yamaguchi, F.; Izumori, K.; Tokuda, M. Potent inhibitory effects of D-tagatose on the acid production and water-insoluble glucan synthesis of Streptococcus mutans GS5 in the presence of sucrose. Acta Med. Okayama 2015, 69, 105–111. [Google Scholar] [PubMed]
- A Study of the Effects on Oral Bacteria with D-Tagatose Chewing Gum. Available online: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000030134 (accessed on 31 December 2024).
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, J.; Zhou, X.; Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 2021, 47, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Pitchika, V.; Standl, M.; Harris, C.; Thiering, E.; Hickel, R.; Heinrich, J.; Kühnisch, J. Association of sugar-sweetened drinks with caries in 10- and 15-year-olds. BMC Oral Health 2020, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.; Zinn, C.; Schofield, G. The consumption of processed sugar- and starch-containing foods, and dental caries: A systematic review. Eur. J. Oral Sci. 2020, 128, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.N.; Choi, H.M.; Jeon, J.G. Relationship between sucrose concentration and bacteria proportion in a multispecies biofilm: Short title: Sucrose challenges to a multispecies biofilm. J. Oral Microbiol. 2021, 13, 1910443. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Twetman, S.; Fisher, J.; Marsh, P.D. Understanding dental caries as a non-communicable disease. Br. Dent. J. 2021, 231, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef]
- Moores, C.J.; Kelly, S.A.M.; Moynihan, P.J. Systematic Review of the Effect on Caries of Sugars Intake: Ten-Year Update. J. Dent. Res. 2022, 101, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, A.D.E.; Grootaert, C.; Van Camp, J. Rare Mono-and Disaccharides as Healthy Alternative for Traditional Sugars and Sweeteners? Crit. Rev. Food Sci. Nutr. 2021, 61, 713–741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Rodríguez, A. Production of glucosyltransferase B and glucans by Streptococcus mutans strains isolated from caries-free individuals. Acta Odontol. Latinoam. 2011, 24, 258–264. [Google Scholar]
- Chahed, A.; Nesler, A.; Aziz, A.; Barka, E.A.; Pertot, I.; Perazzolli, M. A Review of Knowledge on the Mechanisms of Action of the Rare Sugar d-tagatose against Phytopathogenic Oomycetes. Plant Pathol. 2021, 70, 1979–1986. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Poppe, J.; Deyaert, S.; Laurie, I.; Otto Gravert, T.K.; Abrahamsson, A.; Baudot, A.; Karnik, K.; Risso, D. Low-no-calorie sweeteners exert marked compound-specific impact on the human gut microbiota ex vivo. Int. J. Food Sci. Nutr. 2023, 74, 630–644. [Google Scholar] [CrossRef]
- Ahmed, A.; Khan, T.A.; Ramdath, D.D.; Kendall, C.W.C.; Sievenpiper, J.L. Rare Sugars and Their Health Effects in Humans: A Systematic Review and Narrative Synthesis of the Evidence from Human Trials. Nutr. Rev. 2022, 80, 255–270. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Haas, M.J.; Onstead-Haas, L.; Tani, Y.; Iida, T.; Tokuda, M. Naturally Occurring Rare Sugars Are Free Radical Scavengers and Can Ameliorate Endoplasmic Reticulum Stress. Int. J. Vitam. Nutr. Res. 2020, 90, 210–220. [Google Scholar] [CrossRef]
- Ensor, M.; Banfield, A.B.; Smith, R.R.; Williams, J.; Lodder, R.A. Safety and Efficacy of D-Tagatose in Glycemic Control in Subjects with Type 2 Diabetes. J. Endocrinol. Diabetes Obes. 2015, 3, 1065. [Google Scholar]
- Ercan, N.; Erdemir, E.O.; Ozkan, S.Y.; Hendek, M.K. The comparative effect of propolis in two different vehicles; mouthwash and chewing-gum on plaque accumulation and gingival inflammation. Eur. J. Dent. 2015, 9, 272–276. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. eSPen 2017, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.I.; Talati, R.; White, C.M. A clinician’s perspective on rating the strength of evidence in a systematic review. Pharmacotherapy 2009, 29, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Chou, R. Using evidence in pain practice: Part I: Assessing quality of systematic reviews and clinical practice guidelines. Pain Med. 2008, 9, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Li, L.; Yasmin, F.; Khan, S.U.; Bajaj, N.S.; Pandey, A.; Murad, M.H.; Fonarow, G.C.; Butler, J.; Vaduganathan, M. Assessment of Heterogeneity in Heart Failure-Related Meta-Analyses. Circ. Heart Fail. 2020, 13, e007070. [Google Scholar] [CrossRef] [PubMed]
| Property | Description |
|---|---|
| Antimicrobial property | Inhibits the growth of pathogenic bacteria in the oral cavity, particularly Streptococcus spp. |
| Plaque reduction | Reduces bacterial biofilm formation on teeth, potentially reducing plaque accumulation. |
| Effect on oral health | Prevents dental caries and periodontal diseases by inhibiting harmful bacteria. |
| Low glycemic index | Does not significantly increase blood glucose levels, making it suitable for diabetics. |
| Antioxidant effect | May reduce oxidative stress and inflammation in oral tissues. |
| Effect on metabolism | Aids in weight management by preventing fat accumulation. |
| Characteristics | Nagamine et al. [7] | Urrutia-Espinosa et al. [10] | Zakis et al. [13] |
|---|---|---|---|
| Country | Japan | Chile | Netherlands |
| Sample | 19 healthy volunteers (21–49 years) | 30 students (18–30 years) | 65 healthy volunteers (18–55 years) |
| Design | RCT | RCT | RCT |
| Outcome | CFU/mL | CFU/mL Salivary pH | Beta diversity of oral biofilm bacterial taxa Salivary pH. |
| Instruments | Microbiological crop. Polymerase chain reaction | Microbiological crop. Calibrated pH meter | 16S rRNA gene amplicon sequencing, red fluorescence of plaque, and a calibrated pH meter. |
| Characteristics | Nagamine et al. [7] | Urrutia-Espinosa et al. [10] | Zakis et al. [13] |
|---|---|---|---|
| Intervention | EG1: chewing gum with xylitol (5%). EG2: chewing gum with D-tagatose (5%). EG3: D-tagatose gum (2.5%) + 2.5% xylitol (2.5%). CG: placebo. | EG1: D-tagatose mouthwashes (6.4%). EG2: stevia mouthwashes (6.4%). CG: sucrose mouthwashes (6.4%). | EG1: D-tagatose mouthwashes (10%). EG2: Glucose mouthwashes (10%). EG3: Inulin mouthwashes (10%). EG4: Isomaltulose mouthwashes (10%). EG5: Trehalose mouthwashes (10%). |
| Duration of Study | 4 wk | 48 h | 4 wk |
| Nagamine et al. [7] | Urrutia-Espinosa et al. [10] | Zakis et al. [13] | |
|---|---|---|---|
| Results | D-tagatose + xylitol significantly decreased CFU/mL compared to control group (p < 0.01). | D-tagatose significantly decreased CFU/mL in comparison to sucrose (p < 0.001). D-Tagatose did not show significant changes in CFU/mL compared to stevia (p = 0.137). | D-tagatose shows no difference significant on salivary pH or microbiome composition compared to baseline. |
| Outcome | Comparison | Time | MD | 95% CI | Evidence Level (GRADE) |
|---|---|---|---|---|---|
| CFU/mL | D-tagatose vs. stevia | 30 min after | 22.60 | −16.99 to 62.19 | Very low |
| CFU/mL | D-tagatose vs. sucrose | 30 min after | −204.6 | −237.85 to −171.35 | Very low |
| Salivary pH | D-tagatose vs. sucrose | 30 min after | −0.08 | −0.47 to 0.31 | Very low |
| Salivary pH | D-tagatose vs. stevia | 30 min after | −0.06 | −0.31 to 0.19 | Very low |
| Salivary pH | D-tagatose vs. sucrose | 48 h after | 0.68 | 0.19 to 1.17 | Very low |
| Salivary PH | D-tagatose vs. stevia | 48 h after | 0.23 | −0.03 to 0.49 | Very low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angarita-Davila, L.; Fuentes-Barría, H.; Rojas-Gómez, D.; Aguilera-Eguía, R.; Alarcón-Rivera, M.; Guzmán-Muñoz, E. Effects of D-Tagatose on Cariogenic Risk: A Systematic Review of Randomized Clinical Trials. Nutrients 2025, 17, 293. https://doi.org/10.3390/nu17020293
Angarita-Davila L, Fuentes-Barría H, Rojas-Gómez D, Aguilera-Eguía R, Alarcón-Rivera M, Guzmán-Muñoz E. Effects of D-Tagatose on Cariogenic Risk: A Systematic Review of Randomized Clinical Trials. Nutrients. 2025; 17(2):293. https://doi.org/10.3390/nu17020293
Chicago/Turabian StyleAngarita-Davila, Lissé, Héctor Fuentes-Barría, Diana Rojas-Gómez, Raúl Aguilera-Eguía, Miguel Alarcón-Rivera, and Eduardo Guzmán-Muñoz. 2025. "Effects of D-Tagatose on Cariogenic Risk: A Systematic Review of Randomized Clinical Trials" Nutrients 17, no. 2: 293. https://doi.org/10.3390/nu17020293
APA StyleAngarita-Davila, L., Fuentes-Barría, H., Rojas-Gómez, D., Aguilera-Eguía, R., Alarcón-Rivera, M., & Guzmán-Muñoz, E. (2025). Effects of D-Tagatose on Cariogenic Risk: A Systematic Review of Randomized Clinical Trials. Nutrients, 17(2), 293. https://doi.org/10.3390/nu17020293







