Effect of Ficus pumila L. on Improving Insulin Secretory Capacity and Resistance in Elderly Patients Aged 80 Years Old or Older Who Develop Diabetes After COVID-19 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Extract
2.2. Data on the Patients
2.3. Method and Equipment
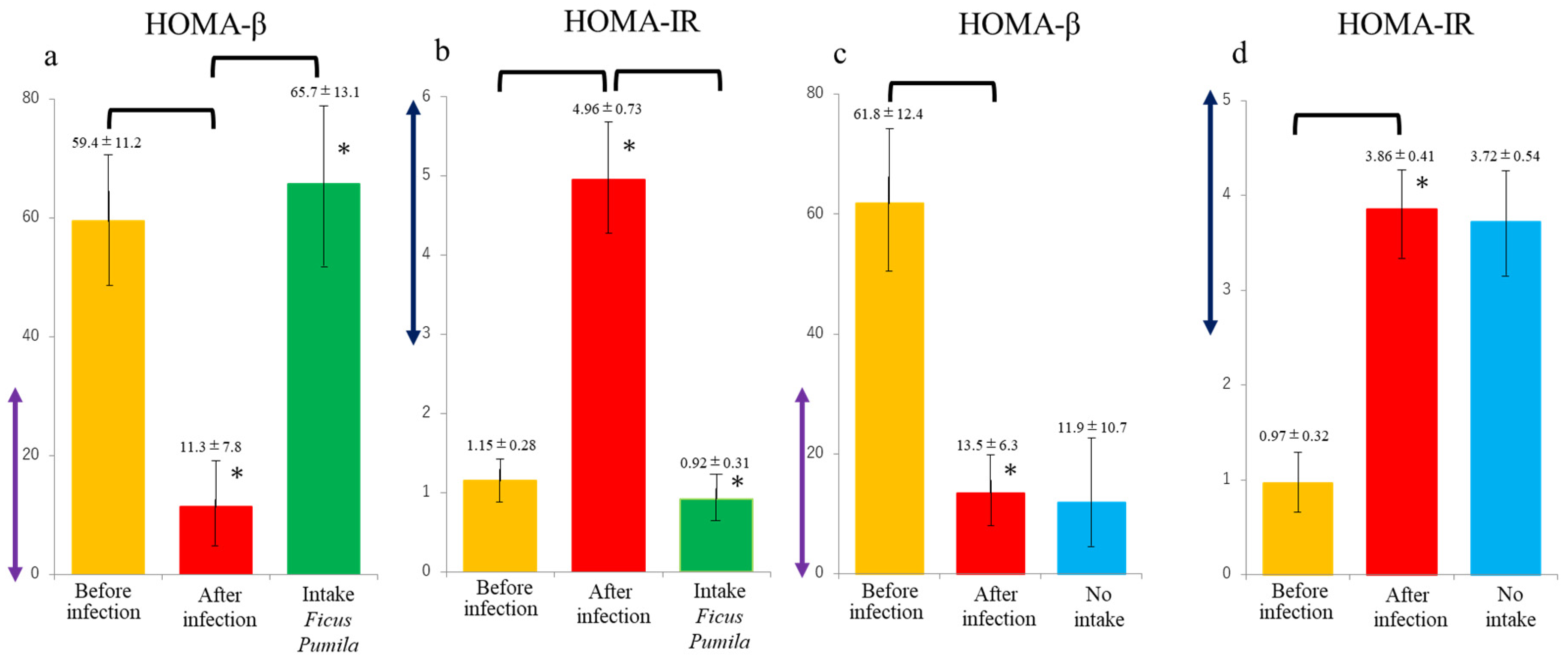
3. Results
4. Discussion
4.1. Pathogenesis of the Development of Diabetes Mellitus in Coronavirus Infection
4.2. Antioxidant Targets in the Treatment of COVID-19 and Complications
4.3. Arguments Related to the Consequences of Treating Diabetes Post-COVID-19 with Antioxidant Therapy According to the Literature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Wander, P.L.; Lowy, E.; Beste, L.A.; Tulloch-Palomino, L.; Korpak, A.; Peterson, A.C.; Kahn, S.E.; Boyko, E.J. The Incidence of Diabetes Among 2,777,768 Veterans With and Without Recent SARS-CoV-2 Infection. Diabetes Care 2022, 45, 782–788. [Google Scholar] [CrossRef]
- Suzuki, K.; Gonda, K.; Kishimoto, Y.; Katsumoto, Y.; Takenoshita, S. Potential curing and beneficial effects of Ooitabi (Ficus pumila L.) on hypertension and dyslipidaemia in Okinawa. J. Hum. Nutr. Diet. 2021, 34, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Gonda, K.; Kanazawa, H.; Maeda, G.; Matayoshi, C.; Hirose, N.; Katsumoto, Y.; Kono, K.; Takenoshita, S. Ingestion of Okinawa Island Vegetables Increases IgA Levels and Prevents the Spread of Influenza RNA Viruses. Nutrients 2021, 13, 1773. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mei, M.S.; Xu, Y.; Xiong, S.; Zhao, Y.; Liu, R.; Shi, S.; Wang, H.; Wang, S. The impact of the methyl esters of homogalacturonan on cellular uptake dependent hypoglycemic activity in IR-HepG2 cells. Carbohydr. Polym. 2022, 293, 119741. [Google Scholar] [CrossRef]
- Qi, Z.-Y.; Zhao, J.-Y.; Lin, F.-J.; Zhou, W.-L.; Gan, R.-Y. Bioactive Compounds, Therapeutic Activities, and Applications of Ficus pumila L. Agronomy 2021, 11, 89. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021, 33, 1565–1576. [Google Scholar] [CrossRef]
- Ji, N.; Zhang, M.; Ren, L.; Wang, Y.; Hu, B.; Xiang, J.; Gong, Y.; Wu, C.; Qu, G.; Ding, W.; et al. SARS-CoV-2 in the pancreas and the impaired islet function in COVID-19 patients. Emerg. Microbes Infect. 2022, 11, 1115–1125. [Google Scholar] [CrossRef]
- Costa, R.; Rodrigues, I.; Guardão, L.; Lima, J.Q.; Sousa, E.; Soares, R.; Negrão, R. Modulation of VEGF signaling in a mouse model of diabetes by xanthohumol and 8-prenylnaringenin: Unveiling the angiogenic paradox and metabolism interplay. Mol. Nutr. Food Res. 2017, 61, 201600488. [Google Scholar] [CrossRef] [PubMed]
- Drzymała, A. The Functions of SARS-CoV-2 Receptors in Diabetes-Related Severe COVID-19. Int. J. Mol. Sci. 2024, 25, 9635. [Google Scholar] [CrossRef]
- Fignani, D.; Pedace, E.; Licata, G.; Grieco, G.E.; Aiello, E.; de Luca, C.; Marselli, L.; Marchetti, P.; Sebastiani, G.; Dotta, F. Angiotensin I-converting enzyme type 2 expression is increased in pancreatic islets of type 2 diabetic donors. Diabetes Metab. Res. Rev. 2023, 39, e3696. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef] [PubMed]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin signalling and GLUT4 trafficking in insulin resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.; Khaliq, O.P.; Moodley, J.; Naicker, T. Insulin resistance in COVID-19 and diabetes. Prim. Care Diabetes. 2021, 15, 629–634. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Voor, M.J.; Tyagi, S.C.; Tyagi, N. Diabetic COVID-19 severity: Impaired glucose tolerance and pathologic bone loss. Biochem. Biophys. Res. Commun. 2022, 620, 180–187. [Google Scholar] [CrossRef]
- Pescaru, C.C.; Marițescu, A.; Costin, E.O.; Trăilă, D.; Marc, M.S.; Trușculescu, A.A.; Pescaru, A.; Oancea, C.I. The Effects of COVID-19 on Skeletal Muscles, Muscle Fatigue and Rehabilitation Programs Outcomes. Medicina 2022, 58, 1199. [Google Scholar] [CrossRef]
- Knudsen, J.R.; Persson, K.W.; Henriquez-Olguin, C.; Li, Z.; Di Leo, N.; Hesselager, S.A.; Raun, S.H.; Hingst, J.R.; Trouillon, R.; Wohlwend, M.; et al. Microtubule-mediated GLUT4 trafficking is disrupted in insulin-resistant skeletal muscle. Elife 2023, 12, e83338. [Google Scholar] [CrossRef]
- Suručić, R.; Radović Selgrad, J.; Kundaković-Vasović, T.; Lazović, B.; Travar, M.; Suručić, L.; Škrbić, R. In Silico and In Vitro Studies of Alchemilla viridiflora Rothm-Polyphenols’ Potential for Inhibition of SARS-CoV-2 Internalization. Molecules 2022, 27, 5174. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.L.; Germolec, D.R.; Zheng, J.F.; Kooistra, L.; Auttachoat, W.; Smith, M.J.; White, K.L.; Elmore, S.A. Genistein protects female nonobese diabetic mice from developing type 1 diabetes when fed a soy- and alfalfa-free diet. Toxicol. Pathol. 2015, 43, 435–448. [Google Scholar] [CrossRef]
- Liu, X.; Raghuvanshi, R.; Ceylan, F.D.; Bolling, B.W. Quercetin and Its Metabolites Inhibit Recombinant Human Angiotensin-Converting Enzyme 2 (ACE2) Activity. J. Agric. Food Chem. 2020, 68, 13982–13989. [Google Scholar] [CrossRef]
- Rashid, Z.; Fatima, A.; Khan, A.; Matthew, J.; Yousaf, M.Z.; Nadeem, N.; Hasan, T.N.; Rehman, M.U.; Naqvi, S.S.; Khan, S.J. Drug repurposing: Identification of SARS-CoV-2 potential inhibitors by virtual screening and pharmacokinetics strategies. J. Infect. Dev. Ctries. 2024, 18, 520–531. [Google Scholar] [CrossRef]
- Tang, X.; Uhl, S.; Zhang, T.; Xue, D.; Li, B.; Vandana, J.J.; Acklin, J.A.; Bonnycastle, L.L.; Narisu, N.; Erdos, M.R.; et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 2021, 33, 1577–1591. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, E.; Rao, S.; Adnan, M.; Pais, M.L.J.; Naik, T.S.; D’Cunha, R.; D’souza, R.; Baliga, M.S. Chapter 1—Polyphenols in the Prevention of Acute Pancreatitis in Preclinical Systems of Study: A Revisit. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–9. [Google Scholar]
- Akdad, M.; Ameziane, R.; Khallouki, F.; Bakri, Y.; Eddouks, M. Antidiabetic Phytocompounds Acting as Glucose Transport Stimulators. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 147–168. [Google Scholar] [PubMed]
- Ortiz-Barragán, E.; Estrada-Soto, S.; Giacoman-Martínez, A.; Alarcón-Aguilar, F.J.; Fortis-Barrera, Á.; Marquina-Rodríguez, H.; Gaona-Tovar, E.; Lazzarini-Lechuga, R.; Suárez-Alonso, A.; Almanza-Pérez, J.C. Antihyperglycemic and Hypolipidemic Activities of Flavonoids Isolated from Smilax Dominguensis Mediated by Peroxisome Proliferator-Activated Receptors. Pharmaceuticals 2024, 17, 1451. [Google Scholar] [CrossRef]
- Eddouks, M.; Akdad, M.; Ameziane, R.; Khallouki, F.; Bakri, Y. Kaempferol 3-O-rutinoside from Antidesma acidum Retz. Stimulates glucose uptake through SIRT1 induction followed by GLUT4 translocation in skeletal muscle L6 cells. J. Ethnopharmacol. 2023, 301, 115788. [Google Scholar]
- Thabah, D.; Syiem, D.; Pakyntein, C.L.; Banerjee, S.; Kharshiing, C.E.; Bhattacharjee, A. Potentilla fulgens upregulate GLUT4, AMPK, AKT and insulin in alloxan-induced diabetic mice: An in vivo and in silico study. Arch. Physiol. Biochem. 2023, 129, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Sharif, A.; Awan, S.J.; Akhtar, B.; Akhtar, M.F.; Ali, S.; Shahnaz. Ficus johannis Boiss. leaves ethanolic extract ameliorate streptozotocin-induced diabetes in rats by upregulating the expressions of GCK, GLUT4, and IGF and downregulating G6P. Environ. Sci. Pollut. Res. Int. 2023, 30, 49108–49124. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cao, C.; Shi, M.; Hong, S.; Guo, S.; Li, J.; Liang, T.; Song, P.; Xu, R.; Li, N. Kaempferol inhibits SARS-CoV-2 invasion by impairing heptad repeats-mediated viral fusion. Phytomedicine 2023, 118, 154942. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M. The Effects of Propolis on Viral Respiratory Diseases. Molecules 2023, 28, 359. [Google Scholar] [CrossRef]
- Zarei, A.; Ramazani, A.; Rezaei, A.; Moradi, S. Screening of honey bee pollen constituents against COVID-19: An emerging hot spot in targeting SARS-CoV-2-ACE-2 interaction. Nat. Prod. Res. 2023, 37, 974–980. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonda, K.; Hai, T.; Suzuki, K.; Ozaki, A.; Shibusa, T.; Takenoshita, S.; Maejima, Y.; Shimomura, K. Effect of Ficus pumila L. on Improving Insulin Secretory Capacity and Resistance in Elderly Patients Aged 80 Years Old or Older Who Develop Diabetes After COVID-19 Infection. Nutrients 2025, 17, 290. https://doi.org/10.3390/nu17020290
Gonda K, Hai T, Suzuki K, Ozaki A, Shibusa T, Takenoshita S, Maejima Y, Shimomura K. Effect of Ficus pumila L. on Improving Insulin Secretory Capacity and Resistance in Elderly Patients Aged 80 Years Old or Older Who Develop Diabetes After COVID-19 Infection. Nutrients. 2025; 17(2):290. https://doi.org/10.3390/nu17020290
Chicago/Turabian StyleGonda, Kenji, Takeshi Hai, Kouichi Suzuki, Akihiko Ozaki, Takashi Shibusa, Seiichi Takenoshita, Yuko Maejima, and Kenjyu Shimomura. 2025. "Effect of Ficus pumila L. on Improving Insulin Secretory Capacity and Resistance in Elderly Patients Aged 80 Years Old or Older Who Develop Diabetes After COVID-19 Infection" Nutrients 17, no. 2: 290. https://doi.org/10.3390/nu17020290
APA StyleGonda, K., Hai, T., Suzuki, K., Ozaki, A., Shibusa, T., Takenoshita, S., Maejima, Y., & Shimomura, K. (2025). Effect of Ficus pumila L. on Improving Insulin Secretory Capacity and Resistance in Elderly Patients Aged 80 Years Old or Older Who Develop Diabetes After COVID-19 Infection. Nutrients, 17(2), 290. https://doi.org/10.3390/nu17020290





