Profiles of 71 Human Milk Oligosaccharides and Novel Sub-Clusters of Type I Milk: Results from the Ulm SPATZ Health Study
Abstract
1. Introduction
2. Materials and Methods
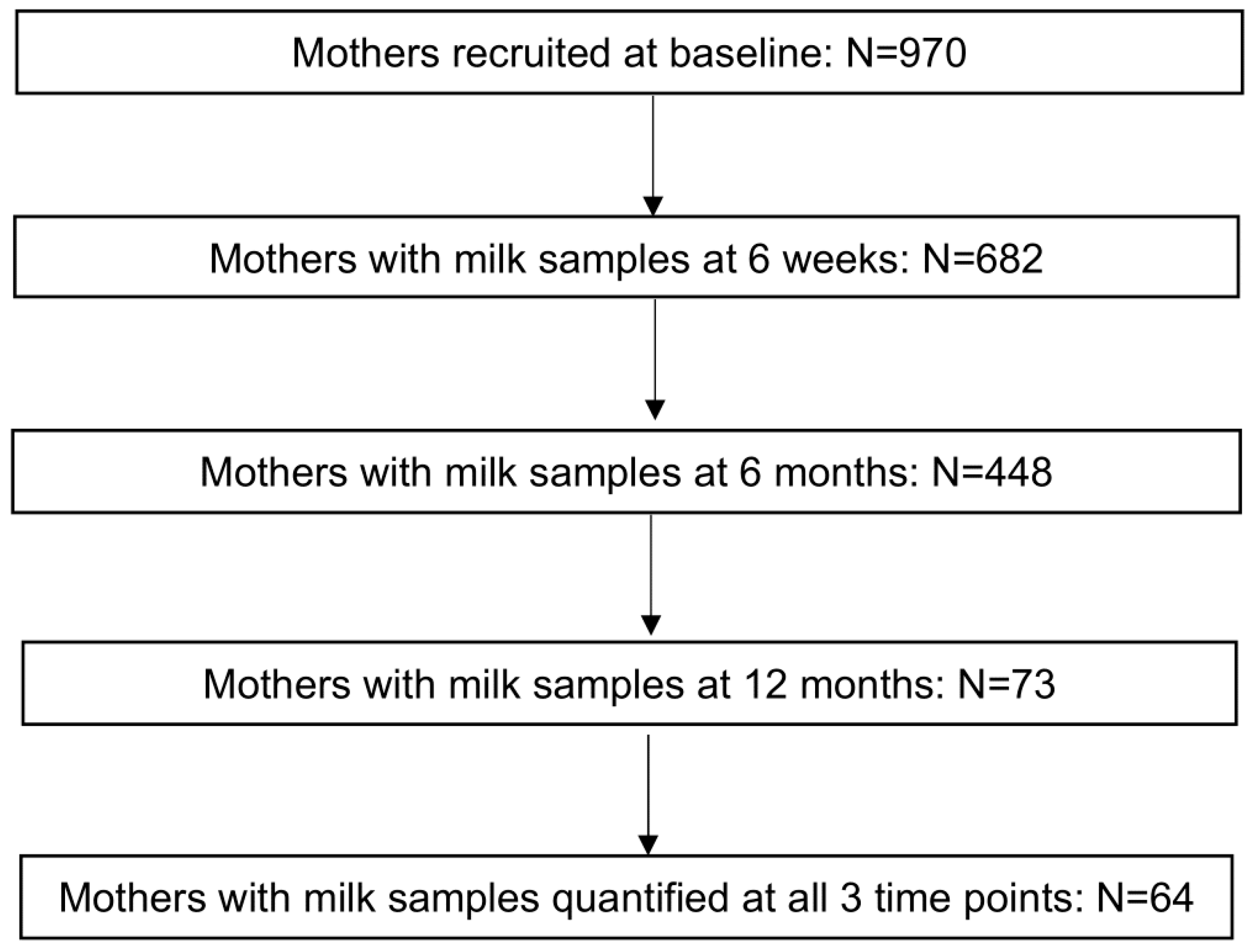
2.1. Cohort Overview
2.2. Human Milk Samples and HMO Quantification
2.3. Statistical Analysis
3. Results
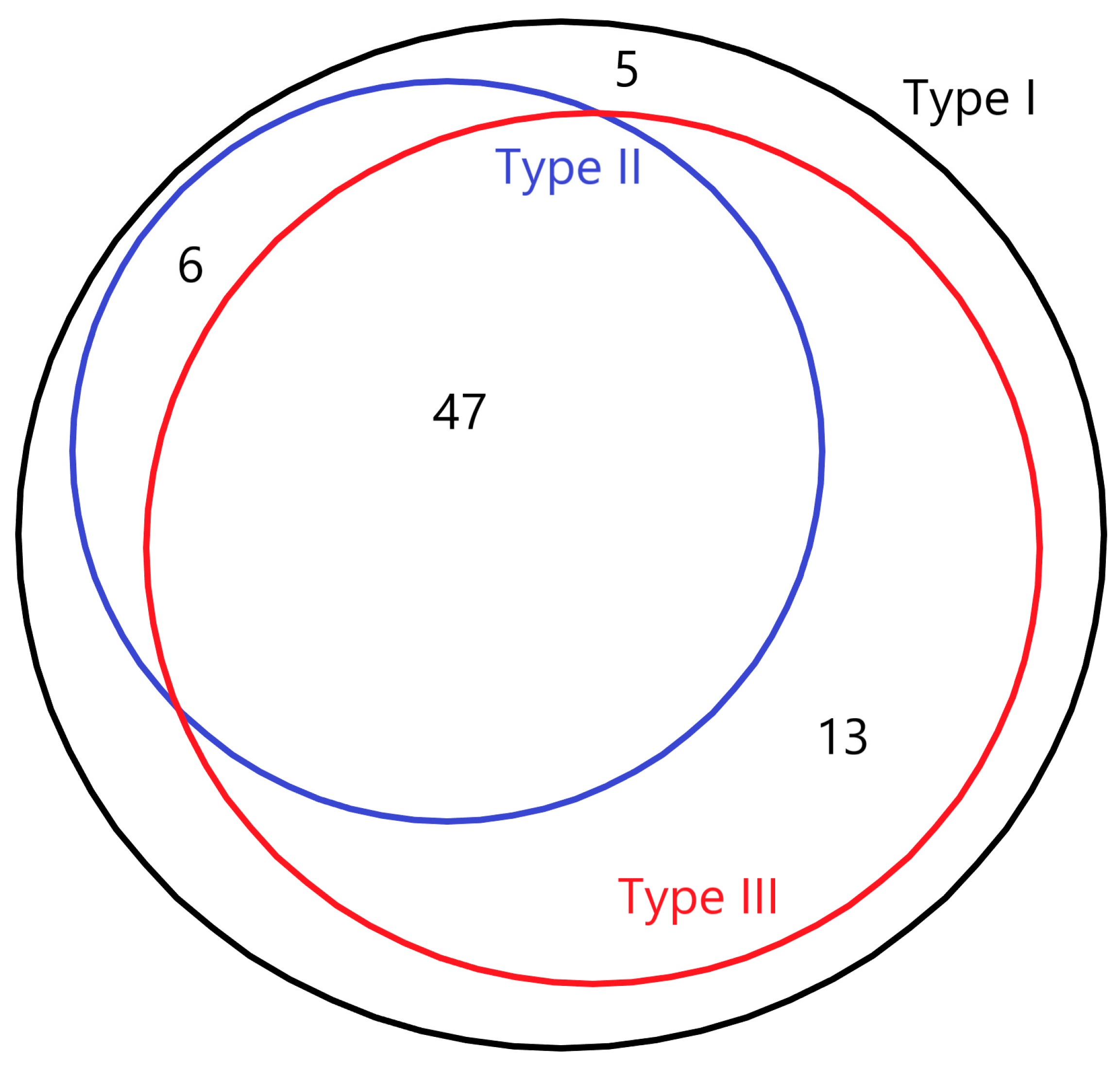
3.1. Basic Characteristics
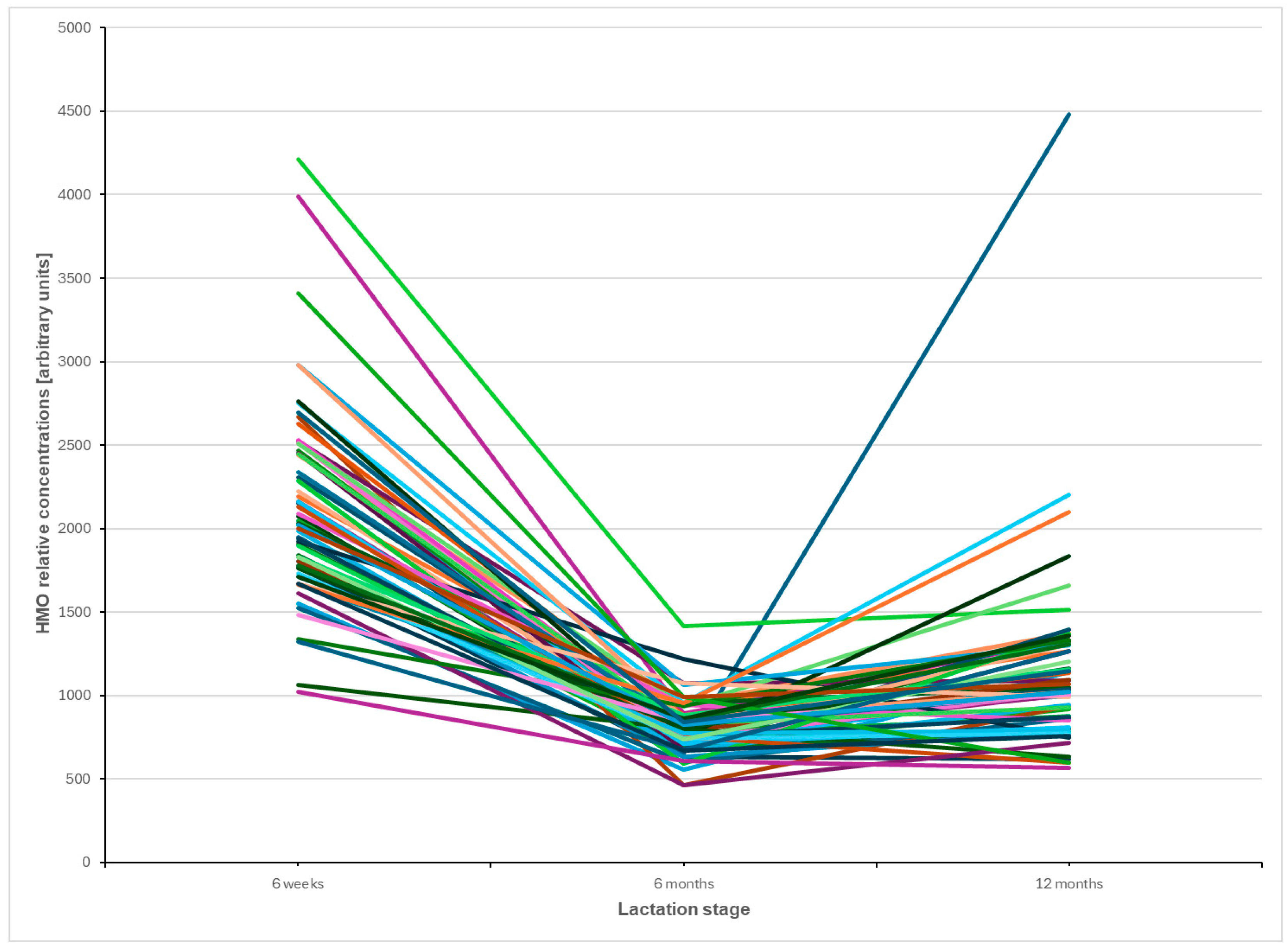
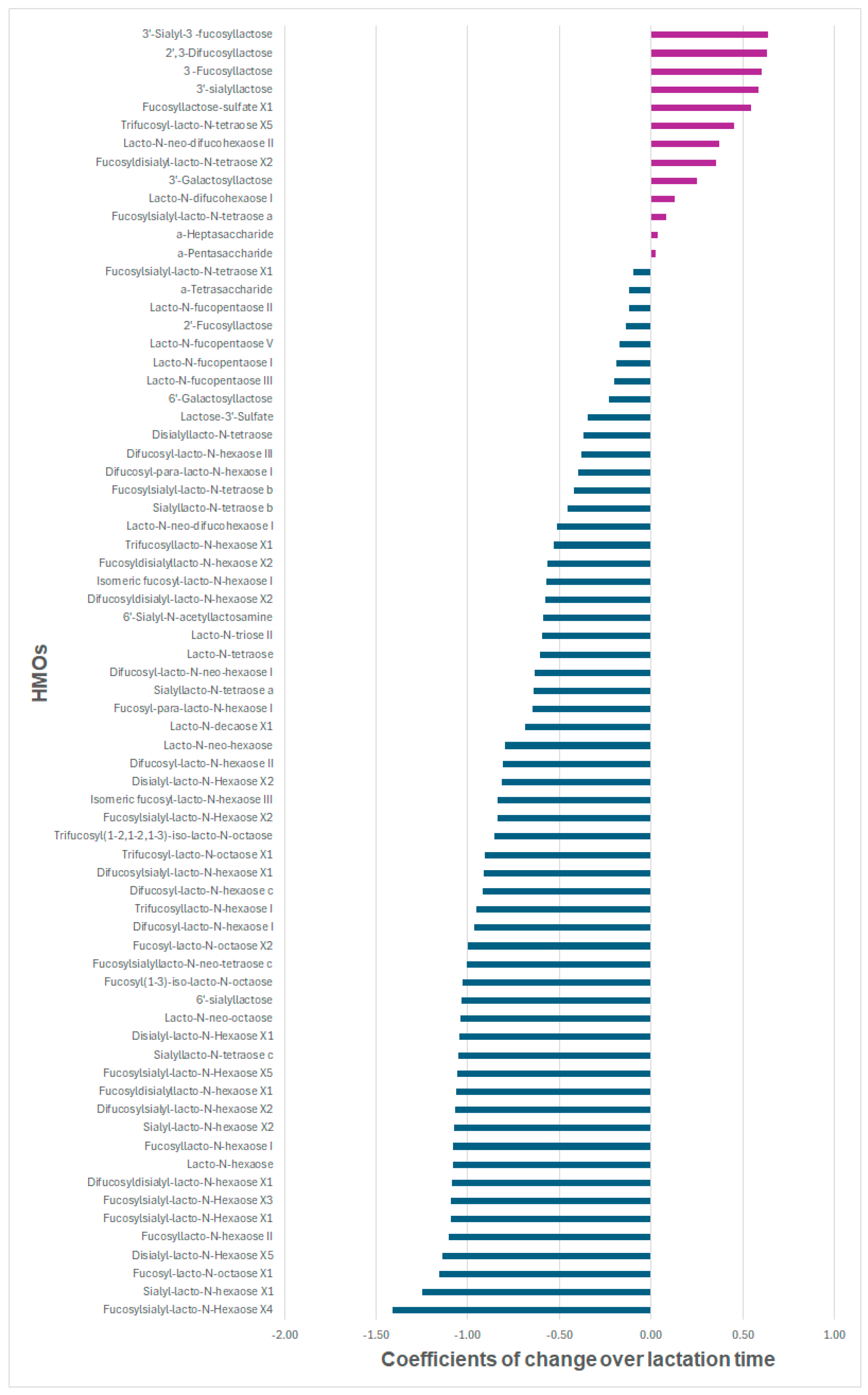
3.2. Trajectories of Individual HMOs over Time
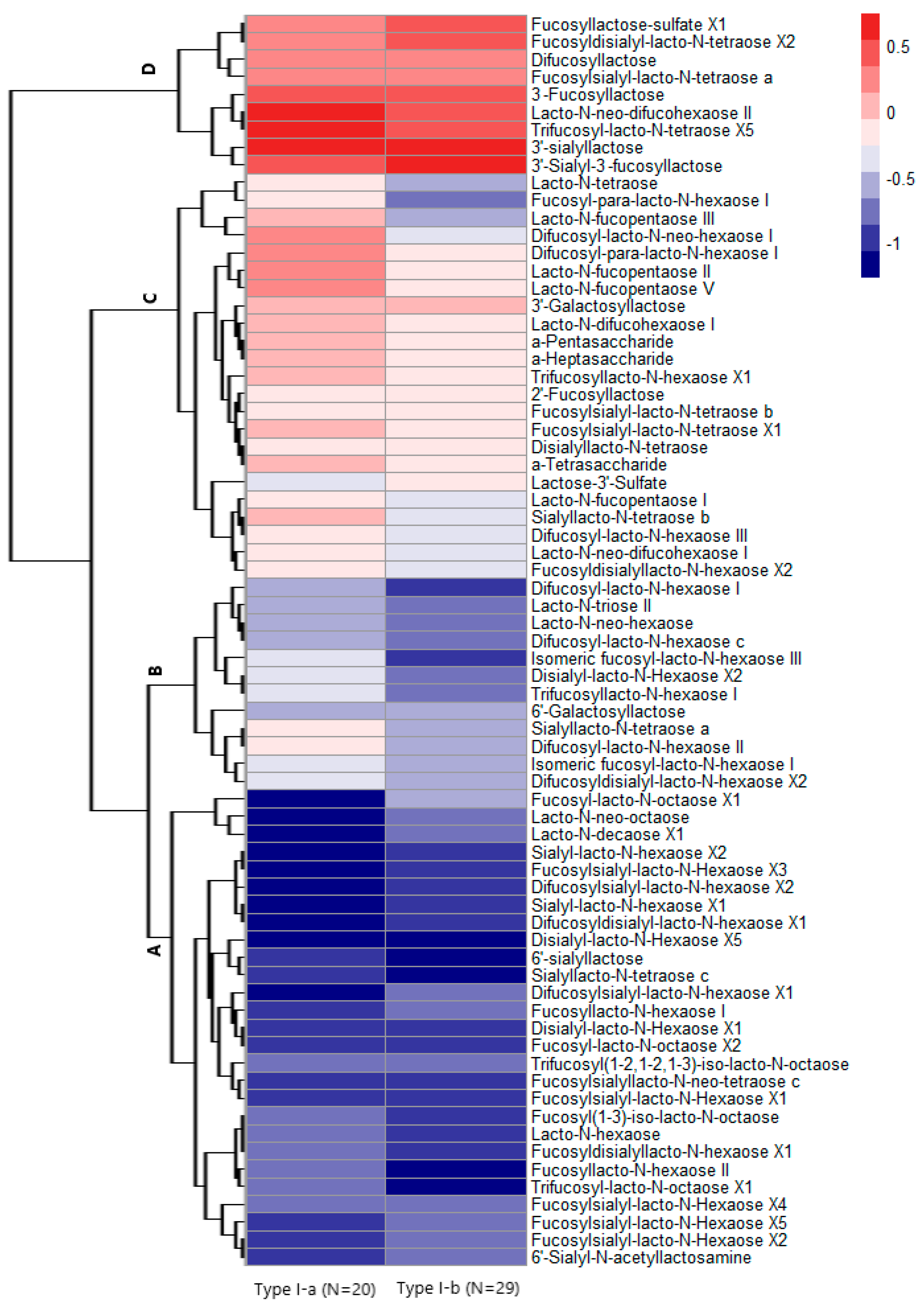
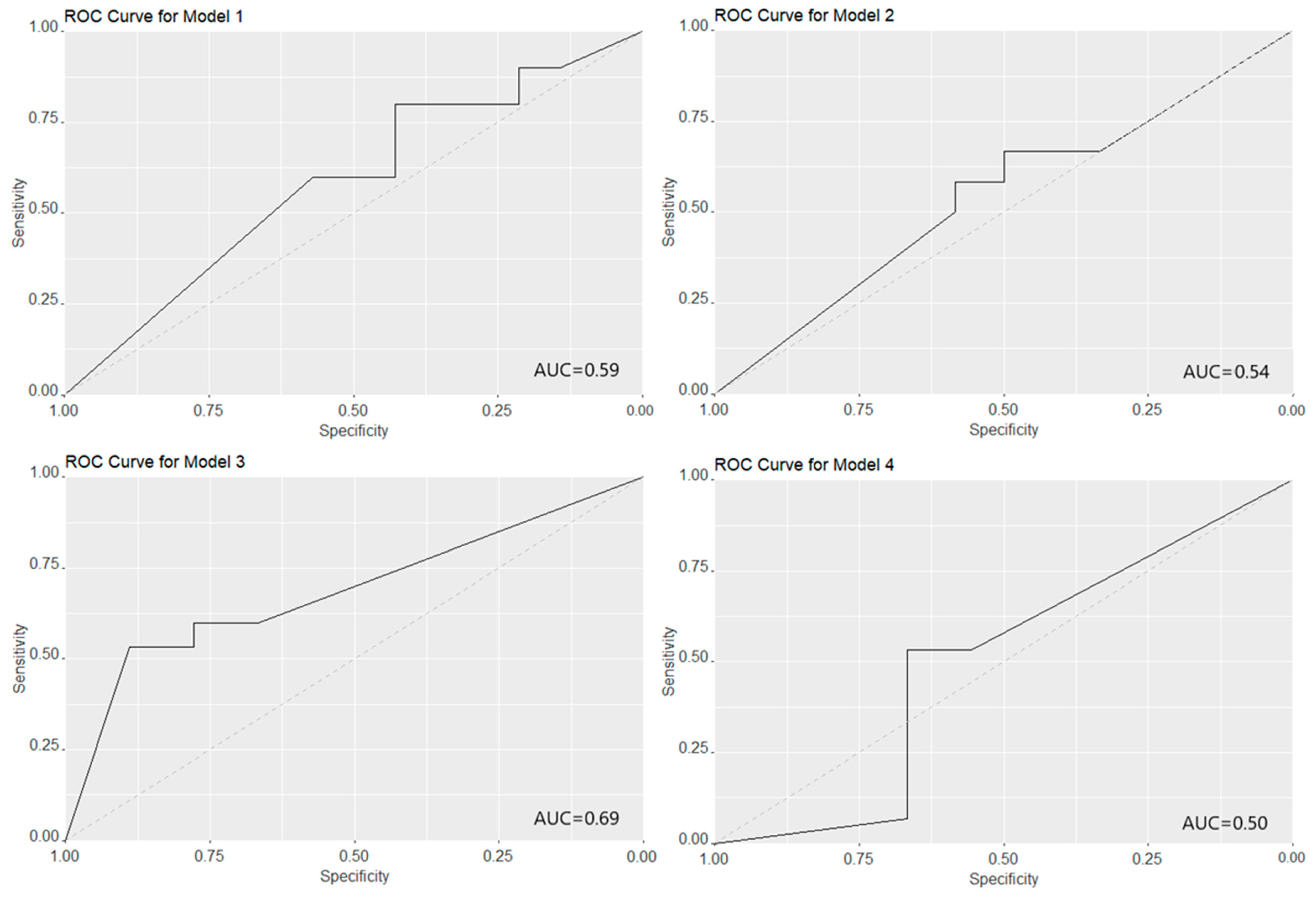
3.3. HMO Clustering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, R.G. Handbook of Milk Composition; Academic Press: San Diego, CA, USA, 1995; pp. 273–349. [Google Scholar]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Ayechu-Muruzabal, V.; van Stigt, A.H.; Mank, M.; Willemsen, L.E.M.; Stahl, B.; Garssen, J.; Van’t Land, B. Diversity of Human Milk Oligosaccharides and Effects on Early Life Immune Development. Front. Pediatr. 2018, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; McMath, A.L.; Donovan, S.M. Review on the Impact of Milk Oligosaccharides on the Brain and Neurocognitive Development in Early Life. Nutrients 2023, 15, 3743. [Google Scholar] [CrossRef]
- Bajic, D.; Wiens, F.; Wintergerst, E.; Deyaert, S.; Baudot, A.; Abbeele, P.V.d. HMOs Impact the Gut Microbiome of Children and Adults Starting from Low Predicted Daily Doses. Metabolites 2024, 14, 239. [Google Scholar] [CrossRef]
- Mulinge, M.M.; Abisi, H.K.; Kabahweza, H.M.; Okutoyi, L.; Wamalwa, D.C.; Nduati, R.W. The Role of Maternal Secretor Status and Human Milk Oligosaccharides on Early Childhood Development: A Systematic Review and Meta-Analysis. Breastfeed. Med. 2024, 19, 409–424. [Google Scholar] [CrossRef]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Altaye, M.; Jiang, X.; Guerrero, M.L.; Meinzen-Derr, J.K.; Farkas, T.; Chaturvedi, P.; Pickering, L.K.; Newburg, D.S. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J. Pediatr. 2004, 145, 297–303. [Google Scholar] [CrossRef]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human milk oligosaccharide 2′-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS ONE 2020, 15, e0228323. [Google Scholar] [CrossRef]
- Thurl, S.; Henker, J.; Siegel, M.; Tovar, K.; Sawatzki, G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj. J. 1997, 14, 795–799. [Google Scholar] [CrossRef]
- Urashima, T.; Asakuma, S.; Leo, F.; Fukuda, K.; Messer, M.; Oftedal, O.T. The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv. Nutr. 2012, 3, 473S–482S. [Google Scholar] [CrossRef]
- Henry, S.; Oriol, R.; Samuelsson, B. Lewis histo-blood group system and associated secretory phenotypes. Vox Sang. 1995, 69, 166–182. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Vervoort, J.; Pan, J.; Gao, P.; Zhu, H.; Wang, X.; Zhang, Y.; Chen, B.; Liu, Y.; Li, Y.; et al. Comparison of twelve human milk oligosaccharides in mature milk from different areas in China in the Chinese Human Milk Project (CHMP) study. Food Chem. 2022, 395, 133554. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef]
- Han, S.M.; Derraik, J.G.B.; Binia, A.; Sprenger, N.; Vickers, M.H.; Cutfield, W.S. Maternal and Infant Factors Influencing Human Milk Oligosaccharide Composition: Beyond Maternal Genetics. J. Nutr. 2021, 151, 1383–1393. [Google Scholar] [CrossRef]
- Soyyılmaz, B.; Mikš, M.H.; Röhrig, C.H.; Matwiejuk, M.; Meszaros-Matwiejuk, A.; Vigsnæs, L.K. The Mean of Milk: A Review of Human Milk Oligosaccharide Concentrations throughout Lactation. Nutrients 2021, 13, 2737. [Google Scholar] [CrossRef]
- Urashima, T.; Hirabayashi, J.; Sato, S.; Kobata, A. Human Milk Oligosaccharides as Essential Tools for Basic and Application Studies on Galectins. Trends Glycosci. Glycotechnol. 2018, 30, SE51–SE65. [Google Scholar] [CrossRef]
- Lundstrøm, J.; Bojar, D. The evolving world of milk oligosaccharides: Biochemical diversity understood by computational advances. Carbohydr. Res. 2024, 537, 109069. [Google Scholar] [CrossRef]
- Masi, A.C.; Embleton, N.D.; Lamb, C.A.; Young, G.; Granger, C.L.; Najera, J.; Smith, D.P.; Hoffman, K.L.; Petrosino, J.F.; Bode, L.; et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut 2021, 70, 2273–2282. [Google Scholar] [CrossRef]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Siziba, L.P.; Mank, M.; Stahl, B.; Gonsalves, J.; Blijenberg, B.; Rothenbacher, D.; Genuneit, J. Human Milk Oligosaccharide Profiles over 12 Months of Lactation: The Ulm SPATZ Health Study. Nutrients 2021, 13, 1973. [Google Scholar] [CrossRef] [PubMed]
- Mainardi, F.; Binia, A.; Rajhans, P.; Austin, S.; Deoni, S.; Schneider, N. Human milk oligosaccharide composition and associations with growth: Results from an observational study in the US. Front. Nutr. 2023, 10, 1239349. [Google Scholar] [CrossRef]
- Mank, M.; Welsch, P.; Heck, A.J.R.; Stahl, B. Label-free targeted LC-ESI-MS2 analysis of human milk oligosaccharides (HMOS) and related human milk groups with enhanced structural selectivity. Anal. Bioanal. Chem. 2019, 411, 231–250. [Google Scholar] [CrossRef]
- Logan, C.A.; Bornemann, R.; Koenig, W.; Reister, F.; Walter, V.; Fantuzzi, G.; Weyermann, M.; Brenner, H.; Genuneit, J.; Rothenbacher, D. Gestational Weight Gain and Fetal-Maternal Adiponectin, Leptin, and CRP: Results of two birth cohorts studies. Sci. Rep. 2017, 7, 41847. [Google Scholar] [CrossRef]
- Kienast-von Einem, C.; Panter, J.; Reid, A. A longitudinal area classification of migration in Great Britain: Testing the application of Group-Based Multi-Trajectory Modelling. Popul. Space Place 2023, 29, e2694. [Google Scholar] [CrossRef]
- Magrini, A. Assessment of agricultural sustainability in European Union countries: A group-based multivariate trajectory approach. AStA Adv. Stat. Anal. 2022, 106, 673–703. [Google Scholar] [CrossRef]
- Thum, C.; Wall, C.R.; Weiss, G.A.; Wang, W.; Szeto, I.M.-Y.; Day, L. Changes in HMO Concentrations throughout Lactation: Influencing Factors, Health Effects and Opportunities. Nutrients 2021, 13, 2272. [Google Scholar] [CrossRef]
- Austin, S.; de Castro, C.A.; Bénet, T.; Hou, Y.; Sun, H.; Thakkar, S.K.; Vinyes-Pares, G.; Zhang, Y.; Wang, P. Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers. Nutrients 2016, 8, 346. [Google Scholar] [CrossRef]
- Plows, J.F.; Berger, P.K.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Najera, J.A.; Khwajazada, S.; Bode, L.; Goran, M.I. Longitudinal Changes in Human Milk Oligosaccharides (HMOs) Over the Course of 24 Months of Lactation. J. Nutr. 2021, 151, 876–882. [Google Scholar] [CrossRef]
- Xu, G.; Davis, J.C.; Goonatilleke, E.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Absolute Quantitation of Human Milk Oligosaccharides Reveals Phenotypic Variations during Lactation. J. Nutr. 2017, 147, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.R.B.M.; Mank, M.; Kottler, R.; Hoffmann, X.-K.; Behne, A.; Rapp, E.; Stahl, B.; Mearin, M.L.; Koletzko, B. Presence and Levels of Galactosyllactoses and Other Oligosaccharides in Human Milk and Their Variation during Lactation and According to Maternal Phenotype. Nutrients 2021, 13, 2324. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; de Castro, C.A.; Sprenger, N.; Binia, A.; Affolter, M.; Garcia-Rodenas, C.L.; Beauport, L.; Tolsa, J.-F.; Fischer Fumeaux, C.J. Human Milk Oligosaccharides in the Milk of Mothers Delivering Term versus Preterm Infants. Nutrients 2019, 11, 1282. [Google Scholar] [CrossRef]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Grabarics, M.; Csernák, O.; Balogh, R.; Béni, S. Analytical characterization of human milk oligosaccharides—Potential applications in pharmaceutical analysis. J. Pharm. Biomed. Anal. 2017, 146, 168–178. [Google Scholar] [CrossRef]
| Maternal Characteristics | n (%) or Means ± SDs | |
|---|---|---|
| In this Analysis | Baseline Cohort | |
| Total | 64 (100) | 970 (100) |
| Milk Types | ||
| I | 49 (76.6) | - |
| II | 10 (15.6) | - |
| III | 5 (7.88) | - |
| IV | 0 (0) | - |
| Age at baseline, years | 34.3 ± 4.0 | 32.7 ± 4.8 |
| Education level | ||
| University degree or higher | 49 (77.8) | 561 (59.0) |
| High school or lower | 14 (22.2) | 390 (41.0) |
| Smoking before pregnancy | ||
| Yes | 7 (11.1) | 259 (27.0) |
| No | 56 (88.9) | 700 (73.0) |
| BMI before pregnancy | 24.2 ± 4.4 | 24.8 ± 5.1 |
| Gestational age, weeks | 39.2 ± 1.4 | 38.8 ± 1.7 |
| Parity | ||
| 1 | 27 (42.2) | 522 (53.9) |
| >1 | 37 (57.8) | 447 (46.1) |
| Delivery mode | ||
| Vaginal (spontaneous or assisted) | 50 (78.1) | 709 (73.2) |
| Cesarean (emergency or elective) | 14 (21.9) | 260 (26.8) |
| HMOs | Coef. a | 95% CIs | PFDR b |
|---|---|---|---|
| All 9 HMOs with increasing trends | |||
| 3′-Sialyl-3-fucosyllactose | 0.64 | 0.52; 0.75 | 2.35E-19 |
| 2′,3-Difucosyllactose | 0.63 | 0.50; 0.77 | 4.73E-15 |
| 3-Fucosyllactose | 0.60 | 0.52; 0.68 | 7.55E-29 |
| 3′-Sialyllactose | 0.59 | 0.47; 0.71 | 5.86E-17 |
| Fucosyllactose-sulfate X1 | 0.54 | 0.37; 0.72 | 2.75E-08 |
| Trifucosyl-lacto-N-tetraose X5 | 0.45 | 0.27; 0.64 | 9.43E-06 |
| Lacto-N-neo-difucohexaose II | 0.37 | 0.26; 0.49 | 8.41E-09 |
| Fucosyldisialyl-lacto-N-tetraose X2 | 0.35 | 0.23; 0.48 | 3.30E-07 |
| 3′-Galactosyllactose | 0.25 | 0.07; 0.43 | 9.95E-03 |
| Top 10 HMOs with decreasing trends | |||
| Fucosylsialyl-lacto-N-Hexaose X4 | −1.41 | −1.93; −0.90 | 1.70E-04 |
| Sialyl-lacto-N-hexaose X1 | −1.25 | −1.63; −0.87 | 2.43E-06 |
| Fucosyl-lacto-N-octaose X1 | −1.16 | −1.51; −0.81 | 2.80E-05 |
| Disialyl-lacto-N-Hexaose X5 | −1.14 | −1.22; −1.05 | 2.96E-43 |
| Fucosyllacto-N-hexaose II | −1.10 | −1.21; −0.99 | 2.77E-39 |
| Fucosylsialyl-lacto-N-Hexaose X1 | −1.09 | −1.20; −0.99 | 3.72E-36 |
| Fucosylsialyl-lacto-N-Hexaose X3 | −1.09 | −1.28; −0.91 | 6.87E-14 |
| Difucosyldisialyl-lacto-N-hexaose X1 | −1.09 | −1.21; −0.97 | 8.28E-22 |
| Lacto-N-hexaose | −1.08 | −1.23; −0.93 | 2.45E-23 |
| Fucosyllacto-N-hexaose I | −1.08 | −1.28; −0.88 | 9.16E-13 |
| Maternal Characteristics | No. (%) or Means ± SDs | Test Statistics | Degrees of Freedom | p | |
|---|---|---|---|---|---|
| Type I-a (n = 20) | Type I-b (n = 29) | ||||
| Maternal age at baseline, years | 34.1 ± 2.9 | 34.7 ± 3.9 | 0.33 | 1 | 0.57 |
| Have a university degree | 15 (75.0) | 22 (78.6) | 0.084 | 1 | 0.77 |
| Smoking before pregnancy: yes | 3 (15.0) | 4 (14.3) | 0.0048 | 1 | 1.00 |
| Maternal BMI before pregnancy | 24.5 ± 4.6 | 23.3 ± 3.8 | 0.87 | 1 | 0.35 |
| Gestational age, week | 38.9 ± 1.5 | 39.5 ± 1.2 | 2.44 | 1 | 0.13 |
| Parity > 1 | 10 (50.0) | 18 (62.1) | 0.70 | 1 | 0.40 |
| Cesarean | 6 (30.0) | 5 (17.2) | 1.11 | 1 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Siziba, L.P.; Mank, M.; Stahl, B.; Gonsalves, J.; Wernecke, D.; Rothenbacher, D.; Genuneit, J. Profiles of 71 Human Milk Oligosaccharides and Novel Sub-Clusters of Type I Milk: Results from the Ulm SPATZ Health Study. Nutrients 2025, 17, 280. https://doi.org/10.3390/nu17020280
Peng Z, Siziba LP, Mank M, Stahl B, Gonsalves J, Wernecke D, Rothenbacher D, Genuneit J. Profiles of 71 Human Milk Oligosaccharides and Novel Sub-Clusters of Type I Milk: Results from the Ulm SPATZ Health Study. Nutrients. 2025; 17(2):280. https://doi.org/10.3390/nu17020280
Chicago/Turabian StylePeng, Zhuoxin, Linda P. Siziba, Marko Mank, Bernd Stahl, John Gonsalves, Deborah Wernecke, Dietrich Rothenbacher, and Jon Genuneit. 2025. "Profiles of 71 Human Milk Oligosaccharides and Novel Sub-Clusters of Type I Milk: Results from the Ulm SPATZ Health Study" Nutrients 17, no. 2: 280. https://doi.org/10.3390/nu17020280
APA StylePeng, Z., Siziba, L. P., Mank, M., Stahl, B., Gonsalves, J., Wernecke, D., Rothenbacher, D., & Genuneit, J. (2025). Profiles of 71 Human Milk Oligosaccharides and Novel Sub-Clusters of Type I Milk: Results from the Ulm SPATZ Health Study. Nutrients, 17(2), 280. https://doi.org/10.3390/nu17020280






