Neonatal Circulating Amino Acids and Lipid Metabolites Mediate the Association of Maternal Gestational Diabetes Mellitus with Offspring Neurodevelopment at 1 Year
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Diagnosis of GDM
2.3. Measurements of Neonatal Circulating Metabolites
2.4. Assessment of Offspring Neurodevelopment
2.5. Measurements of Covariates
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Alterations in Neonatal Circulating Metabolites That Associated with Maternal GDM
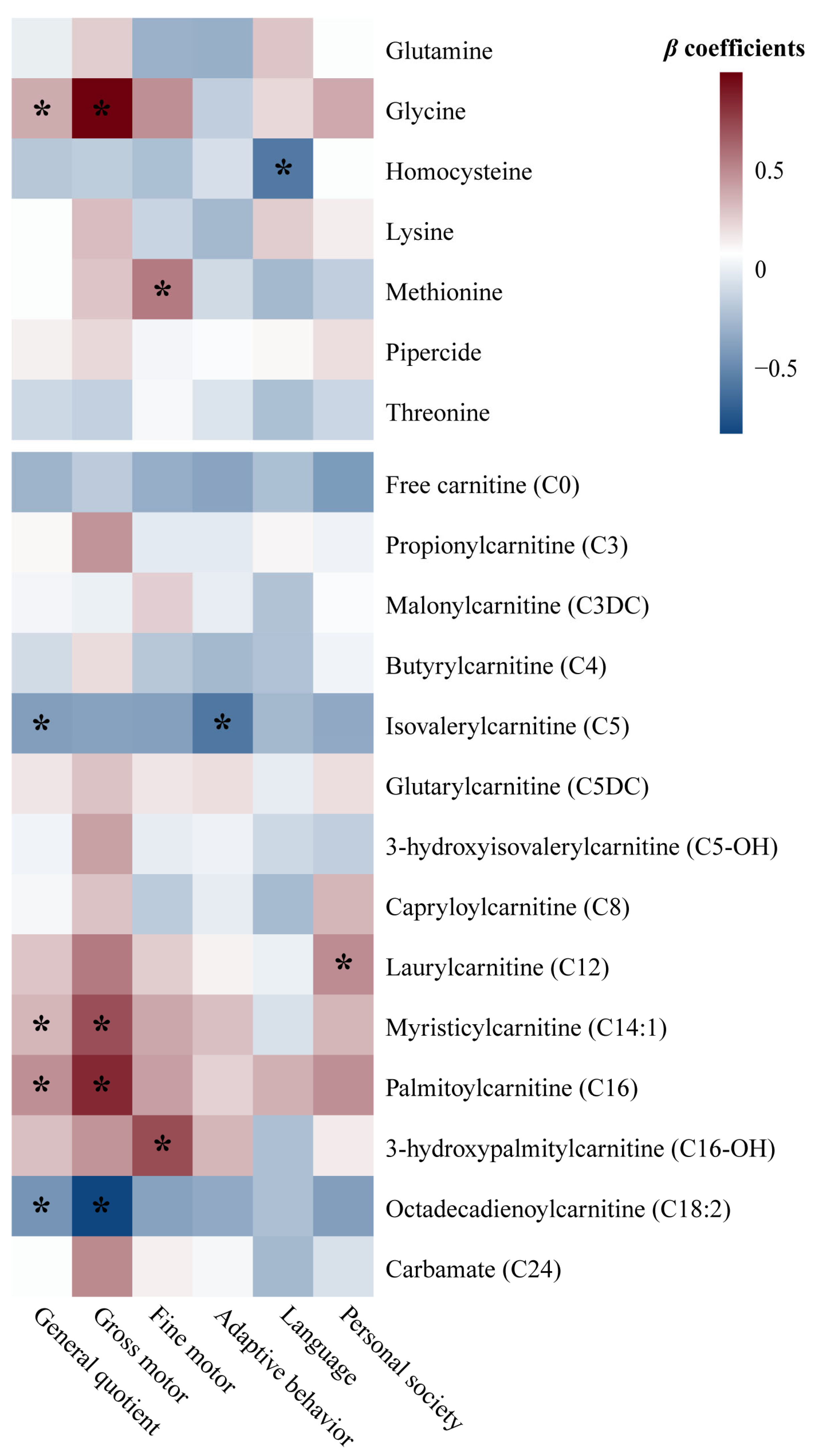
3.3. Relationships Between Neonatal Circulating Metabolites and Offspring Neurodevelopment at 1 Year
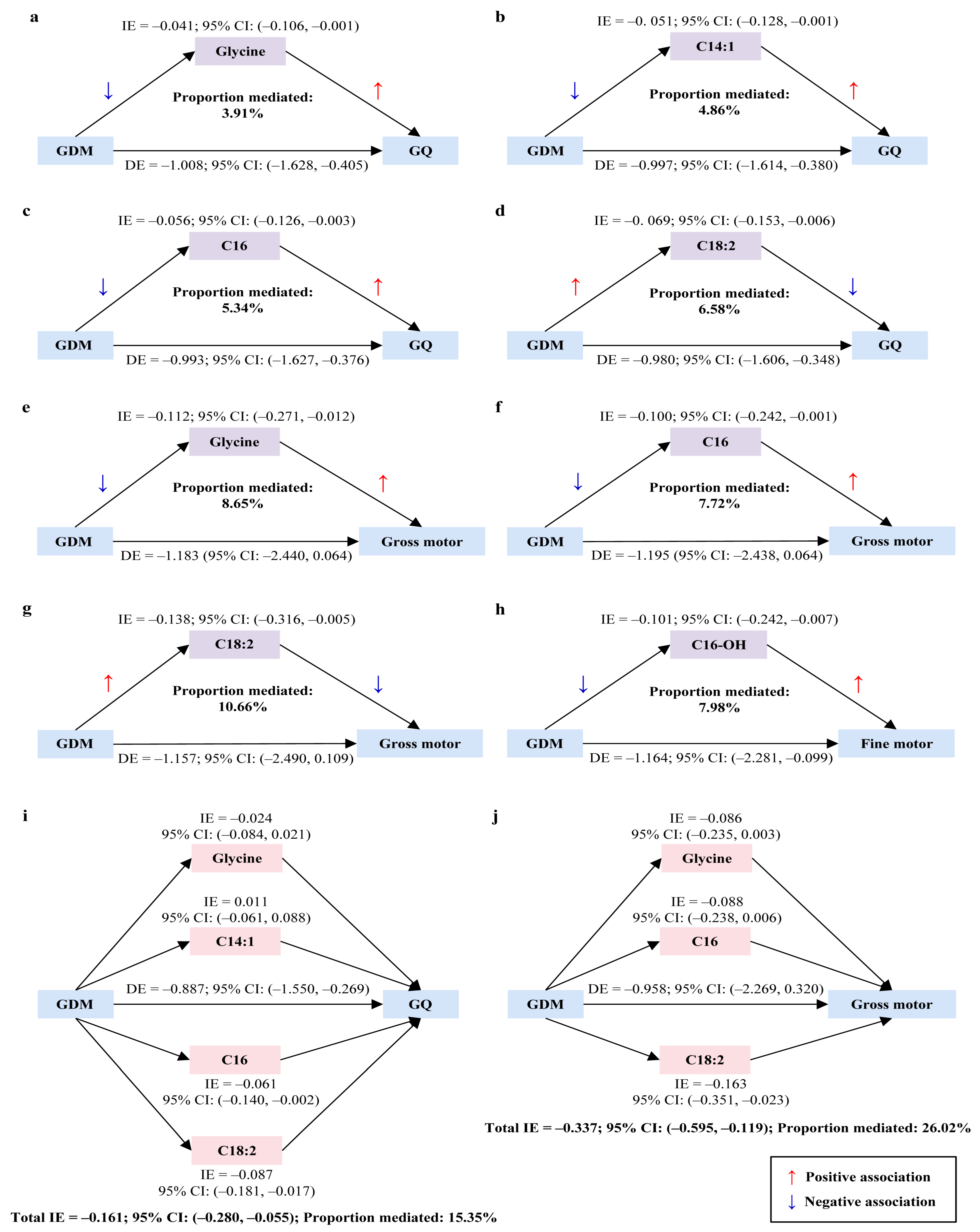
3.4. Mediation Effects of Neonatal Circulating Metabolites in the Relationship Between Maternal GDM and Offspring Neurodevelopment at 1 Year
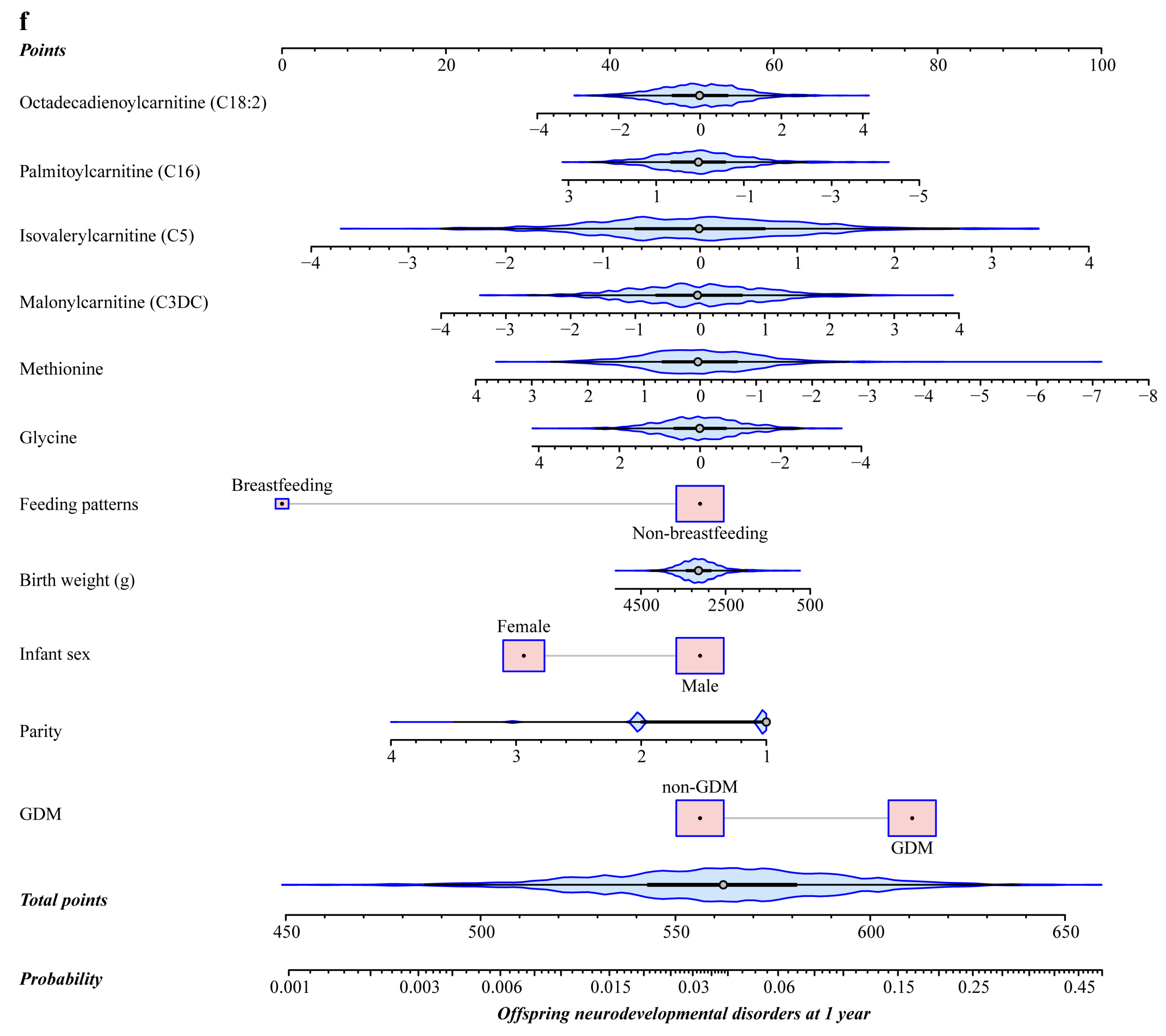
3.5. Predictive Performance of Neonatal Circulating Metabolites for Offspring Neurodevelopmental Disorders at 1 Year Compared to Traditional Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petry, C.J. Gestational Diabetes: Risk Factors and Recent Advances in Its Genetics and Treatment. Br. J. Nutr. 2010, 104, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Reece, E.A.; Pavlinkova, G.; Kappen, C.; Miller, R.K. Effect of Maternal Diabetes on the Embryo, Fetus, and Children: Congenital Anomalies, Genetic and Epigenetic Changes and Developmental Outcomes. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Dionne, G.; Boivin, M.; Séguin, J.R.; Pérusse, D.; Tremblay, R.E. Gestational Diabetes Hinders Language Development in Offspring. Pediatrics 2008, 122, e1073–e1079. [Google Scholar] [CrossRef] [PubMed]
- Adane, A.A.; Mishra, G.D.; Tooth, L.R. Diabetes in Pregnancy and Childhood Cognitive Development: A Systematic Review. Pediatrics 2016, 137, e20154234. [Google Scholar] [CrossRef]
- Wang, P.; Xie, J.; Jiao, X.-C.; Ma, S.-S.; Liu, Y.; Yin, W.-J.; Tao, R.-X.; Hu, H.-L.; Zhang, Y.; Chen, X.-X.; et al. Maternal Glycemia During Pregnancy and Early Offspring Development: A Prospective Birth Cohort Study. J. Clin. Endocrinol. Metab. 2021, 106, 2279–2290. [Google Scholar] [CrossRef]
- Rowland, J.; Wilson, C.A. The Association between Gestational Diabetes and ASD and ADHD: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 5136. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Liu, G.; Han, B.; Wang, J.; Jiang, X. The Association of Maternal Diabetes with Attention Deficit and Hyperactivity Disorder in Offspring: A Meta-Analysis. Neuropsychiatr. Dis. Treat. 2019, 15, 675–684. [Google Scholar] [CrossRef]
- Xu, G.; Jing, J.; Bowers, K.; Liu, B.; Bao, W. Maternal Diabetes and the Risk of Autism Spectrum Disorders in the Offspring: A Systematic Review and Meta-Analysis. J. Autism Dev. Disord. 2014, 44, 766–775. [Google Scholar] [CrossRef]
- Alesi, S.; Ghelani, D.; Rassie, K.; Mousa, A. Metabolomic Biomarkers in Gestational Diabetes Mellitus: A Review of the Evidence. Int. J. Mol. Sci. 2021, 22, 5512. [Google Scholar] [CrossRef]
- Borum, P.R. Carnitine. Annu. Rev. Nutr. 1983, 3, 233–259. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Xu, F.; Wang, M.; Ding, S.; Xu, H.; Dong, F. Comprehensive Analysis of Serum Metabolites in Gestational Diabetes Mellitus by UPLC/Q-TOF-MS. Anal. Bioanal. Chem. 2016, 408, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Batchuluun, B.; Al Rijjal, D.; Prentice, K.J.; Eversley, J.A.; Burdett, E.; Mohan, H.; Bhattacharjee, A.; Gunderson, E.P.; Liu, Y.; Wheeler, M.B. Elevated Medium-Chain Acylcarnitines Are Associated with Gestational Diabetes Mellitus and Early Progression to Type 2 Diabetes and Induce Pancreatic β-Cell Dysfunction. Diabetes 2018, 67, 885–897. [Google Scholar] [CrossRef]
- Leitner, M.; Fragner, L.; Danner, S.; Holeschofsky, N.; Leitner, K.; Tischler, S.; Doerfler, H.; Bachmann, G.; Sun, X.; Jaeger, W.; et al. Combined Metabolomic Analysis of Plasma and Urine Reveals AHBA, Tryptophan and Serotonin Metabolism as Potential Risk Factors in Gestational Diabetes Mellitus (GDM). Front. Mol. Biosci. 2017, 4, 84. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.D.A.; Murgia, A.; Lai, C.; Ferreira, C.S.; Goes, V.A.; Guimarães, D.D.A.B.; Ranquine, L.G.; Reis, D.L.; Struchiner, C.J.; Griffin, J.L.; et al. Sphingolipids and Acylcarnitines Are Altered in Placentas from Women with Gestational Diabetes Mellitus. Br. J. Nutr. 2023, 130, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, Z.; Liu, L.; Zhang, X.; Huang, Q.; Alamdar, A.; Tian, M.; Shen, H. Newborn Meconium and Urinary Metabolome Response to Maternal Gestational Diabetes Mellitus: A Preliminary Case-Control Study. J. Proteome Res. 2015, 14, 1799–1809. [Google Scholar] [CrossRef]
- Cetin, I.; de Santis, M.S.N.; Taricco, E.; Radaelli, T.; Teng, C.; Ronzoni, S.; Spada, E.; Milani, S.; Pardi, G. Maternal and Fetal Amino Acid Concentrations in Normal Pregnancies and in Pregnancies with Gestational Diabetes Mellitus. Am. J. Obstet. Gynecol. 2005, 192, 610–617. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Skalnaya, M.G.; Skalny, A.V. Serum Trace Element and Amino Acid Profile in Children with Cerebral Palsy. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2021, 64, 126685. [Google Scholar] [CrossRef]
- Zhu, J.; Hua, X.; Yang, T.; Guo, M.; Li, Q.; Xiao, L.; Li, L.; Chen, J.; Li, T. Alterations in Gut Vitamin and Amino Acid Metabolism Are Associated with Symptoms and Neurodevelopment in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2022, 52, 3116–3128. [Google Scholar] [CrossRef]
- Li, C.; Shen, K.; Chu, L.; Liu, P.; Song, Y.; Kang, X. Decreased Levels of Urinary Free Amino Acids in Children with Autism Spectrum Disorder. J. Clin. Neurosci. 2018, 54, 45–49. [Google Scholar] [CrossRef]
- Chamtouri, M.; Merghni, A.; Salazar, N.; Redruello, B.; Gaddour, N.; Mastouri, M.; Arboleya, S.; De Los Reyes-Gavilán, C.G. An Overview on Fecal Profiles of Amino Acids and Related Amino-Derived Compounds in Children with Autism Spectrum Disorder in Tunisia. Molecules 2023, 28, 3269. [Google Scholar] [CrossRef]
- Lv, Q.-Q.; You, C.; Zou, X.-B.; Deng, H.-Z. Acyl-Carnitine, C5DC, and C26 as Potential Biomarkers for Diagnosis of Autism Spectrum Disorder in Children. Psychiatry Res. 2018, 267, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Melnyk, S.; Macfabe, D.F. Unique Acyl-Carnitine Profiles Are Potential Biomarkers for Acquired Mitochondrial Disease in Autism Spectrum Disorder. Transl. Psychiatry 2013, 3, e220. [Google Scholar] [CrossRef] [PubMed]
- Moreau, G.B.; Ramakrishnan, G.; Cook, H.L.; Fox, T.E.; Nayak, U.; Ma, J.Z.; Colgate, E.R.; Kirkpatrick, B.D.; Haque, R.; Petri, W.A. Childhood Growth and Neurocognition Are Associated with Distinct Sets of Metabolites. EBioMedicine 2019, 44, 597–606. [Google Scholar] [CrossRef]
- Vinding, R.K.; Rago, D.; Kelly, R.S.; Gürdeniz, G.; Rasmussen, M.A.; Stokholm, J.; Bønnelykke, K.; Litonjua, A.A.; Weiss, S.T.; Lasky-Su, J.; et al. Delayed Motor Milestones Achievement in Infancy Associates with Perturbations of Amino Acids and Lipid Metabolic Pathways. Metabolites 2020, 10, 337. [Google Scholar] [CrossRef]
- Luo, S.-S.; Zou, K.-X.; Zhu, H.; Cheng, Y.; Yan, Y.-S.; Sheng, J.-Z.; Huang, H.-F.; Ding, G.-L. Integrated Multi-Omics Analysis Reveals the Effect of Maternal Gestational Diabetes on Fetal Mouse Hippocampi. Front. Cell Dev. Biol. 2022, 10, 748862. [Google Scholar] [CrossRef]
- Moreno-Giménez, A.; Campos-Berga, L.; Nowak, A.; Sahuquillo-Leal, R.; D’Ocon, A.; Hervás, D.; Navalón, P.; Vento, M.; García-Blanco, A. Impact of Maternal Age on Infants’ Emotional Regulation and Psychomotor Development. Psychol. Med. 2022, 52, 3708–3719. [Google Scholar] [CrossRef] [PubMed]
- Linsell, L.; Malouf, R.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or with Very Low Birth Weight: A Systematic Review. JAMA Pediatr. 2015, 169, 1162–1172. [Google Scholar] [CrossRef]
- Molkenboer, J.F.M.; Roumen, F.J.M.E.; Smits, L.J.M.; Nijhuis, J.G. Birth Weight and Neurodevelopmental Outcome of Children at 2 Years of Age after Planned Vaginal Delivery for Breech Presentation at Term. Am. J. Obstet. Gynecol. 2006, 194, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Naeh, A.; Hallak, M.; Gabbay-Benziv, R. Parity and Interval from Previous Delivery-Influence on Perinatal Outcome in Advanced Maternal Age Parturients. J. Clin. Med. 2021, 10, 460. [Google Scholar] [CrossRef]
- Zhang, M.; Gazimbi, M.M.; Chen, Z.; Zhang, B.; Chen, Y.; Yu, Y.; Tang, J. Association between Birth Weight and Neurodevelopment at Age 1–6 Months: Results from the Wuhan Healthy Baby Cohort. BMJ Open 2020, 10, e031916. [Google Scholar] [CrossRef] [PubMed]
- Badr, L.K.; Bookheimer, S.; Purdy, I.; Deeb, M. Predictors of Neurodevelopmental Outcome for Preterm Infants with Brain Injury: MRI, Medical and Environmental Factors. Early Hum. Dev. 2009, 85, 279–284. [Google Scholar] [CrossRef]
- Woythaler, M. Neurodevelopmental Outcomes of the Late Preterm Infant. Semin. Fetal. Neonatal Med. 2019, 24, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Impact of Maternal Diet on Human Milk Composition and Neurological Development of Infants. Am. J. Clin. Nutr. 2014, 99, 734S–741S. [Google Scholar] [CrossRef]
- Ahrens, A.P.; Hyötyläinen, T.; Petrone, J.R.; Igelström, K.; George, C.D.; Garrett, T.J.; Orešič, M.; Triplett, E.W.; Ludvigsson, J. Infant Microbes and Metabolites Point to Childhood Neurodevelopmental Disorders. Cell 2024, 187, 1853–1873.e15. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association 2. Classification and Diagnosis of Diabetes. Diabetes Care 2015, 39, S13–S22. [Google Scholar] [CrossRef]
- Jin, C. Neuropsychological and Behavioral Examination Scale for Children 2016; Beijing Publishing Group: Beijing, China, 2016; p. 135. ISBN 978-7-200-12294-7. [Google Scholar]
- Kc, K.; Shakya, S.; Zhang, H. Gestational diabetes mellitus and macrosomia: A literature review. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 14–20. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Ekstrand, Y.; Björn, C.; Wennergren, M.; Powell, T.L. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes 2002, 51, 2214–2219. [Google Scholar] [CrossRef]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines Reflecting or Inflicting Insulin Resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Tekola-Ayele, F.; Li, L.-J.; Bremer, A.A.; Lu, R.; Rahman, M.L.; Weir, N.L.; Pang, W.W.; Chen, Z.; et al. Plasma Amino Acids in Early Pregnancy and Midpregnancy and Their Interplay With Phospholipid Fatty Acids in Association With the Risk of Gestational Diabetes Mellitus: Results From a Longitudinal Prospective Cohort. Diabetes Care 2023, 46, 722–732. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, H.-Y.; Fan, Z.-Y.; He, Y.; Yan, Y.-X. Metabolomics Signatures in Type 2 Diabetes: A Systematic Review and Integrative Analysis. J. Clin. Endocrinol. Metab. 2020, 105, dgz240. [Google Scholar] [CrossRef]
- Xiong, H.; Li, S.; Ma, Y.; Gu, X.; Xiao, X.; Hao, H. Analysis of amino acid and acylcarnitine profile in full-term newborns small for gestational age. Chin. J. Appl. Clin. Pediatr. 2020, 35, 1346–1350. [Google Scholar] [CrossRef]
- Xu, T.-L.; Gong, N. Glycine and Glycine Receptor Signaling in Hippocampal Neurons: Diversity, Function and Regulation. Prog. Neurobiol. 2010, 91, 349–361. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic Biomarkers of Increased Oxidative Stress and Impaired Methylation Capacity in Children with Autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef]
- Guo, B.-Q.; Li, H.-B.; Ding, S.-B. Blood Homocysteine Levels in Children with Autism Spectrum Disorder: An Updated Systematic Review and Meta-Analysis. Psychiatry Res. 2020, 291, 113283. [Google Scholar] [CrossRef]
- Virmani, A. Role of Carnitine Esters in Brain Neuropathology. Mol. Aspects Med. 2004, 25, 533–549. [Google Scholar] [CrossRef]
- Adeva-Andany, M.; Souto-Adeva, G.; Ameneiros-Rodríguez, E.; Fernández-Fernández, C.; Donapetry-García, C.; Domínguez-Montero, A. Insulin Resistance and Glycine Metabolism in Humans. Amino Acids 2018, 50, 11–27. [Google Scholar] [CrossRef]
- Yan-Do, R.; MacDonald, P.E. Impaired “Glycine”-Mia in Type 2 Diabetes and Potential Mechanisms Contributing to Glucose Homeostasis. Endocrinology 2017, 158, 1064–1073. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.S.; DeLany, J.P. Increased Levels of Plasma Acylcarnitines in Obesity and Type 2 Diabetes and Identification of a Marker of Glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef]
- Anand, N.S.; Ji, Y.; Wang, G.; Hong, X.; van der Rijn, M.; Riley, A.; Pearson, C.; Zuckerman, B.; Wang, X. Maternal and Cord Plasma Branched-Chain Amino Acids and Child Risk of Attention-Deficit Hyperactivity Disorder: A Prospective Birth Cohort Study. J. Child Psychol. Psychiatry 2021, 62, 868–875. [Google Scholar] [CrossRef]
- Polanska, K.; Kaluzny, P.; Aubert, A.M.; Bernard, J.Y.; Duijts, L.; El Marroun, H.; Hanke, W.; Hébert, J.R.; Heude, B.; Jankowska, A.; et al. Dietary Quality and Dietary Inflammatory Potential during Pregnancy and Offspring Emotional and Behavioral Symptoms in Childhood: An Individual Participant Data Meta-Analysis of Four European Cohorts. Biol. Psychiatry 2021, 89, 550–559. [Google Scholar] [CrossRef]
- Handzlik, M.K.; Gengatharan, J.M.; Frizzi, K.E.; McGregor, G.H.; Martino, C.; Rahman, G.; Gonzalez, A.; Moreno, A.M.; Green, C.R.; Guernsey, L.S.; et al. Insulin-Regulated Serine and Lipid Metabolism Drive Peripheral Neuropathy. Nature 2023, 614, 118–124. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Overall a N = 1228 | GDM a n = 614 | Non-GDM a n = 614 | p-Value b |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age at delivery, years | 31.2 ± 4.8 | 31.2 ± 4.8 | 31.2 ± 4.8 | 0.895 |
| Education level, n (%) | 0.566 | |||
| High-school and below | 553 (45.0) | 282 (45.9) | 271 (44.1) | |
| College and above | 675 (55.0) | 332 (54.1) | 343 (55.9) | |
| Gestational hypertension, n (%) | 17 (1.4) | 7 (1.1) | 10 (1.6) | 0.625 |
| Thalassemia, n (%) | 99 (8.1) | 46 (7.5) | 53 (8.6) | 0.529 |
| Primigravid, n (%) | 412 (33.6) | 218 (35.5) | 194 (31.6) | 0.165 |
| Multiparous, n (%) | 584 (47.6) | 272 (44.3) | 312 (50.8) | 0.026 |
| Natural conception, n (%) | 1108 (90.2) | 541 (88.1) | 567 (92.3) | 0.016 |
| Spontaneous vaginal delivery, n (%) | 792 (64.5) | 389 (63.4) | 403 (65.6) | 0.438 |
| Offspring characteristics at birth | ||||
| Male sex, n (%) | 698 (56.8) | 349 (56.8) | 349 (56.8) | 1.000 |
| Gestational age, weeks | 38.9 (38.1–39.7) | 38.9 (38.0–39.6) | 39.0 (38.1–39.9) | 0.003 |
| Birth weight, g | 3103.1 ± 485.5 | 3082.2 ± 480.6 | 3124.0 ± 489.8 | 0.132 |
| Birth length, cm | 49.0 (48.0–50.0) | 49.0 (48.0–50.0) | 49.0 (48.0–50.0) | 0.447 |
| Birth head circumference, cm | 32.8 ± 1.9 | 32.8 ± 1.8 | 32.7 ± 2.0 | 0.655 |
| Offspring characteristics at 1 year | ||||
| Breastfeeding, n (%) | 86 (7.0) | 41 (6.7) | 45 (7.3) | 0.737 |
| Duration of outdoor activities, hours/day | 1.0 (0.0–1.0) | 1.0 (0.0–1.0) | 1.0 (0.0–1.0) | 0.880 |
| Neurodevelopmental disorders c, n (%) | 67 (5.46) | 47 (7.65) | 20 (3.26) | 0.001 |
| Metabolites | Median (IQR) a | p-Value b | VIP c | β (95% CI) d | ||
|---|---|---|---|---|---|---|
| GDM n = 614 | Non-GDM n = 614 | Crude | Adjusted e | |||
| Amino acids | ||||||
| Glutamine | 22.614 (10.001) | 23.786 (10.181) | 0.039 | 1.050 | −0.118 (−0.230, −0.006) | −0.126 (−0.238, −0.014) |
| Glycine | 196.751 (50.194) | 202.277 (50.910) | 0.047 | 1.010 | −0.113 (−0.225, −0.001) | −0.117 (−0.229, −0.005) |
| Homocysteine | 13.670 (2.588) | 13.482 (2.455) | 0.041 | 1.040 | 0.117 (0.005, 0.228) | 0.123 (0.010, 0.235) |
| Lysine | 20.003 (8.522) | 21.221 (8.554) | 0.041 | 1.038 | −0.116 (−0.228, −0.005) | −0.127 (−0.239, −0.014) |
| Methionine | 27.220 (9.139) | 26.683 (8.688) | 0.042 | 1.032 | 0.116 (0.004, 0.228) | 0.108 (−0.004, 0.220) |
| Pipercide | 138.644 (36.911) | 149.254 (40.572) | <0.001 | 1.904 | −0.2124 (−0.325, −0.102) | −0.214 (−0.326, −0.102) |
| Threonine | 29.807 (10.814) | 28.474 (9.448) | 0.001 | 1.638 | 0.184 (0.072, 0.295) | 0.173 (0.063, 0.283) |
| Carnitines | ||||||
| Free carnitine (C0) | 26.389 (9.192) | 25.584 (8.909) | 0.010 | 1.304 | 0.146 (0.035, 0.258) | 0.134 (0.023, 0.244) |
| Propionylcarnitine (C3) | 2.068 (0.959) | 1.857 (0.922) | <0.001 | 2.226 | 0.250 (0.139, 0.361) | 0.254 (0.142, 0.365) |
| Malonylcarnitine (C3DC) | 0.059 (0.023) | 0.061 (0.024) | 0.028 | 1.118 | −0.125 (−0.237, −0.014) | −0.125 (−0.237, −0.013) |
| Butyrylcarnitine (C4) | 0.246 (0.090) | 0.239 (0.099) | 0.031 | 1.095 | 0.123 (0.011, 0.235) | 0.120 (0.008, 0.232) |
| Isovalerylcarnitine (C5) | 0.117(0.053) | 0.112(0.046) | 0.034 | 1.078 | 0.121 (0.009, 0.233) | 0.096 (−0.011, 0.202) |
| Glutarylcarnitine (C5DC) | 0.081 (0.036) | 0.084 (0.040) | 0.017 | 1.218 | −0.137 (−0.248, −0.025) | −0.134 (−0.246, −0.022) |
| 3-hydroxyisovalerylcarnitine (C5-OH) | 0.220 (0.081) | 0.205 (0.082) | <0.001 | 1.878 | 0.211 (0.099, 0.322) | 0.203 (0.092, 0.314) |
| Capryloylcarnitine (C8) | 0.068 (0.029) | 0.070 (0.030) | 0.032 | 1.090 | −0.122 (−0.234, −0.010) | −0.122 (−0.234, −0.010) |
| Laurylcarnitine (C12) | 0.085 (0.041) | 0.094 (0.047) | <0.001 | 2.440 | −0.274 (−0.385, −0.163) | −0.247 (−0.355, −0.139) |
| Myristicylcarnitine (C14:1) | 0.117 (0.063) | 0.127 (0.066) | 0.001 | 1.753 | −0.197 (−0.308, −0.085) | −0.174 (−0.284, −0.064) |
| Palmitoylcarnitine (C16) | 2.440 (0.969) | 2.520 (1.083) | 0.011 | 1.297 | −0.145 (−0.257, −0.034) | −0.125 (−0.233, −0.016) |
| 3-hydroxypalmitylcarnitine (C16-OH) | 0.026 (0.013) | 0.028 (0.012) | 0.003 | 1.518 | −0.170 (−0.282, −0.059) | −0.151 (−0.261, −0.040) |
| Octadecadienoylcarnitine (C18:2) | 1.132 (0.574) | 1.048 (0.583) | <0.001 | 1.839 | 0.206 (0.095, 0.318) | 0.179 (0.072, 0.285) |
| Carbamate (C24) | 0.061 (0.027) | 0.063 (0.027) | 0.010 | 1.311 | −0.147 (−0.259, −0.035) | −0.136 (−0.248, −0.023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Chen, X.; Li, T.; Gao, P.; Huang, S.; Wang, X.; Lin, Z.; Huang, F.; Zhu, L.; Lu, Y.; et al. Neonatal Circulating Amino Acids and Lipid Metabolites Mediate the Association of Maternal Gestational Diabetes Mellitus with Offspring Neurodevelopment at 1 Year. Nutrients 2025, 17, 258. https://doi.org/10.3390/nu17020258
Zhou Y, Chen X, Li T, Gao P, Huang S, Wang X, Lin Z, Huang F, Zhu L, Lu Y, et al. Neonatal Circulating Amino Acids and Lipid Metabolites Mediate the Association of Maternal Gestational Diabetes Mellitus with Offspring Neurodevelopment at 1 Year. Nutrients. 2025; 17(2):258. https://doi.org/10.3390/nu17020258
Chicago/Turabian StyleZhou, Yueqin, Xiaoyan Chen, Tianze Li, Pingming Gao, Saijun Huang, Xiaotong Wang, Zongyu Lin, Fenglian Huang, Lewei Zhu, Yeling Lu, and et al. 2025. "Neonatal Circulating Amino Acids and Lipid Metabolites Mediate the Association of Maternal Gestational Diabetes Mellitus with Offspring Neurodevelopment at 1 Year" Nutrients 17, no. 2: 258. https://doi.org/10.3390/nu17020258
APA StyleZhou, Y., Chen, X., Li, T., Gao, P., Huang, S., Wang, X., Lin, Z., Huang, F., Zhu, L., Lu, Y., & Zhu, Y. (2025). Neonatal Circulating Amino Acids and Lipid Metabolites Mediate the Association of Maternal Gestational Diabetes Mellitus with Offspring Neurodevelopment at 1 Year. Nutrients, 17(2), 258. https://doi.org/10.3390/nu17020258








