Total Cholesterol and Mortality in Older Adults: A Sex-Stratified Cohort Study
Abstract
1. Background
2. Methods
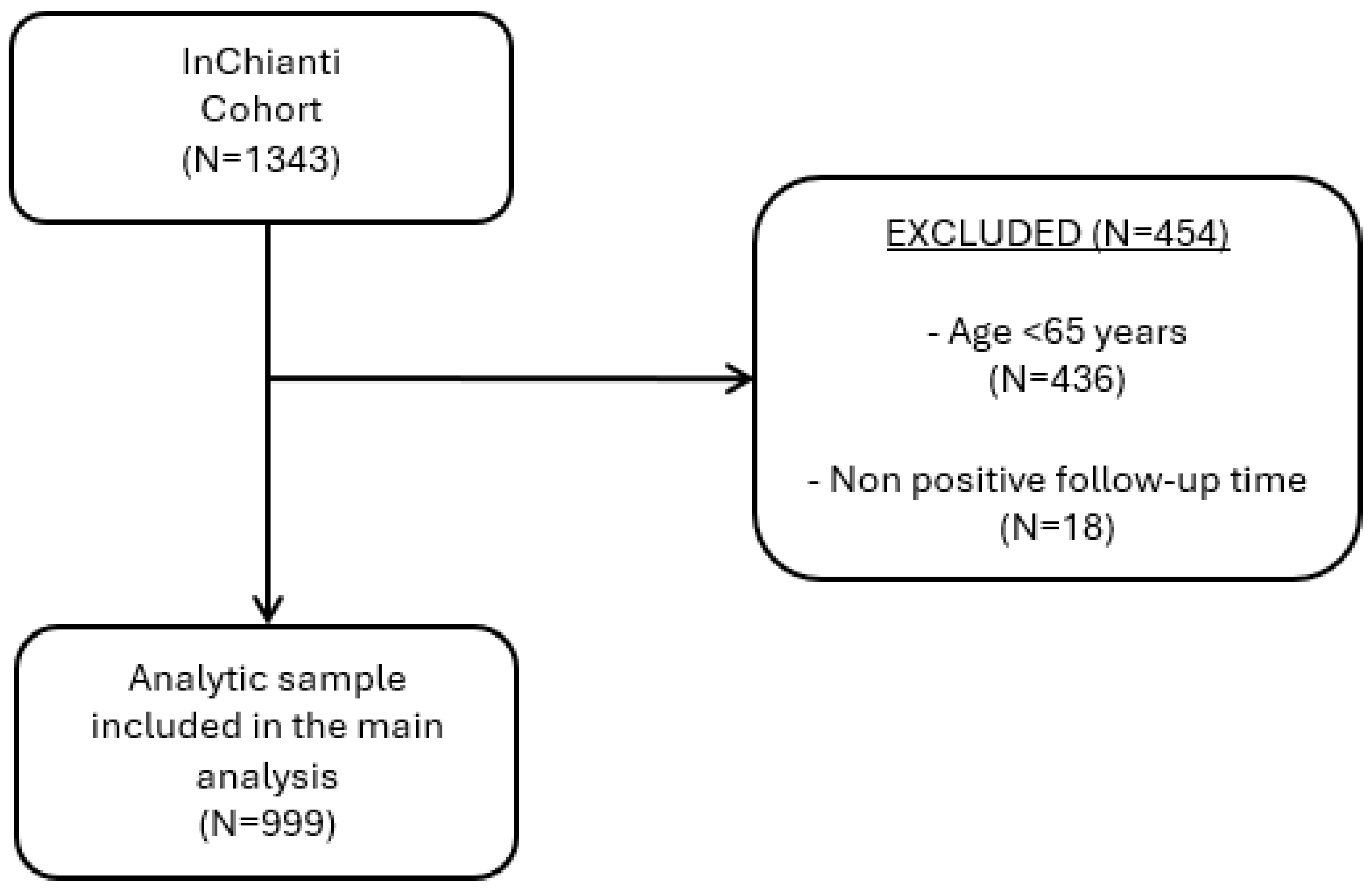
2.1. Data Sources and Sample Selection
2.2. Study Variables
2.3. Study Outcome
2.4. Statistical Analysis
3. Results
3.1. Study Selection and General Characteristics of the Population
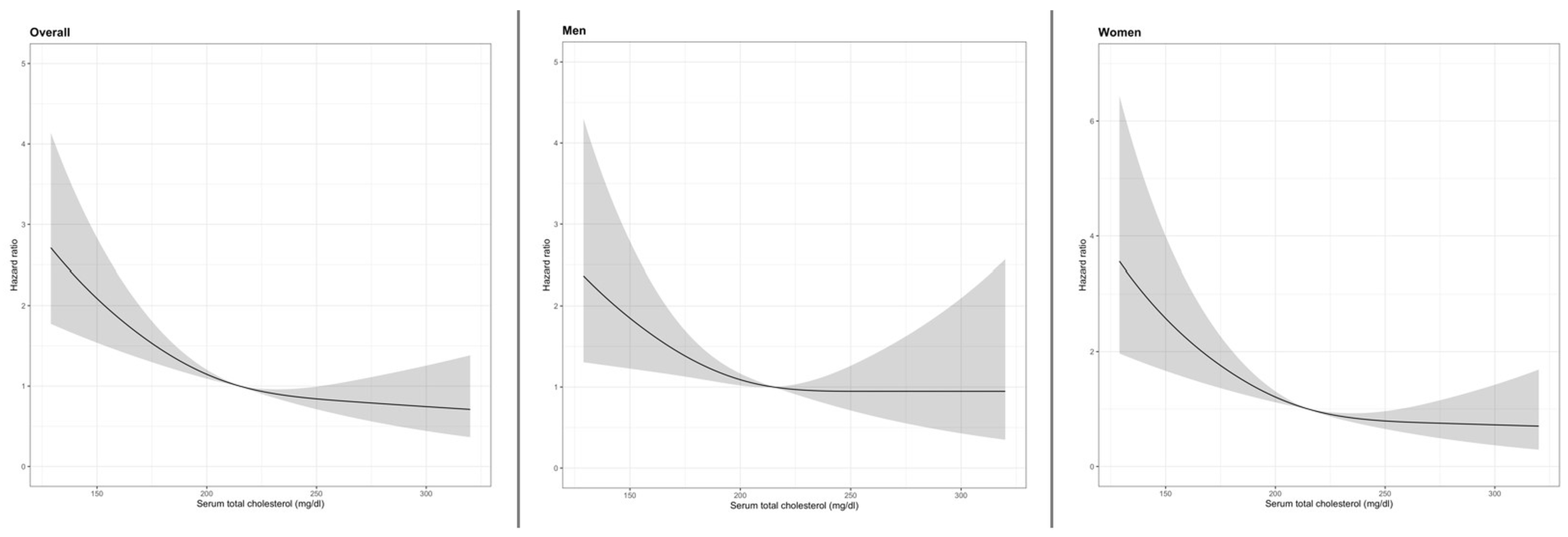
3.2. Total Cholesterol Levels and All-Cause Mortality in Overall Population
3.3. Total Cholesterol Levels and All-Cause Mortality in Older Men
3.4. Total Cholesterol Levels and All-Cause Mortality in Older Women
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okamura, T.; Tanaka, H.; Miyamatsu, N.; Hayakawa, T.; Kadowaki, T.; Kita, Y.; Nakamura, Y.; Okayama, A.; Ueshima, H. The Relationship between Serum Total Cholesterol and All-Cause or Cause-Specific Mortality in a 17.3-Year Study of a Japanese Cohort. Atherosclerosis 2007, 190, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.M.; Castelli, W.P.; Levy, D. Cholesterol and mortality. 30 years of follow-up from the Framingham study. JAMA 1987, 257, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Aronow, W.S.; Ahn, C.; Kronzon, I. Comparison of Incidences of Congestive Heart Failure in Older African-Americans, Hispanics, and Whites. Am. J. Cardiol. 1999, 84, 611–612, A9. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, E1046–E1081. [Google Scholar] [CrossRef]
- Mach, F.; Koskinas, K.C.; Roeters van Lennep, J.E.; Tokgözoğlu, L.; Badimon, L.; Baigent, C.; Benn, M.; Binder, C.J.; Catapano, A.L.; De Backer, G.G.; et al. 2025 Focused Update of the 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2025, 120479. [Google Scholar] [CrossRef]
- Schupf, N.; Costa, R.; Luchsinger, J.; Tang, M.; Lee, J.H.; Mayeux, R. Relationship Between Plasma Lipids and All-Cause Mortality in Nondemented Elderly. J. Am. Geriatr. Soc. 2005, 53, 219–226. [Google Scholar] [CrossRef]
- Murata, S.; Ebeling, M.; Meyer, A.C.; Schmidt-Mende, K.; Hammar, N.; Modig, K. Blood Biomarker Profiles and Exceptional Longevity: Comparison of Centenarians and Non-Centenarians in a 35-Year Follow-up of the Swedish AMORIS Cohort. GeroScience 2023, 46, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pereira, S.; Luo, M.; Matheson, E. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
- Shi, J.; Liu, T.; Ge, Y.; Liu, C.; Zhang, Q.; Xie, H.; Ruan, G.; Lin, S.; Zheng, X.; Chen, Y.; et al. Cholesterol-Modified Prognostic Nutritional Index (CPNI) as an Effective Tool for Assessing the Nutrition Status and Predicting Survival in Patients with Breast Cancer. BMC Med. 2023, 21, 512. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.-W.; Yi, J.-J.; Ohrr, H. Total Cholesterol and All-Cause Mortality by Sex and Age: A Prospective Cohort Study among 12.8 Million Adults. Sci. Rep. 2019, 9, 1596. [Google Scholar] [CrossRef]
- Phelps, T.; Snyder, E.; Rodriguez, E.; Child, H.; Harvey, P. The Influence of Biological Sex and Sex Hormones on Bile Acid Synthesis and Cholesterol Homeostasis. Biol. Sex Differ. 2019, 10, 52. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. In Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity; Mauvais-Jarvis, F., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 1043, pp. 227–256. ISBN 978-3-319-70177-6. [Google Scholar]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation 2020, 142, 506–532. [Google Scholar] [CrossRef]
- Conforto, R.; Rizzo, V.; Russo, R.; Mazza, E.; Maurotti, S.; Pujia, C.; Succurro, E.; Arturi, F.; Ferro, Y.; Sciacqua, A.; et al. Advances in Body Composition and Gender Differences in Susceptibility to Frailty Syndrome: Role of Osteosarcopenic Obesity. Metabolism 2024, 161, 156052. [Google Scholar] [CrossRef]
- Ferrucci, L.; Bandinelli, S.; Benvenuti, E.; Di Iorio, A.; Macchi, C.; Harris, T.B.; Guralnik, J.M. Subsystems Contributing to the Decline in Ability to Walk: Bridging the Gap Between Epidemiology and Geriatric Practice in the InCHIANTI Study. J. Am. Geriatr. Soc. 2000, 48, 1618–1625. [Google Scholar] [CrossRef]
- Katz, S. Assessing Self-maintenance: Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9 Pt 1, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol.—Ser. Biol. Sci. Med. Sci. 2001, 56, 146–157. [Google Scholar] [CrossRef]
- Nicholson, K.; Liu, W.; Fitzpatrick, D.; Hardacre, K.A.; Roberts, S.; Salerno, J.; Stranges, S.; Fortin, M.; Mangin, D. Prevalence of Multimorbidity and Polypharmacy among Adults and Older Adults: A Systematic Review. Lancet Healthy Longev. 2024, 5, e287–e296. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by Representatives of 10 Societies and by Invited Experts)Developed with the Special Contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Available online: https://www.ahajournals.org/doi/epdf/10.1161/circ.106.25.3143 (accessed on 24 September 2025).
- Carroll, M.D.; Fryar, C.D.; Gwira, J.A.; Iniguez, M. Total and High-Density Lipoprotein Cholesterol in Adults: United States, August 2021–August 2023. In NCHS Data Briefs; National Center for Health Statistics: Hyattsville, MD, USA, 2024. [Google Scholar]
- Kromhout, D.; Nissinen, A.; Menotti, A.; Bloemberg, B.; Pekkanen, J.; Giampaoli, S. Total and HDL Cholesterol and Their Correlates in Elderly Men in Finland, Italy, and The Netherlands. Am. J. Epidemiol. 1990, 131, 855–863. [Google Scholar] [CrossRef]
- Trevisan, C.; Capodaglio, G.; Ferroni, E.; Fedeli, U.; Noale, M.; Baggio, G.; Manzato, E.; Maggi, S.; Corti, M.C.; Sergi, G. Cardiovascular Risk Profiles and 20-Year Mortality in Older People: Gender Differences in the Pro.V.A. Study. Eur. J. Ageing 2022, 19, 37–47. [Google Scholar] [CrossRef]
- Roshanravan, B.; Robinson-Cohen, C.; Patel, K.V.; Ayers, E.; Littman, A.J.; De Boer, I.H.; Ikizler, T.A.; Himmelfarb, J.; Katzel, L.I.; Kestenbaum, B.; et al. Association between Physical Performance and All-Cause Mortality in CKD. J. Am. Soc. Nephrol. 2013, 24, 822–830. [Google Scholar] [CrossRef]
- Petersen, L.K.; Christensen, K.; Kragstrup, J. Lipid-Lowering Treatment to the End? A Review of Observational Studies and RCTs on Cholesterol and Mortality in 80+-Year Olds. Age Ageing 2010, 39, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Tuikkala, P.; Hartikainen, S.; Korhonen, M.J.; Lavikainen, P.; Kettunen, R.; Sulkava, R.; Enlund, H. Serum Total Cholesterol Levels and All-Cause Mortality in a Home-Dwelling Elderly Population: A Six-Year Follow-Up. Scand. J. Prim. Health Care 2010, 28, 121–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, F.; Wang, Z.; Liu, Y.; Gao, Y.; Liu, S.; Xu, C.; Wang, Y.; Cai, Y. Association between Total Cholesterol and All-Cause Mortality in Oldest Old: A National Longitudinal Study. Front. Endocrinol. 2024, 15, 1405283. [Google Scholar] [CrossRef]
- Turusheva, A.; Vaes, B.; Degryse, J.-M.; Frolova, E. Low Cholesterol Levels Are Associated with a High Mortality Risk in Older Adults without Statins Therapy: An Externally Validated Cohort Study. Arch. Gerontol. Geriatr. 2020, 90, 104180. [Google Scholar] [CrossRef]
- Truijen, S.P.M.; Hayhoe, R.P.G.; Hooper, L.; Schoenmakers, I.; Forbes, A.; Welch, A.A. Predicting Malnutrition Risk with Data from Routinely Measured Clinical Biochemical Diagnostic Tests in Free-Living Older Populations. Nutrients 2021, 13, 1883. [Google Scholar] [CrossRef]
- Ng, T.P.; Nyunt, M.S.Z.; Gao, Q.; Wee, S.L.; Yap, P.; Yap, K.B. Elderly Nutritional Indicators for Geriatric Malnutrition Assessment (ENIGMA): Development and Validation of a Nutritional Prognostic Index. Clin. Nutr. ESPEN 2017, 22, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Cabré, M.; Ferreiro, C.; Arus, M.; Roca, M.; Palomera, E.; Serra-Prat, M. Evaluation of Conut for Clinical Malnutrition Detection and Short-Term Prognostic Assessment in Hospitalized Elderly People. J. Nutr. Health Aging 2015, 19, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, H.; Kelleher, C.; Diem, G.; Concin, H. Why Eve Is Not Adam: Prospective Follow-Up in 149,650 Women and Men of Cholesterol and Other Risk Factors Related to Cardiovascular and All-Cause Mortality. J. Womens Health 2004, 13, 41–53. [Google Scholar] [CrossRef]
- Bo, M. Cholesterol and Long-Term Mortality after Acute Myocardial Infarction in Elderly Patients. Age Ageing 1999, 28, 313–315. [Google Scholar] [CrossRef][Green Version]
- Takata, Y.; Ansai, T.; Soh, I.; Awano, S.; Nakamichi, I.; Akifusa, S.; Goto, K.; Yoshida, A.; Fujii, H.; Fujisawa, R.; et al. Serum Total Cholesterol Concentration and 10-Year Mortality in an 85-Year-Old Population. Clin. Interv. Aging 2014, 9, 293–300. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex Differences in Lipid and Lipoprotein Metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Castel, H.; Shahar, D.; Harman-Boehm, I. Gender Differences in Factors Associated with Nutritional Status of Older Medical Patients. J. Am. Coll. Nutr. 2006, 25, 128–134. [Google Scholar] [CrossRef]
- Chataut, J.; Jonche, S.; Ghimire, M.; Tamrakar, D.; Singh Bhandari, M. Prevalence of Malnutrition among Elderly People Living in a Rural Area of Nepal. J. Nepal Med. Assoc. 2021, 59, 146–151. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liao, J.K. Pleiotropic Effects of Statins—Basic Research and Clinical Perspectives. Circ. J. 2010, 74, 818–826. [Google Scholar] [CrossRef] [PubMed]

| Total (N:999) | Men (N: 443) | Women (N: 556) | |
|---|---|---|---|
| Age (years) | 74.9 (7) | 74.2 (6.9) | 75.5 (7.5) |
| Education (years) | 5.4 (3) | 6.1 (3.6) | 4.7 (2.8) |
| Smoking (P/Y) | 12.5 (21) | 24.6 (24.6) | 2.8 (9.0) |
| BMI (kg/m2) | 27.4 (4) | 27.1 (3.3) | 27.7 (4.6) |
| Lost ADL | 0.24 (0.96) | 0.22 (0.9) | 0.26 (1.0) |
| Lost IADL | 0.92 (2) | 0.72 (2.0) | 1.1 (2.2) |
| Frailty | 82 (8.2%) | 31 (7.0) | 51(9.2) |
| Polypharmacy | 146 (14.6%) | 62 (14.0) | 84 (15.1) |
| Total cholesterol (mg/dL) | 217.5 (40) | 207.8 (39.3) | 225.3 (38.1) |
| HDL cholesterol (mg/dL) | 55.7 (15) | 51.4 (13.3) | 59.2 (15.5) |
| LDL cholesterol (mg/dL) | 136.1 (34) | 130.2 (34.0) | 140.7 (33.9) |
| Albumin (g/dL) | 58.6 (4) | 59.1 (3.6) | 58.2 (3.4) |
| hs-CRP (mg/L) | 5.3 (10) | 6.1 (12.3) | 4.7 (6.4) |
| eGFR (ml/min/1.73 m2) | 70.9 (14) | 73.7 (13.4) | 68.6 (14.4) |
| Diabetes | 128 (12.8%) | 62 (14.0%) | 66 (11.9%) |
| Hypertension | 667 (66.8%) | 271 (61.2%) | 396 (71.2%) |
| COPD | 119 (11.9%) | 90 (20.3%) | 29 (5.2%) |
| Alcohol consumption (g/day) | 14.3 (20) | 24.1 (25.1) | 6.5 (9.6) |
| Thyroid disease | 81 (8.3%) | 12 (2.8%) | 68 (12.6%) |
| Chronic liver disease | 17 (1.7%) | 11 (2.5%) | 6 (1.1%) |
| Low physical activity | 633 (63.7%) | 214 (48.6%) | 419 (75.6%) |
| Statin use | 44 (4.4%) | 16 (3.6%) | 28 (5.0%) |
| Sex | Total Cholesterol (mg/dL) | HR (Crude) | 95% CI | Adjusted HR *§ | 95% CI *§ |
|---|---|---|---|---|---|
| Overall * | <200 | - | - | - | - |
| 200–239 | 0.61 | 0.46–0.80 | 0.72 | 0.53–0.99 | |
| ≥240 | 0.47 | 0.34–0.66 | 0.71 | 0.49–1.02 | |
| Men ** | <200 | (reference group) | |||
| 200–239 | 0.83 | 0.56–1.22 | 1.04 | 0.68–1.59 | |
| ≥240 | 0.56 | 0.34–0.95 | 0.76 | 0.43–1.35 | |
| Women ** | <200 | (reference group) | |||
| 200–239 | 0.42 | 0.28–0.63 | 0.50 | 0.31–0.89 | |
| ≥240 | 0.39 | 0.25–0.60 | 0.64 | 0.39–1.05 | |
| Sex | Total Cholesterol (mg/dL) | HR (Crude) | 95% CI | Adjusted HR *§ | 95% CI *§ |
|---|---|---|---|---|---|
| Overall | <200 | - | - | - | - |
| 200–239 | 0.60 | 0.45–0.79 | 0.68 | 0.50–0.93 | |
| ≥240 | 0.46 | 0.33–0.65 | 0.65 | 0.45–0.93 | |
| Men | <200 | (reference group) | |||
| 200–239 | 0.84 | 0.56–1.22 | 1.05 | 0.69–1.62 | |
| ≥240 | 0.56 | 0.33–0.94 | 0.76 | 0.43–1.34 | |
| Women | <200 | (reference group) | |||
| 200–239 | 0.41 | 0.27–0.62 | 0.47 | 0.29–0.78 | |
| ≥240 | 0.38 | 0.24–0.60 | 0.63 | 0.38–1.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iuorio, M.S.; Lelli, D.; Bandinelli, S.; Ferrucci, L.; Pedone, C.; Antonelli Incalzi, R. Total Cholesterol and Mortality in Older Adults: A Sex-Stratified Cohort Study. Nutrients 2025, 17, 3128. https://doi.org/10.3390/nu17193128
Iuorio MS, Lelli D, Bandinelli S, Ferrucci L, Pedone C, Antonelli Incalzi R. Total Cholesterol and Mortality in Older Adults: A Sex-Stratified Cohort Study. Nutrients. 2025; 17(19):3128. https://doi.org/10.3390/nu17193128
Chicago/Turabian StyleIuorio, Maria Serena, Diana Lelli, Stefania Bandinelli, Luigi Ferrucci, Claudio Pedone, and Raffaele Antonelli Incalzi. 2025. "Total Cholesterol and Mortality in Older Adults: A Sex-Stratified Cohort Study" Nutrients 17, no. 19: 3128. https://doi.org/10.3390/nu17193128
APA StyleIuorio, M. S., Lelli, D., Bandinelli, S., Ferrucci, L., Pedone, C., & Antonelli Incalzi, R. (2025). Total Cholesterol and Mortality in Older Adults: A Sex-Stratified Cohort Study. Nutrients, 17(19), 3128. https://doi.org/10.3390/nu17193128







