Abstract
Objective: Vitamin D deficiency (VDD) is common in pregnancy and may affect lipid metabolism. The underlying mechanisms are multifactorial, but most evidence so far comes from non-pregnant populations. This study aims to identify metabolites and metabolic patterns associated with VDD in early pregnancy and to evaluate their relationships with maternal lipid profiles. Methods: A nested case–control research was carried out in the Zhoushan Pregnant Women Cohort (ZPWC). Cases were defined as women with VDD (25(OH)D < 20 ng/mL), and controls (≥20 ng/mL) were matched 1:1 using propensity scores based on age, pre-pregnancy BMI, gestational week, and calendar year at blood sampling. The untargeted metabolomics of first-trimester maternal plasma were measured. Metabolic profiles were analyzed using partial least squares-discriminant analysis (PLS-DA). Principal component analysis (PCA) was applied to visualize group separation, and metabolite set enrichment analysis (MSEA) was performed to reveal biologically relevant metabolic patterns. Associations between VDD-related metabolite components in early pregnancy and lipid levels in mid-pregnancy were assessed using linear regression models. Results: 44 cases and 44 controls were selected for the study. There were 60 metabolites identified as being connected to VDD. Among these, 26 metabolites, primarily glycerophospholipids and fatty acyls, exhibited decreased levels in the VDD group. In contrast, 34 metabolites showed increased levels, mainly comprising benzene derivatives, carboxylic acids, and organooxygen compounds. PCA based on these metabolites explained 52.8% of the total variance (R2X = 0.528) across the first six principal components (PC1: 16.4%, PC2: 10.6%, PC3: 9.2%, PC4: 6.3%, PC5: 5.7%, PC6: 4.6%). PC2, dominated by lineolic acids and derivatives, was negatively associated with total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) (all p < 0.01). PC3, dominated by glycerophosphocholines, was negatively associated with TC, TG, and high-density lipoprotein cholesterol (HDL-C) (all p < 0.05). MSEA revealed significant enrichment of the pantothenate and CoA biosynthesis pathway after multiple testing correction (FDR < 0.05). Conclusions: This study reveals distinct metabolic alterations linked to VDD and suggests potential mechanisms underlying its association with maternal lipid metabolism in early pregnancy.
1. Introduction
Vitamin D is a fat-soluble vitamin with diverse physiological functions beyond skeletal health, including roles in immune regulation and metabolic processes [1]. It has been acknowledged that vitamin D deficiency (VDD) is a widespread global public health issue, with an even higher prevalence among pregnant women [2]. The prevalence of VDD among pregnant women in China is approximately 41.96%, and we observed an even higher prevalence rate of 65.26% in coastal regions of eastern China [3,4]. Meanwhile, there is growing evidence that vitamin D status is linked to dyslipidemia in pregnant women. For example, lower levels of 25(OH)D in early pregnancy were linked to higher levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and total cholesterol to high-density lipoprotein cholesterol ratios (TC/HDL-C) during gestation, according to a prospective cohort study conducted on pregnant Brazilian women [5]. Consistent with these results, a prior study found a negative relationship between TC, triglycerides (TG), and LDL-C in mid-pregnancy and serum 25(OH)D levels in early pregnancy, which suggests that higher maternal vitamin D levels may help improve lipid profiles during pregnancy [6]. Previous research has mostly concentrated on how vitamin D’s antioxidant and anti-inflammatory mechanisms may affect lipid metabolism; however, additional underlying mechanisms remain to be explored [7].
Metabolomics examines endogenous and exogenous metabolites to generate a comprehensive view of biological systems, offering a powerful means to uncover disruptions in metabolic pathways associated with phenotypic variation and to identify novel targets for therapeutic intervention. The relationship between maternal 25(OH)D and metabolome during pregnancy has been investigated in earlier research. The U.S. study (n = 30), conducted in a cohort of adolescent pregnant women, found that several metabolites related to inflammation and fatty acid oxidation were decreased in the vitamin D sufficient group during mid-pregnancy [8]. In contrast, in a Chinese study (n = 111), based on early-pregnancy blood samples from an adult cohort, vitamin D insufficiency or deficiency women showed higher concentrations of glycerophospholipids as well as aromatic and branched-chain amino acids [9]. The Danish study identified 42 metabolites associated with maternal 25(OH)D levels at one week postpartum with the method of linear regression, with these metabolites significantly enriched in the sphingolipid subpathway [10]. Another study focusing on fatty acids reported that maternal 25(OH)D levels were positively associated with plasma omega-6 fatty acids, and total polyunsaturated fatty acids, while being inversely associated with total saturated fatty acids and total monounsaturated fatty acids at delivery [11]. In addition, a study (n = 80) in Turkey demonstrated that VDD was associated with altered profiles of bile acids [12]. Although these studies were conducted across diverse populations and different stages of pregnancy, they collectively suggest a potential link between vitamin D status, lipid-related metabolites, and metabolic pathways. However, no study has yet elucidated the relationship between VDD, metabolite alterations, and their potential contribution to dyslipidemia during pregnancy.
In this study, a nested case–control design was employed to conduct untargeted metabolomic profiling of maternal plasma samples collected in early pregnancy, with the aim of identifying metabolites and metabolic pathways associated with VDD and assessing their associations with maternal lipid levels.
2. Methods
2.1. Study Design and Participants
The Zhoushan Pregnant Women Cohort (ZPWC), an ongoing prospective cohort that was started in August 2011 at Zhoushan Maternal and Child Health Care Hospital in China, served as the basis for the nested case–control research design of this investigation. Eligibility for ZPWC required participants to: (1) provide informed consent; (2) be enrolled between 8 and 12 gestational weeks; (3) complete routine perinatal examinations and plan to deliver at the study hospital; (4) be between 18 and 45 years of age; (5) have no family history of psychiatric illness. Women were excluded if they had: (1) a history of major chronic or acute diseases; (2) a history of psychiatric disorders before pregnancy; (3) threatened abortion; (4) fetal malformations or abnormal fetal growth; or (5) difficulties completing questionnaires due to cognitive impairment. During the ZPWC study, trained nurses conducted in-person interviews to gather participants’ sociodemographic details, lifestyle habits, and health-related behaviors. Laboratory examination results were taken from the electronic medical record system of the hospital. Fasting blood samples in each trimester were also collected. The Zhejiang University School of Medicine’s Research Ethics Committee gave its approval to the study protocol (Approval No. 2011-1-002).
For the present analysis, we first identified all women with VDD (serum 25(OH)D < 20 ng/mL) in early stage of pregnancy from our cohort. Among these, 44 women were randomly selected as cases. Controls were drawn from participants with adequate vitamin D levels (25(OH)D ≥ 20 ng/mL) at the same gestational stage. To minimize potential confounding, cases and controls were matched 1:1 using propensity score matching based on maternal age, pre-pregnancy BMI, calendar year, and gestational week at blood sampling, yielding 44 matched pairs (n = 88).
2.2. Clinical Data Collection
2.2.1. Measurement of 25(OH)D
Plasma concentrations of 25(OH)D2 (ng/mL) and 25(OH)D3 (ng/mL) were quantified using liquid chromatography–tandem mass spectrometry (LC–MS/MS) with an API 3200MD system (Applied Biosystems/MDS Sciex, Waltham, MA, USA). Plasma 25(OH)D levels (ng/mL) were obtained by summing 25(OH)D2 and 25(OH)D3.
2.2.2. Measurement of Blood Lipids
Serum levels of TC, TG, HDL-C, and LDL-C in the mid-pregnancy were measured using enzymatic methods on an AU5800 automated analyzer (Beckman Coulter, Brea, CA, USA). All assays followed manufacturer protocols, with inter-assay coefficients of variation < 5%.
2.2.3. Variable Definition
Self-reported height and weight data were used to calculate the maternal pre-pregnancy BMI (kg/m2). Reproductive history included gravidity (1, 2, or ≥3 pregnancies) and parity (nulliparous vs. multiparous). Education levels were stratified into three categories: (1) junior high school and below, (2) senior high school, and (3) college and above. Seasonal variations in vitamin D assessment were adjusted for differences in sunlight exposure: spring (March to May), summer (June to August), autumn (September to November), and winter (December to February). Missing data were coded as “unknown”.
2.3. LC–MS Metabolomics Data Acquisition and Preprocessing
Untargeted metabolomic analysis was performed using LC-MS/MS with a Q-Exactive series Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Raw data preprocessing included the following steps: (1) Missing Value Handling: Features with > 50% missing values in any group were removed, and the remaining entries were imputed as half of the minimum observed value for each metabolite. (2) Features showing > 90% relative standard deviation (RSD) excluded from further analysis. (3) To reduce the effects of noise and the high variance of the variables, the data were scaled and logarithmically processed. After preprocessing, 14,661 features were retained, of which 648 were retained for secondary qualitative substances.
2.4. Data Analysis
According to their non-normal distribution, all continuous variables were summarized with the median [IQR]. The frequency of categorical variables was displayed as a percentage. Between-group comparisons were conducted using the chi-square test for categorical data and the Mann–Whitney U test for continuous data. Fisher’s exact test was utilized if the expected frequency of any cell was below five.
Multivariate analysis was conducted using partial least squares-discriminant analysis (PLS-DA). Metabolites with variable importance in projection (VIP) scores >1.0 and statistically significant differences (p < 0.05) were identified as VDD-associated metabolites. These discriminant metabolites were subsequently analyzed using principal component analysis (PCA) to visualize group separations. Metabolite set enrichment analysis (MSEA) was performed using the MetaboAnalyst 6.0 platform (https://www.metaboanalyst.ca/, accessed on 30 December 2024) to identify biologically relevant metabolic patterns, in order to reveal significant enrichment of key pathways associated with vitamin D metabolism. Linear regression modeling was used to analyze the association between VDD-related metabolites and lipid levels in mid-pregnancy. The results were visualized using forest plots, with β coefficients and 95% confidence intervals representing the effect sizes.
The level of p < 0.05 was considered as statistically significant. All statistical analyses were performed in R software (version 4.3.2).
3. Results
3.1. Baseline Characteristics
44 participants were included in each of the control and case groups, and no significant differences were observed in baseline demographics, gestational week or season of sampling between VDD and non-VDD participants (all p > 0.05), while 25(OH)D and 25(OH)D3 levels were significantly lower in the VDD group (p < 0.001) (Table 1).

Table 1.
The distribution of the relevant characteristics between subjects with VDD and Non-VDD.
3.2. VDD and Maternal Metabolic Profile in Early Pregnancy
The score plot for multivariate modeling using the PLS-DA method is shown in Figure 1A, defining metabolites with VIP > 1 and p < 0.05 as VDD-associated metabolites. The model shows good goodness-of-fit (R2Y = 0.897) and moderate predictive ability (Q2Y = 0.325), as well as a low explained variance in metabolic profiles (R2X = 0.064). The volcano plots depicting increases and decreases in VDD-associated metabolites (N = 60) as well as their belonging to the Superclass (Figure 1B). Among the identified metabolites, 21 of them were categorized as lipid and lipid-like molecules, 11 as benzenoids, and the remaining included organic acids and derivatives, organoheterocyclic compounds, and organic oxygen compounds. At the class level, a total of 26 metabolites, mainly represented by 8 glycerophospholipids and 4 fatty acyls, were decreased in the VDD group. In contrast, 34 increased metabolites in the VDD group were primarily classified as benzene and substituted derivatives (n = 4), carboxylic acids and derivatives (n = 4), and organooxygen compounds (n = 4) (More details in Supplementary Table S1).
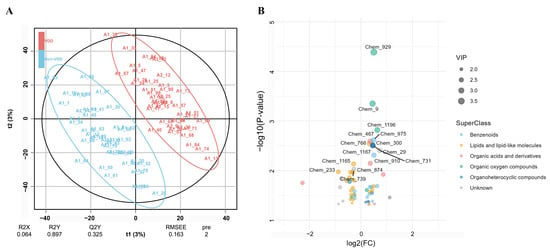
Figure 1.
VDD-associated metabolites by the PLS-DA method. (A) The score plot for multivariate modeling using the PLS-DA approach reveals an overlapping of metabolome between VDD and non-VDD participants. The score plot depicts percentage of response variable explained by the first and second predictors (t1 and t2). R2X (predictors explained by the model), R2Y (grouping explained by the model), and Q2Y (predictive ability estimated via cross-validation) are listed on the figure and indicate overfitting. (B) Volcano plot depicting increased and decreased VDD-related metabolites.
Based on VDD-associated metabolites obtained from PLS-DA, unsupervised PCA modeling captured 52.8% of the cumulative variance in the metabolic profiles across the first six principal components (PC1: 16.4%, PC2: 10.6%, PC3: 9.2%, PC4: 6.3%, PC5: 5.7%, PC6: 4.6%) (Figure 2).
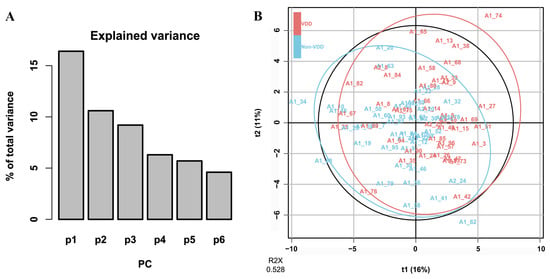
Figure 2.
PCA results based on VDD-associated metabolites. (A) The proportion of total variance explained by the PCs. (B) The Score plot of multivariate modeling by the PCA approach.
Table 2 presents the metabolites that had primary influence on the top three principal components (absolute value of score > 0.2). PC1 exhibited a complex composition, dominated by benzenoids, lipids and lipid-like molecules; PC2 was dominated by lipids and lipid-like molecules, including three lineolic acids and derivatives; and PC3 was dominated by organoheterocyclic compounds, lipids and lipid-like molecules, with five glycerophosphocholines.

Table 2.
Primary metabolites of TOP 3 PCs.
To explore the systemic metabolic impact of VDD, we conducted MSEA using the KEGG pathway library (Table 3). Among all evaluated pathways, only pantothenate and CoA biosynthesis (p = 0.0002) and unsaturated fatty acid biosynthesis (p = 0.0387) showed statistically significant enrichment. After multiple-test corrections, only the pantothenate and CoA biosynthesis remained significant at an FDR threshold of 5% (p = 0.0118).

Table 3.
Metabolite set enrichment analysis (MSEA) of VDD versus non-VDD participants.
3.3. VDD-Associated Metabolites and Lipid Levels
In Figure 3, linear regression analysis showed that PC2, which is dominated by lineolic acids and derivatives, was significantly inversely related to TC (β = −0.130, 95%CI: −0.201, −0.059), TG (β = −0.063, 95%CI: −0.100, −0.026) and LDL-C (β = −0.081, 95%CI: −0.136, −0.026). Conversely, PC3 mainly composed of glycerophosphocholines showed significant negative correlation with TC (β = −0.097, 95%CI: −0.175, −0.019), TG (β = −0.072, 95%CI: −0.111, −0.033) and HDL-C (β = −0.032, 95%CI: −0.057, −0.007).
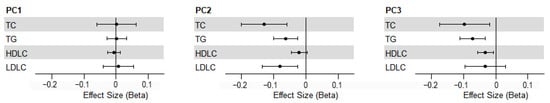
Figure 3.
Associations between TOP 3 PCs and lipid levels. TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
4. Discussion
This study identified 60 metabolites associated with VDD in early pregnancy. In the VDD group, the down-regulated metabolites were predominantly glycerophospholipids and fatty acyls, while the up-regulated metabolites mainly consisted of various benzenoids, organic acids and derivatives, and organic oxygen compounds. Constructed with VDD-associated metabolites, PC2, which is dominated by lineolic acids and derivatives, showed a negative correlation with TC, TG, and LDL-C; PC3, mainly composed by glycerophosphocholines, exhibited an inverse relationship with TC, TG, and HDL-C. MSEA identified significant enrichment of the pantothenate and CoA biosynthesis pathway.
As for the exploration of vitamin D-related differential metabolites, in comparison with previous metabolomics studies, our findings both support and extend existing evidence. For instance, Li et al. [9] collected early pregnancy serum samples and applied a targeted metabolomics profiling method, which revealed that several fatty acyls, glycerolipids, glycerophospholipids and sterol lipids were downregulated in the VDD group. The ATBC Study in middle-aged men found that 25(OH)D levels correlate with several lipid metabolites including erucoyl sphingomyelin, eicosapentaenoate (EPA) and docosahexaenoate (DHA), and so on [13]. Also, the Hong Kong Osteoporosis Study identified correlations between 13 metabolites and 25(OH)D, while DHA and EPA had the highest correlations [14]. In patients with critical illness, Amrein et al. [15] found that as the 25(OH)D levels increased, the plasma levels of plasmalogen, lysoplasmalogen, and lysophospholipid metabolite classes also increased simultaneously, suggesting that vitamin D status is associated with glycerophospholipids and fatty acids across different populations, while the exact metabolite species involved may vary between studies. Another study using pregnant mice as the experimental model showed that the metabolite pathways of unsaturated fatty acids and glycerophospholipids were significantly enriched in the VDD diet group, indicating that vitamin D status profoundly affects the homeostasis of glycerophospholipids [16]. However, beyond these lipid-related findings, our study identified additional metabolite changes that were not reported in earlier investigations. Specifically, upregulated metabolites in the VDD group included various benzenoids, organic acids and derivatives, and organic oxygen compounds, suggesting that VDD may also disturb aromatic compound metabolism and broader energy-related pathways. To further explain these findings, we will discuss them in greater detail below, integrating evidence from the PCA and MSEA results.
In our study, glycerophosphocholines contributed most to PC3. As we mentioned before, the association between vitamin D and glycerophospholipid metabolites has been widely investigated in non-pregnant populations, with several studies providing mechanistic insights [13,14,17]. The effects of a natural vitamin D formulation on hepatocyte lipotoxicity were investigated in vitro. The results showed that the phosphatidylcholine pool’s chain length and number of double bonds changed significantly, as well as the metabolism of glycerophospholipids [18]. There are several hypotheses can be proposed to explain the finding. First, vitamin D/vitamin D receptor (VDR) activation may regulate the expression of peroxisome proliferator-activated receptor gamma (PPAR-γ). Activated PPARα promotes the expression of fatty acid transporters and oxidases, thereby accelerating fatty acid catabolism and ultimately reducing the availability of substrates for TG synthesis [19]. Second, it has been revealed that 25(OH)D inhibits the activation of the Sterol Regulatory Element-Binding Protein (SREBP), SREBP1c and SREBP2 are critical transcription factors engaged in TG and cholesterol biosynthesis, and the inhibition of their activity directly reduces the hepatic production of endogenous TG and cholesterol [20,21,22]. Additionally, vitamin D may improve the uptake and utilization of free fatty acids by modulating insulin signaling, thereby indirectly influencing hepatic TG synthesis and very-low-density lipoprotein (VLDL) secretion. The association between vitamin D and glycerophospholipids is also likely to be indirect. Previous studies have discovered associations between calcium, magnesium, phosphate, and metabolites [23,24,25]. Among them, phosphate plays a direct role in glycerophospholipid biosynthesis, while calcium and magnesium are also essential cofactors in numerous enzymatic and metabolic processes [26,27,28,29]. Moreover, decreased intestinal absorption of calcium, magnesium, and phosphate is a recognized potential consequence of vitamin D insufficiency [30,31]. Therefore, the lower glycerophospholipid levels observed in the VDD group in our study may be indirectly explained by disturbances in mineral homeostasis, such as reduced phosphate absorption leading to lower serum phosphate concentrations, together with potential contributions from altered calcium and magnesium availability.
Different from glycerophospholipids, which have been the primary focus in most studies examining associations between vitamin D status and metabolites, linoleic acid and its derivatives (contributed most to PC2) have been less frequently reported in non-pregnant population, suggesting their potential importance may be specific to pregnancy. An experimental study also found that linoleic acid levels were sharply reduced in both the plasma and liver of pregnant rats with VDD, suggesting that as an essential fatty acid, linoleic acid is more susceptible to amplified effects of both synthesis and consumption mechanisms under conditions of VDD [11]. The level of linoleic acid in the mother is very important for the growth and development of the fetus. Studies have shown an inverted U-shaped connection among the birth weight of the offspring and the mother’s consumption of linoleic acid, indicating that both insufficient and excessive intake may adversely affect birth outcomes [32]. Additionally, linoleic acid and its derivatives can influence placental function and fetal neurodevelopment by modulating inflammatory cytokines and cellular signaling pathways [33]. Given this, whether vitamin D can influence maternal health and neonatal birth outcomes by regulating linoleic acid and its metabolic pathways warrants further in-depth and systematic investigation.
This study also found elevated levels of various benzenoids, Organic acids and derivatives, and organic oxygen compounds in the VDD group. These findings suggest that vitamin D status may exert broader effects beyond classical lipid and energy metabolism pathways, potentially influencing biochemical clearance processes and host–microbiome homeostasis. For example, low vitamin D levels have been linked to downregulated expression and activity of key cytochrome P450 enzymes, which may slow the metabolism of various drugs and organic compounds, leading to their accumulation in the body [34].
MSEA revealed a significant enrichment in the pantothenate and CoA biosynthesis pathway, while unsaturated fatty acids biosynthetic pathways lost statistical significance after correction. The pantothenate and CoA biosynthesis pathway is a critical hub for energy production and biosynthesis in the human body. CoA is a necessary cofactor in several enzyme-catalyzed reactions, including fatty acid β-oxidation, triglyceride synthesis, and cholesterol biosynthesis. Vitamin D may regulate pantothenate kinase 4 through the modulation of PPARγ, thereby influencing the efficiency of the conversion of pantothenate to CoA [35]. However, previous studies based on the KEGG database differ from ours and are not entirely consistent with each other. Lasky-Su et al. [36] found a linkage between vitamin D status and the glutathione and glutamate metabolism pathways, while Wang et al. [37] identified differential metabolites concentrated in glycerophospholipid metabolism, fat digestion and absorption, and triglyceride metabolism pathways, and Mousa et al. [38] focused on the sphingolipid pathway. To validate these relationships, more pathway and enrichment analyses with bigger sample sizes are required.
Overall, there is a limited number of metabolomic studies on VDD in pregnant women, and the conclusions remain inconsistent. Additionally, existing studies have not considered the relationship between vitamin D status, metabolites and lipid profiles together. This study provides new proof of vitamin D’s beneficial effect on pregnant women’s lipid levels and offers insights into the underlying mechanisms. This study still has a number of shortcomings, though. First, because it is a cross-sectional study, causal correlations cannot be established. Including mid-pregnancy lipid data for longitudinal analysis would better assess the impact of vitamin D on lipid levels and help elucidate the underlying mechanisms. Second, the statistical power is limited by the small sample size, which may therefore limit how broadly the results can be applied, future studies with larger populations are warranted.
5. Conclusions
Pregnant women with VDD exhibited alterations in 60 metabolites, particularly down-regulation of glycerophospholipids and fatty acyls. A linoleic acid–dominated component (PC2) and a glycerophosphocholine-dominated component (PC3) both showed inverse correlations with maternal lipid levels. MSEA further revealed significant enrichment of the pantothenate and CoA biosynthesis pathway. This study provides new metabolomic evidence for the linkage between vitamin D and maternal lipid levels during pregnancy, along with clues about the mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17193096/s1, Table S1. Detailed information of VDD-associated metabolites.
Author Contributions
Y.Y. is the chief investigator for the study and is responsible for the conceptualization, funding acquisition, supervision and revision of the manuscript. H.L. contributed to conceptualization and manuscript revision. Y.Q. contributed to formal analysis and original draft preparation. B.W. contributed to the validation and manuscript revision. N.X. and S.W. contributed to the methodology and manuscript revision. X.A. and H.C. contributed to the visualization and manuscript revision. D.C. contributed to the funding acquisition and manuscript revision. L.Y. contributed to the manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the National Key R&D Program of China [grant number 2021YFC2701901 and 2022YFC2703505], and 4+X Clinical Research Project of Women’s Hospital, School of Medicine, Zhejiang University [grant number ZDFY2021-4X104].
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of Zhejiang University School of Medicine (Approval No. 2011-1-002, 4 March 2011).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to ethical and privacy restrictions.
Acknowledgments
We appreciate everyone who took part in this study. We acknowledge the support of Zhoushan Maternal and Child Care Hospital and fellows there who conducted and managed the cohort.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, H.; Wang, S.; Tuo, L.; Zhai, Q.; Cui, J.; Chen, D.; Xu, D. Relationship between Maternal Vitamin D Levels and Adverse Outcomes. Nutrients 2022, 14, 4230. [Google Scholar] [CrossRef]
- Varthaliti, A.; Rodolaki, K.; Lygizos, V.; Vlachos, D.E.; Thomakos, N.; Sioutis, D.; Daskalakis, G.; Pergialiotis, V. Neurodevelopmental Outcomes in the Offspring of Women with Vitamin D Deficiency and Women Who Received Vitamin D Supplementation During Pregnancy. Nutrients 2025, 17, 978. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, R.; Mao, D.; Chen, J.; Li, M.; Li, W.; Yang, Y.; Zhao, L.; Zhang, J.; Piao, J.; et al. Vitamin D Nutritional Status of Chinese Pregnant Women, Comparing the Chinese National Nutrition Surveillance (CNHS) 2015-2017 with CNHS 2010-2012. Nutrients 2021, 13, 2237. [Google Scholar] [CrossRef]
- Shen, Y.; Pu, L.; Si, S.; Xin, X.; Mo, M.; Shao, B.; Wu, J.; Huang, M.; Wang, S.; Muyiduli, X.; et al. Vitamin D nutrient status during pregnancy and its influencing factors. Clin. Nutr. 2020, 39, 1432–1439. [Google Scholar] [CrossRef]
- Lepsch, J.; Eshriqui, I.; Farias, D.R.; Vaz; Cunha Figueiredo, A.C.; Adegboye, A.R.A.; Brito, A.; Mokhtar, R.; Allen, L.H.; Holick, M.F.; et al. Association between early pregnancy vitamin D status and changes in serum lipid profiles throughout pregnancy. Metab. Clin. Exp. 2017, 70, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhu, D.-M.; Hu, H.-L.; Yao, M.-N.; Yin, W.-J.; Tao, R.-X.; Zhu, P. Vitamin D status affects the relationship between lipid profile and high-sensitivity C-reactive protein. Nutr. Metab. 2020, 17, 57. [Google Scholar] [CrossRef]
- The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/37895163/ (accessed on 3 July 2025).
- Finkelstein, J.L.; Pressman, E.K.; Cooper, E.M.; Kent, T.R.; Bar, H.Y.; O’Brien, K.O. Vitamin D Status Affects Serum Metabolomic Profiles in Pregnant Adolescents. Reprod. Sci. 2015, 22, 685. [Google Scholar] [CrossRef]
- Li, X.; An, Z.; Yao, A.; Li, R.; Zhang, S.; Yu, S.; Ma, L.; Liu, Y. Targeted metabolomics profiling in pregnancy associated with vitamin D deficiency. BMC Pregnancy Childbirth 2024, 24, 295. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Brustad, N.; Ali, M.; Gürdeniz, G.; Arendt, M.; Litonjua, A.A.; Wheelock, C.E.; Kelly, R.S.; Chen, Y.; Prince, N.; et al. Maternal vitamin D–related metabolome and offspring risk of asthma outcomes. J. Allergy Clin. Immunol. 2023, 152, 1646–1657.e11. [Google Scholar] [CrossRef]
- Nandi, A.; Wadhwani, N.; Joshi, S.R. Vitamin D deficiency influences fatty acid metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 57–63. [Google Scholar] [CrossRef]
- Gençosmanoğlu Türkmen, G.; Vural Yilmaz, Z.; Dağlar, K.; Kara, Ö.; Sanhal, C.Y.; Yücel, A.; Uygur, D. Low serum vitamin D level is associated with intrahepatic cholestasis of pregnancy. J. Obstet. Gynaecol. Res. 2018, 44, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Panagiotou, O.A.; Anic, G.M.; Mondul, A.M.; Männistö, S.; Weinstein, S.J.; Albanes, D. Metabolomics analysis of serum 25-hydroxy-vitamin D in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study. Int. J. Epidemiol. 2016, 45, 1458–1468. [Google Scholar] [CrossRef]
- Leung, R.Y.H.; Li, G.H.Y.; Cheung, B.M.Y.; Tan, K.C.B.; Kung, A.W.C.; Cheung, C.-L. Serum metabolomic profiling and its association with 25-hydroxyvitamin D. Clin. Nutr. 2020, 39, 1179–1187. [Google Scholar] [CrossRef]
- Amrein, K.; Lasky-Su, J.A.; Dobnig, H.; Christopher, K.B. Metabolomic basis for response to high dose vitamin D in critical illness. Clin. Nutr. 2021, 40, 2053–2060. [Google Scholar] [CrossRef]
- Xue, J.; Hutchins, E.K.; Elnagheeb, M.; Li, Y.; Valdar, W.; McRitchie, S.; Sumner, S.; Ideraabdullah, F.Y. Maternal Liver Metabolic Response to Chronic Vitamin D Deficiency Is Determined by Mouse Strain Genetic Background. Curr. Dev. Nutr. 2020, 4, nzaa106. [Google Scholar] [CrossRef] [PubMed]
- McCourt, A.F.; O’Sullivan, A.M. Influence of Vitamin D Status and Supplementation on Metabolomic Profiles of Older Adults. Metabolites 2023, 13, 166. [Google Scholar] [CrossRef]
- Migni, A.; Bartolini, D.; Varfaj, I.; Moscardini, I.F.; Sardella, R.; Garetto, S.; Lucci, J.; Galli, F. Lipidomics reveals different therapeutic potential for natural and synthetic vitamin D formulations in hepatocyte lipotoxicity. Biomed. Pharmacother. 2025, 187, 118068. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Xiang, L.; Zhang, J.; Yang, C.; Zhao, W.; Li, J.; Zhou, Y.; Ma, L. Vitamin D improves hepatic steatosis in NAFLD via regulation of fatty acid uptake and β-oxidation. Front. Endocrinol. 2023, 14, 1138078. [Google Scholar] [CrossRef]
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, M.; Sato, S.-I.; Sakai, J.; Nagasawa, K.; et al. Vitamin D Metabolite, 25-Hydroxyvitamin D, Regulates Lipid Metabolism by Inducing Degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217. [Google Scholar] [CrossRef]
- Xu, X.; So, J.-S.; Park, J.-G.; Lee, A.-H. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin. Liver Dis. 2013, 33, 301–311. [Google Scholar] [CrossRef]
- Madison, B.B. Srebp2: A master regulator of sterol and fatty acid synthesis1. J. Lipid Res. 2016, 57, 333–335. [Google Scholar] [CrossRef]
- Merchant, T.E.; Glonek, T. 31P NMR of tissue phospholipids: Competition for Mg2+, Ca2+, Na+ and K+ cations. Lipids 1992, 27, 551–559. [Google Scholar] [CrossRef]
- Mao, H.; Wang, W.; Shi, L.; Chen, C.; Han, C.; Zhao, J.; Zhuo, Q.; Shen, S.; Li, Y.; Huo, J. Metabolomics and physiological analysis of the effect of calcium supplements on reducing bone loss in ovariectomized rats by increasing estradiol levels. Nutr. Metab. 2021, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, M.; Nakagawa, R.; Tomonaga, S.; Funaba, M.; Matsui, T. Fluctuations in metabolite content in the liver of magnesium-deficient rats. Br. J. Nutr. 2016, 116, 1694–1699. [Google Scholar] [CrossRef]
- Lamari, F.; Rossignol, F.; Mitchell, G.A. Glycerophospholipids: Roles in Cell Trafficking and Associated Inborn Errors. J. Inherit. Metab. Dis. 2025, 48, e70019. [Google Scholar] [CrossRef] [PubMed]
- Hishikawa, D.; Hashidate, T.; Shimizu, T.; Shindou, H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res. 2014, 55, 799–807. [Google Scholar] [CrossRef]
- Dutta, P.; Layton, A.T. Modeling calcium and magnesium balance: Regulation by calciotropic hormones and adaptations under varying dietary intake. iScience 2024, 27, 111077. [Google Scholar] [CrossRef]
- Alsheikh, R.; Aldulaimi, H.; Hinawi, R.; Al-Sadi, F.; Al-Baker, A.; Alkuwari, A.; Sameer, M.; Al-Abdulla, G.; Shi, Z.; Rathnaiah Babu, G. Association of serum magnesium and calcium with metabolic syndrome: A cross-sectional study from the Qatar-biobank. Nutr. Metab. 2025, 22, 8. [Google Scholar] [CrossRef]
- Institute of Medicine (US). Vitamin D. In Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press (US): Washington, DC, USA, 1997. [Google Scholar]
- Kaur, J.; Khare, S.; Sizar, O.; Givler, A. Vitamin D Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Nayyar, D.; Said, J.M.; McCarthy, H.; Hryciw, D.H.; O’Keefe, L.; McAinch, A.J. Effect of a High Linoleic Acid Diet on Pregnant Women and Their Offspring. Nutrients 2024, 16, 3019. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K.; Basak, S. Maternal Fatty Acid Metabolism in Pregnancy and Its Consequences in the Feto-Placental Development. Front. Physiol. 2022, 12, 787848. [Google Scholar] [CrossRef]
- Wang, Z.; Schuetz, E.G.; Xu, Y.; Thummel, K.E. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J. Steroid Biochem. Mol. Biol. 2013, 136, 54–58. [Google Scholar] [CrossRef]
- Ramaswamy, G.; Karim, M.A.; Murti, K.G.; Jackowski, S. PPARalpha controls the intracellular coenzyme A concentration via regulation of PANK1alpha gene expression. J. Lipid Res. 2004, 45, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Lasky-Su, J.; Dahlin, A.; Litonjua, A.A.; Rogers, A.J.; McGeachie, M.J.; Baron, R.M.; Gazourian, L.; Barragan-Bradford, D.; Fredenburgh, L.E.; Choi, A.M.K.; et al. Metabolome alterations in severe critical illness and vitamin D status. Crit. Care 2017, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, H.; Zhang, Z.-Y.; Chang, T.-Z.; Yu, Y.-T.; Miao, D.-S.; Zhang, B.-J.; Qiu, W.-X.; Zhang, C.-Y.; Tong, X. A new perspective on bone development in vitamin D deficiency-associated obese children. Sci. Rep. 2024, 14, 31482. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.; Elrayess, M.A.; Diboun, I.; Jackson, S.K.; Zughaier, S.M. Metabolomics Profiling of Vitamin D Status in Relation to Dyslipidemia. Metabolites 2022, 12, 771. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).