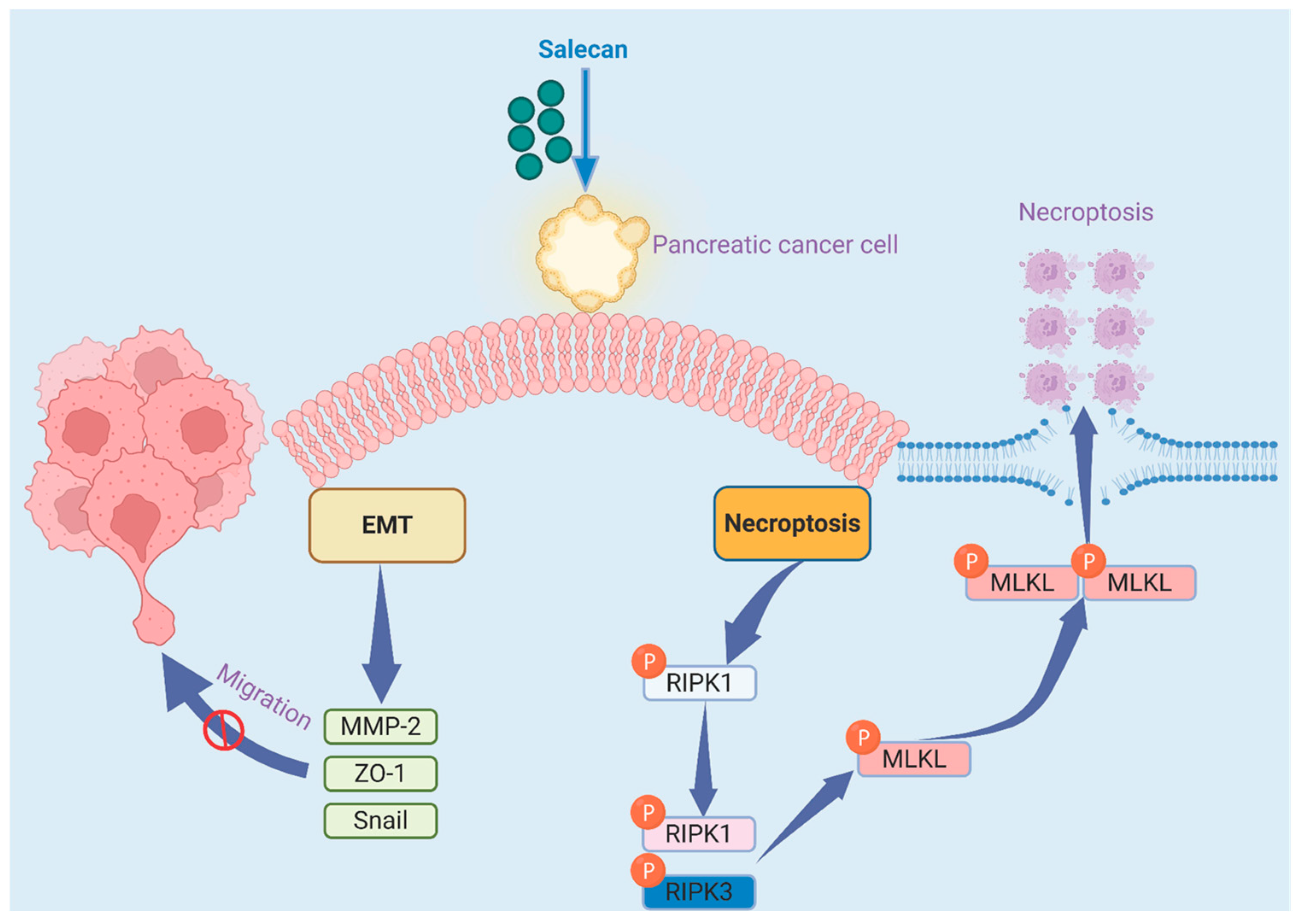
Salecan Suppresses Pancreatic Cancer Progression by Promoting Necroptosis via the RIPK1/MLKL Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. β-Glucan
2.2. Cell Culture
2.3. Cell Proliferation
2.4. Colony Formation Assay
2.5. Wound Healing Assay
2.6. Cell Migration and Invasion Assay
2.7. Flow Cytometry Analysis
2.8. Caspase-3 Activity Measurement
2.9. RNA-seq Analysis
2.10. Functional and Pathway Enrichment Analysis
2.11. Quantitative Real-Time PCR
2.12. Western Blotting
2.13. Statistical Analysis
3. Results
3.1. Salecan Inhibits Pancreatic Cancer Cell Proliferation
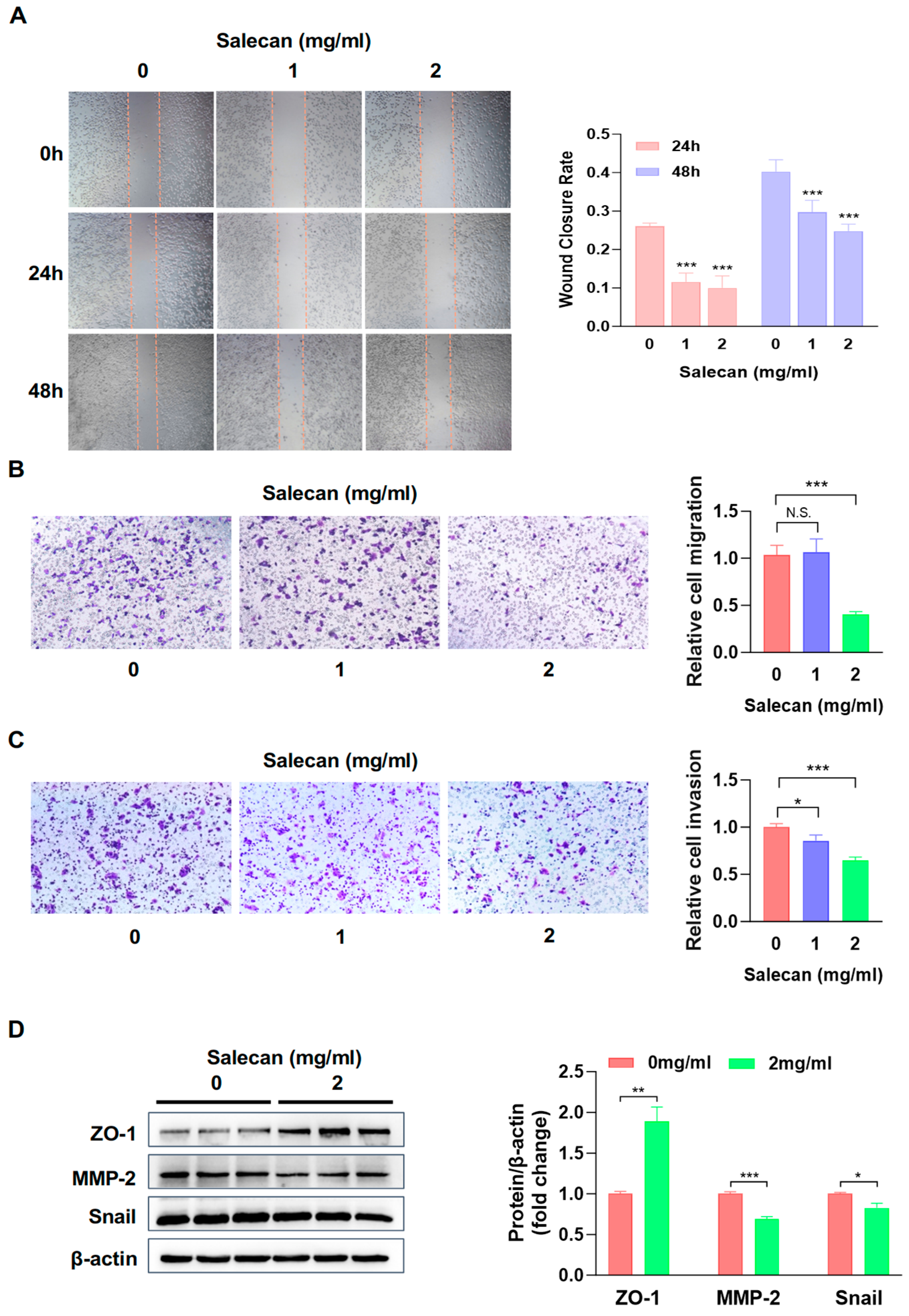
3.2. Salecan Attenuates Pancreatic Cancer Cell Migration and Invasion in a Concentration-Dependent Manner
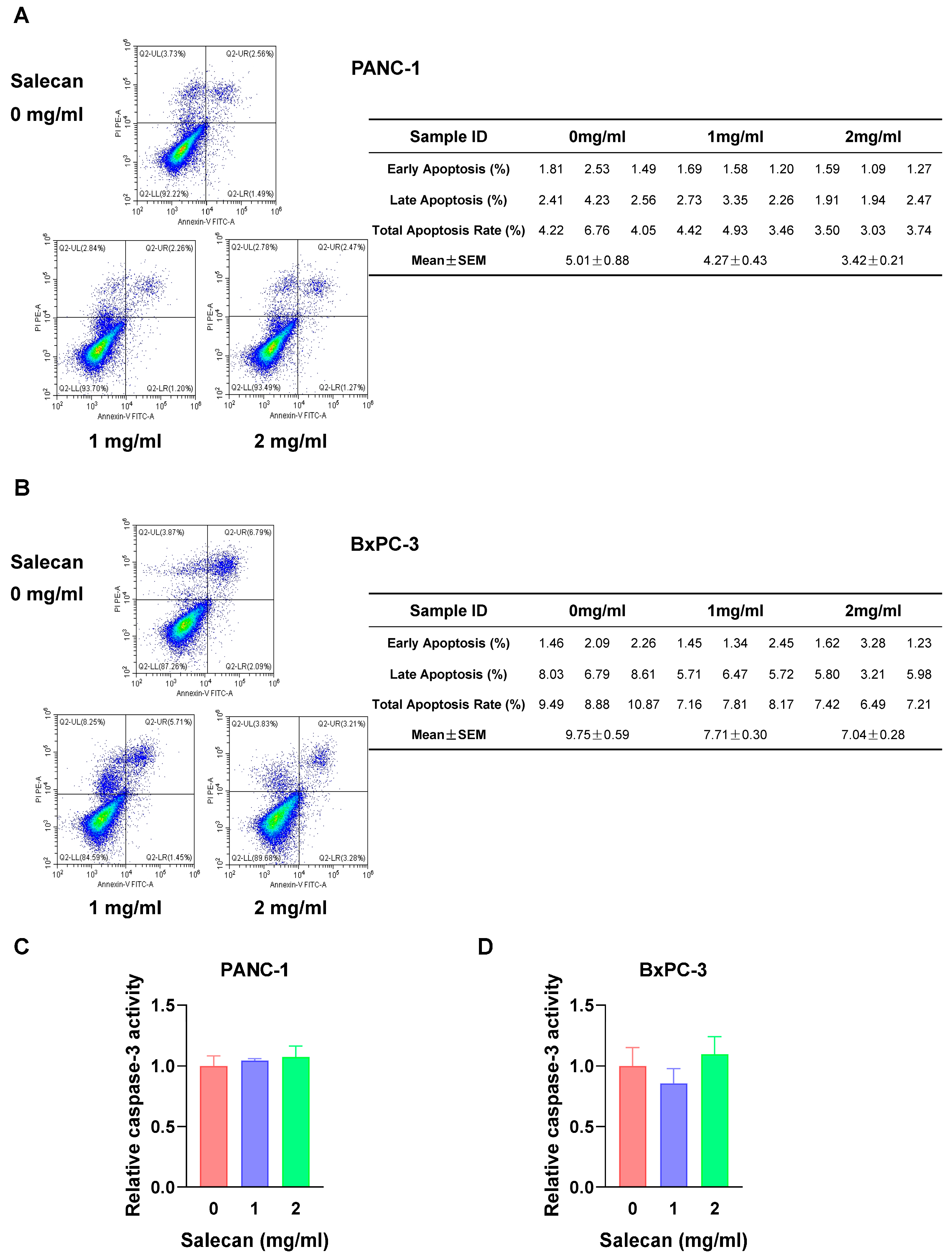
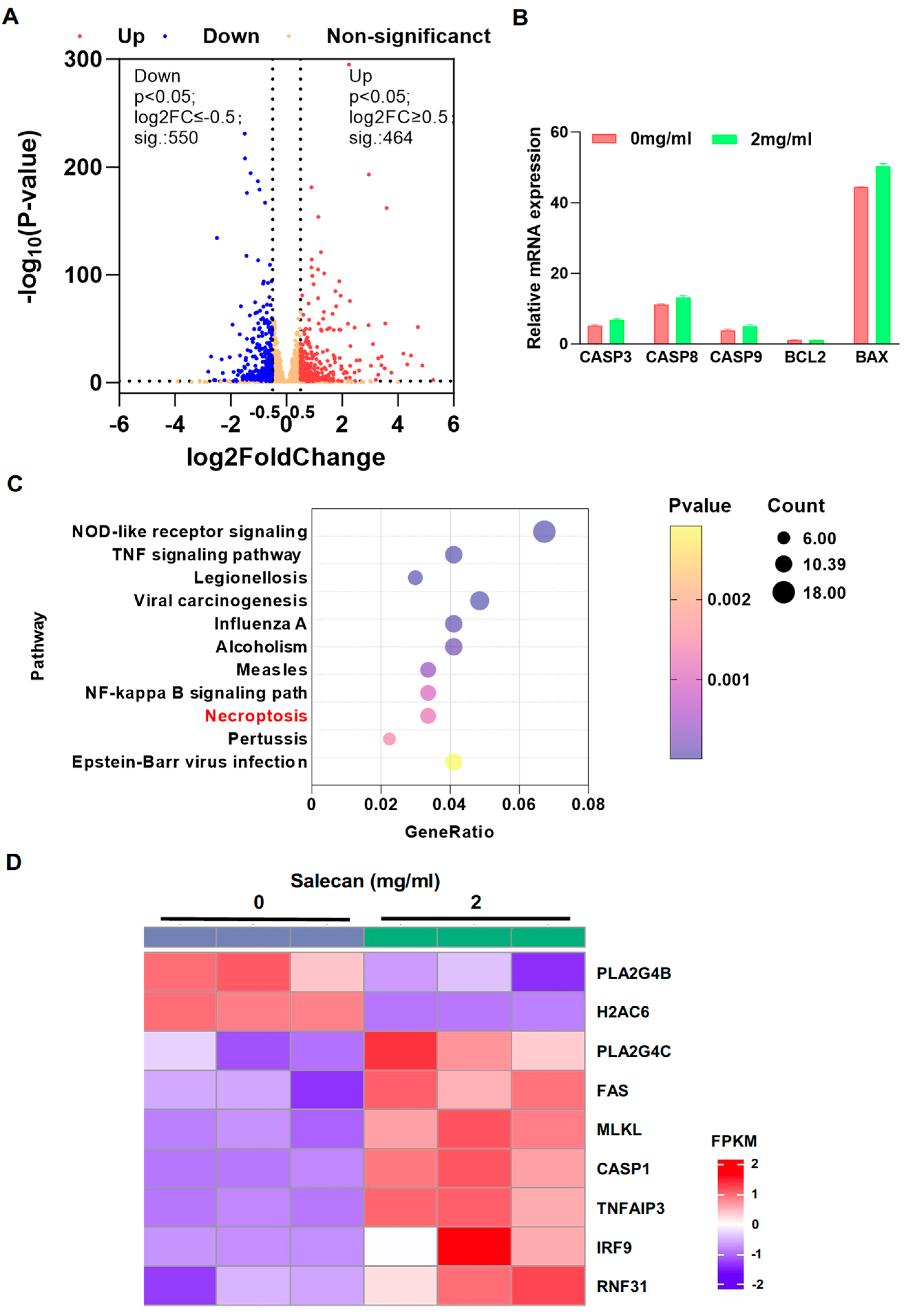
3.3. RNA-seq Analysis Reveals That Salecan Affects the Necroptosis Pathway, Not Apoptosis, in Pancreatic Cancer Cells
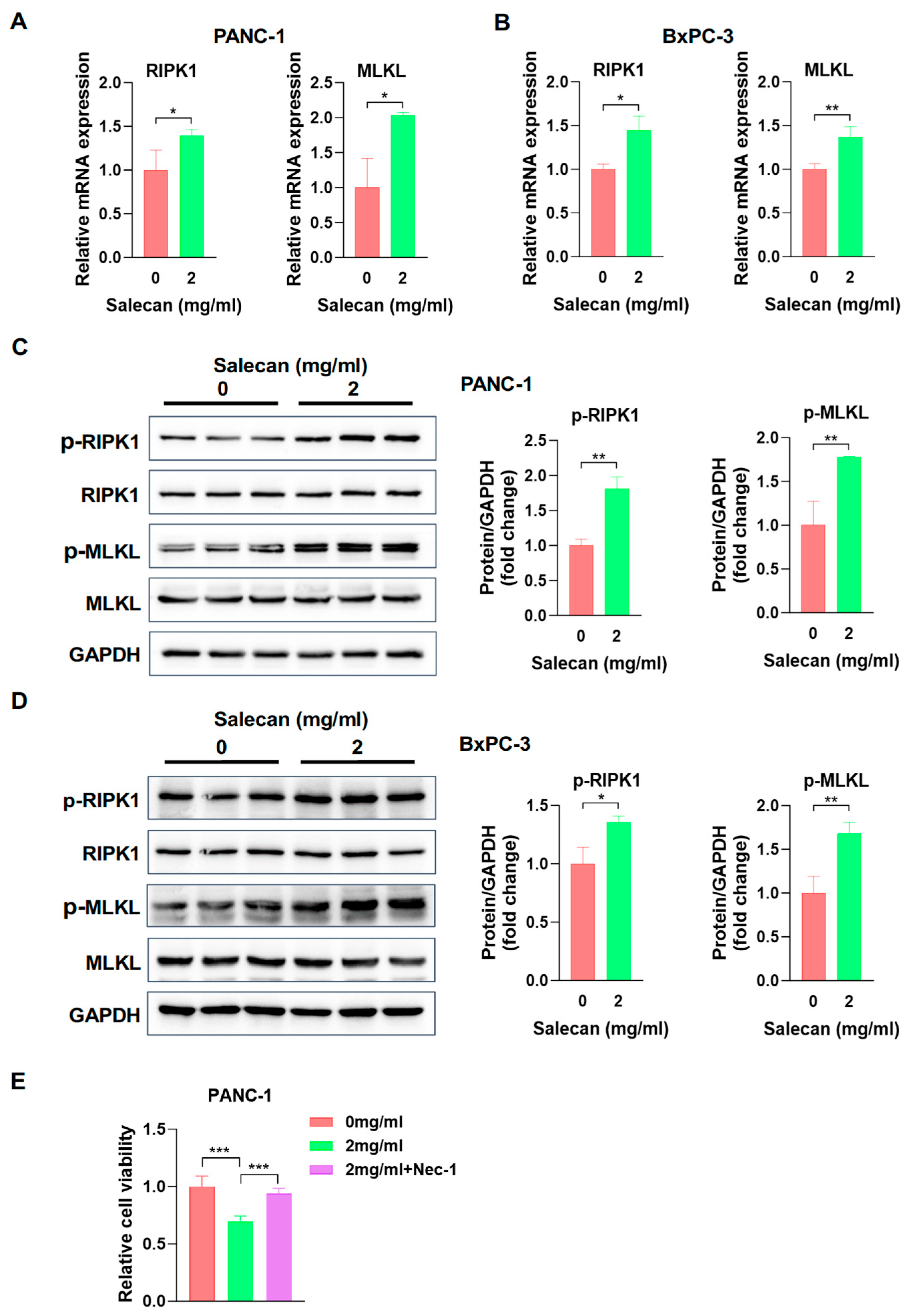
3.4. Salecan Impairs Pancreatic Cancer Cell Proliferation and Metastasis via the MLKL/RIPK1 Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e1. [Google Scholar] [CrossRef]
- Corty, R.W.; Langworthy, B.W.; Fine, J.P.; Buse, J.B.; Sanoff, H.K.; Lund, J.L. Antibacterial Use Is Associated with an Increased Risk of Hematologic and Gastrointestinal Adverse Events in Patients Treated with Gemcitabine for Stage IV Pancreatic Cancer. Oncologist 2020, 25, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Hu, Z.I.; O’Reilly, E.M. Therapeutic developments in pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 7–24. [Google Scholar] [CrossRef]
- Dai, Y.; Guan, X.; Guo, F.; Kong, X.; Ji, S.; Shang, D.; Bai, C.; Zhang, Q.; Zhao, L. Botanical drugs and their natural compounds: A neglected treasury for inhibiting the carcinogenesis of pancreatic ductal adenocarcinoma. Pharm. Biol. 2024, 62, 853–873. [Google Scholar] [CrossRef]
- Sujithra, S.; Arthanareeswaran, G.; Ismail, A.F.; Taweepreda, W. Isolation, purification and characterization of beta-glucan from cereals—A review. Int. J. Biol. Macromol. 2024, 256, 128255. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. beta-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. beta-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar] [CrossRef]
- Mathews, R.; Shete, V.; Chu, Y. The effect of cereal Beta-glucan on body weight and adiposity: A review of efficacy and mechanism of action. Crit. Rev. Food Sci. Nutr. 2023, 63, 3838–3850. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; He, Y.; Zhu, X.; Ai, Q.; Shi, Y. beta-glucan protects against necrotizing enterocolitis in mice by inhibiting intestinal inflammation, improving the gut barrier, and modulating gut microbiota. J. Transl. Med. 2023, 21, 14. [Google Scholar] [CrossRef]
- Muthuramalingam, K.; Kim, Y.; Cho, M. beta-glucan, “the knight of health sector”: Critical insights on physiochemical heterogeneities, action mechanisms and health implications. Crit. Rev. Food Sci. Nutr. 2022, 62, 6908–6931. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.I.; Wu, C.C.; Hsieh, S.L.; Lee, C.L.; Chang, Y.P.; Chang, C.C.; Wang, Y.Z.; Wang, J.J. Anticancer effects on human pancreatic cancer cells of triterpenoids, polysaccharides and 1,3-beta-D-glucan derived from the fruiting body of Antrodia camphorata. Food Funct. 2014, 5, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Xiu, A.; Kong, Y.; Zhou, M.; Zhu, B.; Wang, S.; Zhang, J. The chemical and digestive properties of a soluble glucan from Agrobacterium sp. ZX09. Carbohydr. Polym. 2010, 82, 623–628. [Google Scholar] [CrossRef]
- Xu, X.; Ding, Y.; Yang, Y.; Gao, Y.; Sun, Q.; Liu, J.; Yang, X.; Wang, J.; Zhang, J. beta-glucan Salecan Improves Exercise Performance and Displays Anti-Fatigue Effects through Regulating Energy Metabolism and Oxidative Stress in Mice. Nutrients 2018, 10, 858. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Zhao, J.; Pan, W.; He, Y.; Fu, S.; Liu, Y.; Xu, Y.J. Salecan ameliorates liver injury by regulating gut microbiota and its metabolites. Food Funct. 2022, 13, 11744–11757. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Huang, Q.; Yan, X.; Dai, Y.; Zhao, J.; Xiong, X.; Wang, H.; Chen, X.; Chen, P.; Liu, L. Facile fabrication of a novel, photodetachable salecan-based hydrogel dressing with self-healing, injectable, and antibacterial properties based on metal coordination. Int. J. Biol. Macromol. 2024, 264, 130551. [Google Scholar] [CrossRef]
- Shen, Z.; Dai, J.; Yang, X.; Liu, Y.; Liu, L.; Huang, Y.; Wang, L.; Chen, P.; Chen, X.; Zhang, C.; et al. Comparison of sea buckthorn fruit oil nanoemulsions stabilized by protein-polysaccharide conjugates prepared using beta-glucan from various sources. Food Chem. 2024, 457, 140098. [Google Scholar] [CrossRef]
- Lin, J.; Dai, J.; Yang, Q.; Li, J.; Xiao, J.; Zhang, Y.; Huang, Y.; Wang, L.; Chen, P.; Xu, B.; et al. Preparation and characterization of Salecan beta-glucan-based edible film loaded with lemon essential oil nanoemulsion: Effects on the preservation of chilled pork. Food Chem. 2025, 478, 143598. [Google Scholar] [CrossRef]
- Sun, H.; Shi, K.; Qi, K.; Kong, H.; He, Q.; Zhou, M. Pseudostellaria heterophylla Extract Polysaccharide H-1-2 Suppresses Pancreatic Cancer by Inhibiting Hypoxia-Induced AG2. Mol. Ther. Oncolytics 2020, 17, 61–69. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Z.; Zeng, L.; Yang, X.; Wang, S.; Dong, W.; Jia, A.; Cai, C.; Zhang, J. A novel soluble beta-glucan salecan protects against acute alcohol-induced hepatotoxicity in mice. Biosci. Biotechnol. Biochem. 2011, 75, 1990–1993. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xu, X.; Yang, X.; Weng, D.; Wang, J.; Zhang, J. Salecan protected against concanavalin A-induced acute liver injury by modulating T cell immune responses and NMR-based metabolic profiles. Toxicol. Appl. Pharmacol. 2017, 317, 63–72. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Pan, W.; Zhao, J.; Olnood, C.G.; Liu, Y.; Xu, Y.J. Salecan confers anti-inflammatory effects in liver injury via regulating gut microbiota and its metabolites. Carbohydr. Polym. 2023, 302, 120418. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Q.; Yang, X.; Lu, W.; Zhao, Y.; Ge, W.; Yang, Y.; Xu, X.; Zhang, J. Orally administered salecan ameliorates methotrexate-induced intestinal mucositis in mice. Cancer Chemother. Pharmacol. 2019, 84, 105–116. [Google Scholar] [CrossRef]
- Zhou, M.; Jia, P.; Chen, J.; Xiu, A.; Zhao, Y.; Zhan, Y.; Chen, P.; Zhang, J. Laxative effects of Salecan on normal and two models of experimental constipated mice. BMC Gastroenterol. 2013, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, M.; Yang, X.; Xu, X.; Wang, J.; Zhang, J. Dietary salecan reverts partially the metabolic gene expressions and NMR-based metabolomic profiles from high-fat-diet-induced obese rats. J. Nutr. Biochem. 2017, 47, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cheng, R.; Li, J.; Wang, Y.; Zhu, B.; Ma, S.; Zhang, W.; Dong, W.; Wang, S.; Zhang, J. Identification of substituent groups and related genes involved in salecan biosynthesis in Agrobacterium sp. ZX09. Appl. Microbiol. Biotechnol. 2017, 101, 585–598. [Google Scholar] [CrossRef]
- Si, W.; Liu, X.; Wei, R.; Zhang, Y.; Zhao, Y.; Cui, L.; Hong, T. MTA2-mediated inhibition of PTEN leads to pancreatic ductal adenocarcinoma carcinogenicity. Cell Death Dis. 2019, 10, 206. [Google Scholar] [CrossRef]
- Kaushal, J.B.; Raut, P.; Halder, S.; Alsafwani, Z.W.; Parte, S.; Sharma, G.; Abdullah, K.M.; Seshacharyulu, P.; Lele, S.M.; Batra, S.K.; et al. Oncogenic potential of truncated-Gli3 via the Gsk3beta/Gli3/AR-V7 axis in castration-resistant prostate cancer. Oncogene 2025, 44, 1007–1023. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, J.; Wang, B.; Zhao, M.; Wu, Z.; Liu, X. Establishment of an in Vitro Three-Dimensional Vascularized Micro-Tumor Model and Screening of Chemotherapeutic Drugs. Technol. Cancer Res. Treat. 2024, 23, 15330338241286755. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, H.; Liu, G.; Li, M.; He, X.; Yin, C.; Tu, Q.; Shen, X.; Bai, W.; Wang, Q.; et al. Yeast β-D-glucan exerts antitumour activity in liver cancer through impairing autophagy and lysosomal function, promoting reactive oxygen species production and apoptosis. Redox Biol. 2020, 32, 101495. [Google Scholar] [CrossRef]
- Tao, H.; Chen, X.; Du, Z.; Ding, K. Corn silk crude polysaccharide exerts anti-pancreatic cancer activity by blocking the EGFR/PI3K/AKT/CREB signaling pathway. Food Funct. 2020, 11, 6961–6970. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.-L.; Li, W.-B.; Chen, L.-J.; Wang, W.; Ju, T.-F.; Yin, D.-L. Curcumin inhibits the invasion and migration of pancreatic cancer cells by upregulating TFPI-2 to regulate ERK- and JNK-mediated epithelial–mesenchymal transition. Eur. J. Nutr. 2023, 63, 639–651. [Google Scholar] [CrossRef]
- Ang, H.L.; Mohan, C.D.; Shanmugam, M.K.; Leong, H.C.; Makvandi, P.; Rangappa, K.S.; Bishayee, A.; Kumar, A.P.; Sethi, G. Mechanism of epithelial-mesenchymal transition in cancer and its regulation by natural compounds. Med. Res. Rev. 2023, 43, 1141–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Jiang, P.; Peng, M.; Zhang, X.; Chen, K.; Liu, H.; Bi, H.; Liu, X.; Li, X. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 177. [Google Scholar] [CrossRef]
- Gobin, E.; Bagwell, K.; Wagner, J.; Mysona, D.; Sandirasegarane, S.; Smith, N.; Bai, S.; Sharma, A.; Schleifer, R.; She, J.X. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer 2019, 19, 581. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, S.; Zhong, M.; Yang, M.; Sun, X.; Liu, J.; Kroemer, G.; Lotze, M.; Zeh, H.J., 3rd; Kang, R.; et al. Inhibition of Aurora Kinase A Induces Necroptosis in Pancreatic Carcinoma. Gastroenterology 2017, 153, 1429–1443.e5. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Hsu, S.K.; Jadhao, M.; Liao, W.T.; Chang, W.T.; Hung, C.T.; Chiu, C.C. Culprits of PDAC resistance to gemcitabine and immune checkpoint inhibitor: Tumour microenvironment components. Front. Mol. Biosci. 2022, 9, 1020888. [Google Scholar] [CrossRef]
- Parrasia, S.; Zoratti, M.; Szabo, I.; Biasutto, L. Targeting Pancreatic Ductal Adenocarcinoma (PDAC). Cell Physiol. Biochem. 2021, 55, 61–90. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.; Zhong, K.; Wu, Z.; Wang, C.; Ding, Z.; Chen, S.; Zhang, J. Salecan ameliorates LPS-induced acute lung injury through regulating Keap1-Nrf2/HO-1 pathway in mice. Int. Immunopharmacol. 2024, 128, 111512. [Google Scholar] [CrossRef] [PubMed]
- Stathis, A.; Moore, M.J. Advanced pancreatic carcinoma: Current treatment and future challenges. Nat. Rev. Clin. Oncol. 2010, 7, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Bulle, A.; Lim, K.H. Beyond just a tight fortress: Contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer. Signal Transduct. Target. Ther. 2020, 5, 249. [Google Scholar] [CrossRef]
- Friend, C.; Parajuli, P.; Razzaque, M.S.; Atfi, A. Deciphering epithelial-to-mesenchymal transition in pancreatic cancer. Adv. Cancer Res. 2023, 159, 37–73. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, L.; Zhou, Y.; Shen, W.; Xu, D.; Dou, J.; Shen, B.; Zhou, C. Polysaccharide enhanced NK cell cytotoxicity against pancreatic cancer via TLR4/MAPKs/NF-kappaB pathway in vitro/vivo. Carbohydr. Polym. 2019, 225, 115223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; He, F.; Chen, X.; Ding, K. Isolation and structural characterization of a pectin from Lycium ruthenicum Murr and its anti-pancreatic ductal adenocarcinoma cell activity. Carbohydr. Polym. 2019, 223, 115104. [Google Scholar] [CrossRef]
- Ding, M.; Yang, Y.; Zhang, Z.; Liu, H.; Dai, Y.; Wang, Z.; Ma, S.; Liu, Y.; Wang, Q. Structural characterization of the polysaccharide from the black crystal region of Inonotus obliquus and its effect on AsPC-1 and SW1990 pancreatic cancer cell apoptosis. Int. J. Biol. Macromol. 2024, 268, 131891. [Google Scholar] [CrossRef]
- He, F.; Zhang, S.; Li, Y.; Chen, X.; Du, Z.; Shao, C.; Ding, K. The structure elucidation of novel arabinogalactan LRP1-S2 against pancreatic cancer cells growth in vitro and in vivo. Carbohydr. Polym. 2021, 267, 118172. [Google Scholar] [CrossRef]
- Delma, C.R.; Thirugnanasambandan, S.; Srinivasan, G.P.; Raviprakash, N.; Manna, S.K.; Natarajan, M.; Aravindan, N. Fucoidan from marine brown algae attenuates pancreatic cancer progression by regulating p53—NFkappaB crosstalk. Phytochemistry 2019, 167, 112078. [Google Scholar] [CrossRef]
- Bian, Y.; Zeng, H.; Tao, H.; Huang, L.; Du, Z.; Wang, J.; Ding, K. A pectin-like polysaccharide from Polygala tenuifolia inhibits pancreatic cancer cell growth in vitro and in vivo by inducing apoptosis and suppressing autophagy. Int. J. Biol. Macromol. 2020, 162, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Chen, X.; Zeh, H.J.; Kang, R.; Kroemer, G.; Tang, D. Cell death in pancreatic cancer: From pathogenesis to therapy. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 804–823. [Google Scholar] [CrossRef]
- Hsu, S.K.; Chu, Y.H.; Syue, W.J.; Lin, H.Y.; Chang, W.T.; Chen, J.Y.; Wu, C.Y.; Yen, C.H.; Cheng, K.C.; Chiu, C.C. The Role of Nonapoptotic Programmed Cell Death—Ferroptosis, Necroptosis, and Pyroptosis—In Pancreatic Ductal Adenocarcinoma Treatment. Front. Oncol. 2022, 12, 872883. [Google Scholar] [CrossRef]
- Liao, C.Y.; Li, G.; Kang, F.P.; Lin, C.F.; Xie, C.K.; Wu, Y.D.; Hu, J.F.; Lin, H.Y.; Zhu, S.C.; Huang, X.X.; et al. Necroptosis enhances ‘don’t eat me’ signal and induces macrophage extracellular traps to promote pancreatic cancer liver metastasis. Nat. Commun. 2024, 15, 6043. [Google Scholar] [CrossRef]
- Wu, Y.D.; Huang, X.X.; Zhang, H.X.; Pan, Y.; Xie, C.K.; Li, G.; Lin, C.F.; Lin, X.Q.; Li, Z.Y.; Chen, Y.H.; et al. TRAF3IP2-AS1 Deficiency Induces Necroptosis to Promote Pancreatic Cancer Liver Metastasis. Cancer Res. 2025, 85, 3292–3312. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhao, H.; Xiao, S.; Zheng, L.; Fan, T.; Wang, L.; Zhang, H.; Hu, Y.; Yang, J.; Wang, T.; et al. Single-cell RNA-seq analyses inform necroptosis-associated myeloid lineages influence the immune landscape of pancreas cancer. Front. Immunol. 2023, 14, 1263633. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wan, P.; Choksi, S.; Liu, Z.-G. Necroptosis and tumor progression. Trends Cancer 2022, 8, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, W.; Xu, R.; Chen, P.; Wen, J.; Sun, L.; Chen, X. Salecan Suppresses Pancreatic Cancer Progression by Promoting Necroptosis via the RIPK1/MLKL Pathway. Nutrients 2025, 17, 3090. https://doi.org/10.3390/nu17193090
Du W, Xu R, Chen P, Wen J, Sun L, Chen X. Salecan Suppresses Pancreatic Cancer Progression by Promoting Necroptosis via the RIPK1/MLKL Pathway. Nutrients. 2025; 17(19):3090. https://doi.org/10.3390/nu17193090
Chicago/Turabian StyleDu, Wenya, Rong Xu, Pengfei Chen, Jianxia Wen, Luchuanyang Sun, and Xianggui Chen. 2025. "Salecan Suppresses Pancreatic Cancer Progression by Promoting Necroptosis via the RIPK1/MLKL Pathway" Nutrients 17, no. 19: 3090. https://doi.org/10.3390/nu17193090
APA StyleDu, W., Xu, R., Chen, P., Wen, J., Sun, L., & Chen, X. (2025). Salecan Suppresses Pancreatic Cancer Progression by Promoting Necroptosis via the RIPK1/MLKL Pathway. Nutrients, 17(19), 3090. https://doi.org/10.3390/nu17193090




