Weizmannia coagulans JA845 Postbiotics Alleviate Atherosclerosis via TMAO-Related Gut Microbiota Regulation and JAK/STAT3 Pathway Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. W. coagulans JA845 Culture and Postbiotic Preparation
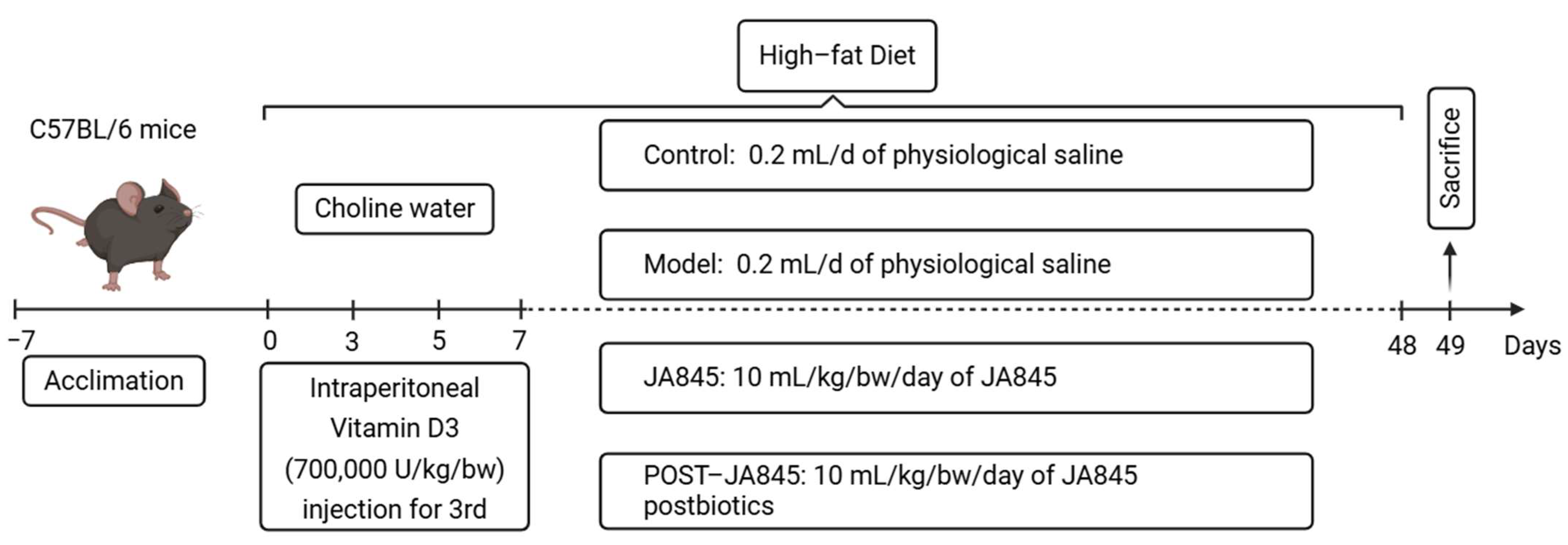
2.2. Animal Experiments
2.3. Histological Examination
2.4. Immunofluorescence
2.5. Biochemistry Analysis
2.6. Western Blotting
2.7. High-Throughput Sequencing of 16S rRNA in Cecum Contents
2.8. Analysis of Differential Metabolites
2.8.1. Metabolite Extraction
2.8.2. LC-MS Detection Conditions
2.9. Statistical Analysis
3. Results
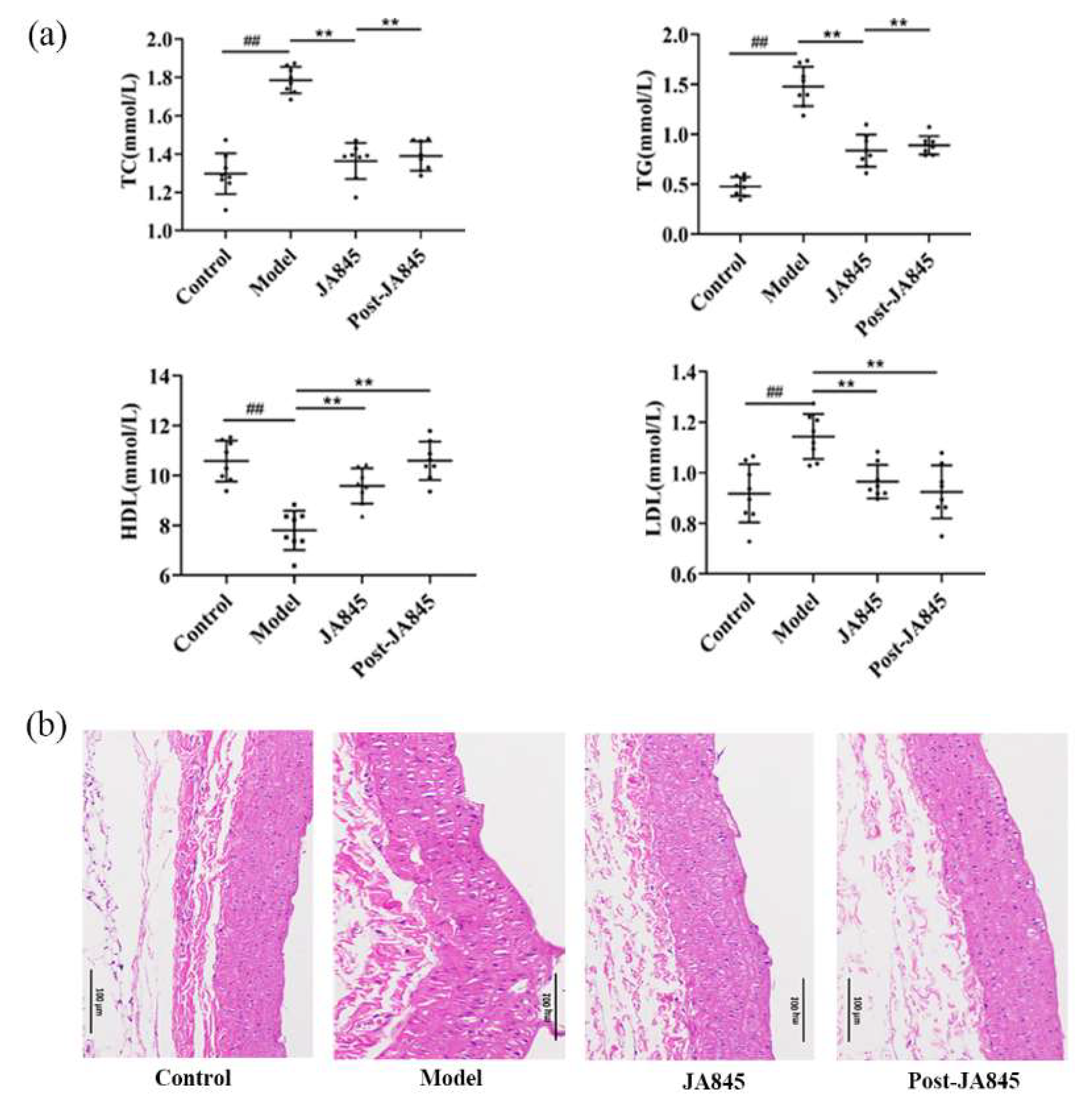
3.1. W. coagulans JA845 Postbiotics Regulates the Serum Lipid Levels in AS Mice
3.2. W. coagulans JA845 Postbiotics Improves the Pathological Characteristics of the Abdominal Aorta in AS Mice
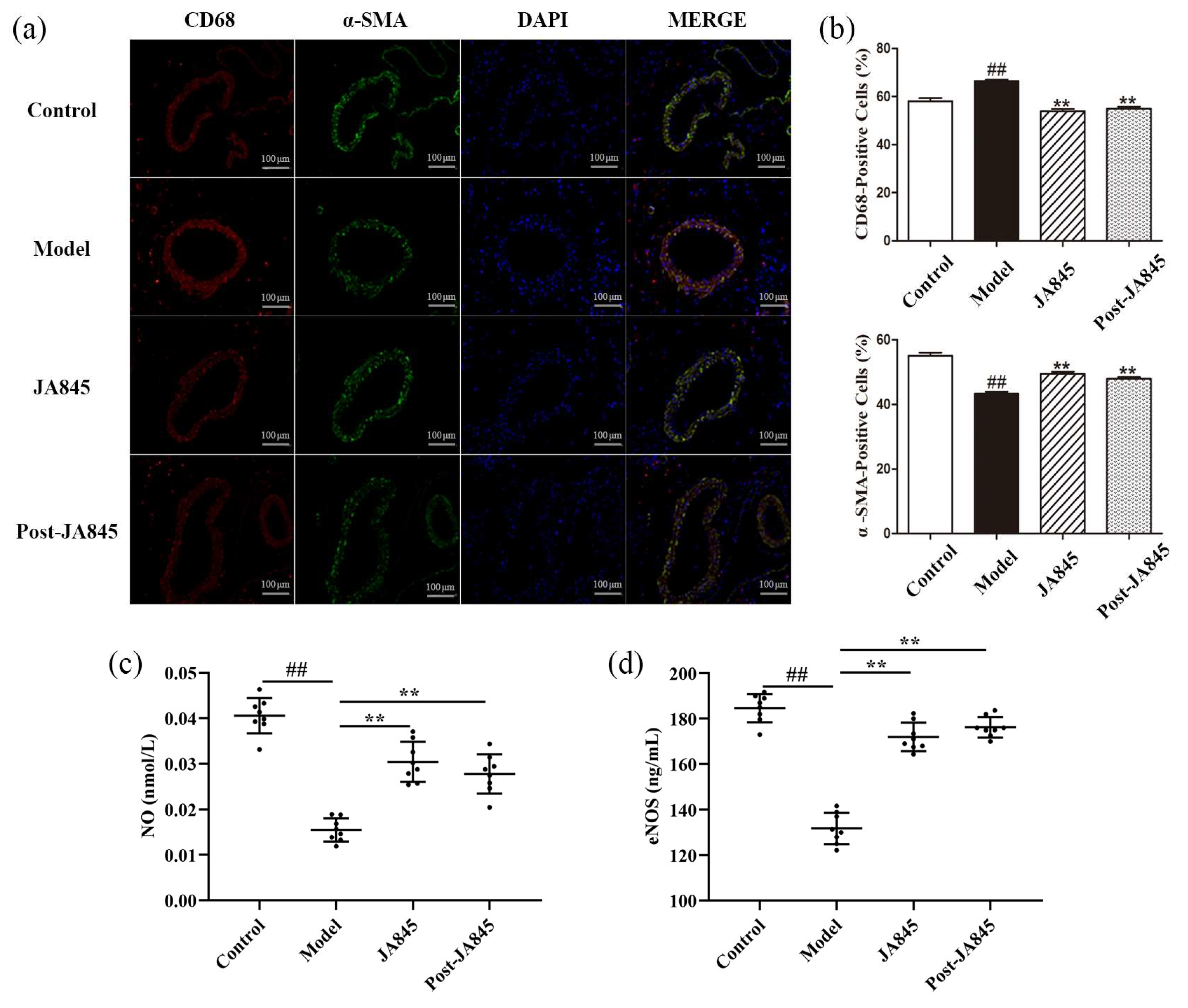
3.3. W. coagulans JA845 Postbiotics Regulates Expression of Immunological Markers in AS Mice
3.4. W. coagulans JA845 Postbiotics Exerts an Impact on the Serum Adhesion Molecules Expression in AS Mice
3.5. W. coagulans JA845 Postbiotics Alleviate Inflammation in AS Mice
3.6. W. coagulans JA845 Suppresses the Activation of the JAK/STAT3 Pathway in AS Mice
3.7. W. coagulans JA845 Postbiotics Improves Gut Microbiota in AS Mice
3.8. W. coagulans JA845 Postbiotics Modulates Variation of Metabolites Between Groups in AS Mice
3.9. W. coagulans JA845 Postbiotics-Mediated Combined Analysis of Intestinal Microbiota and Differential Metabolites in AS Mice
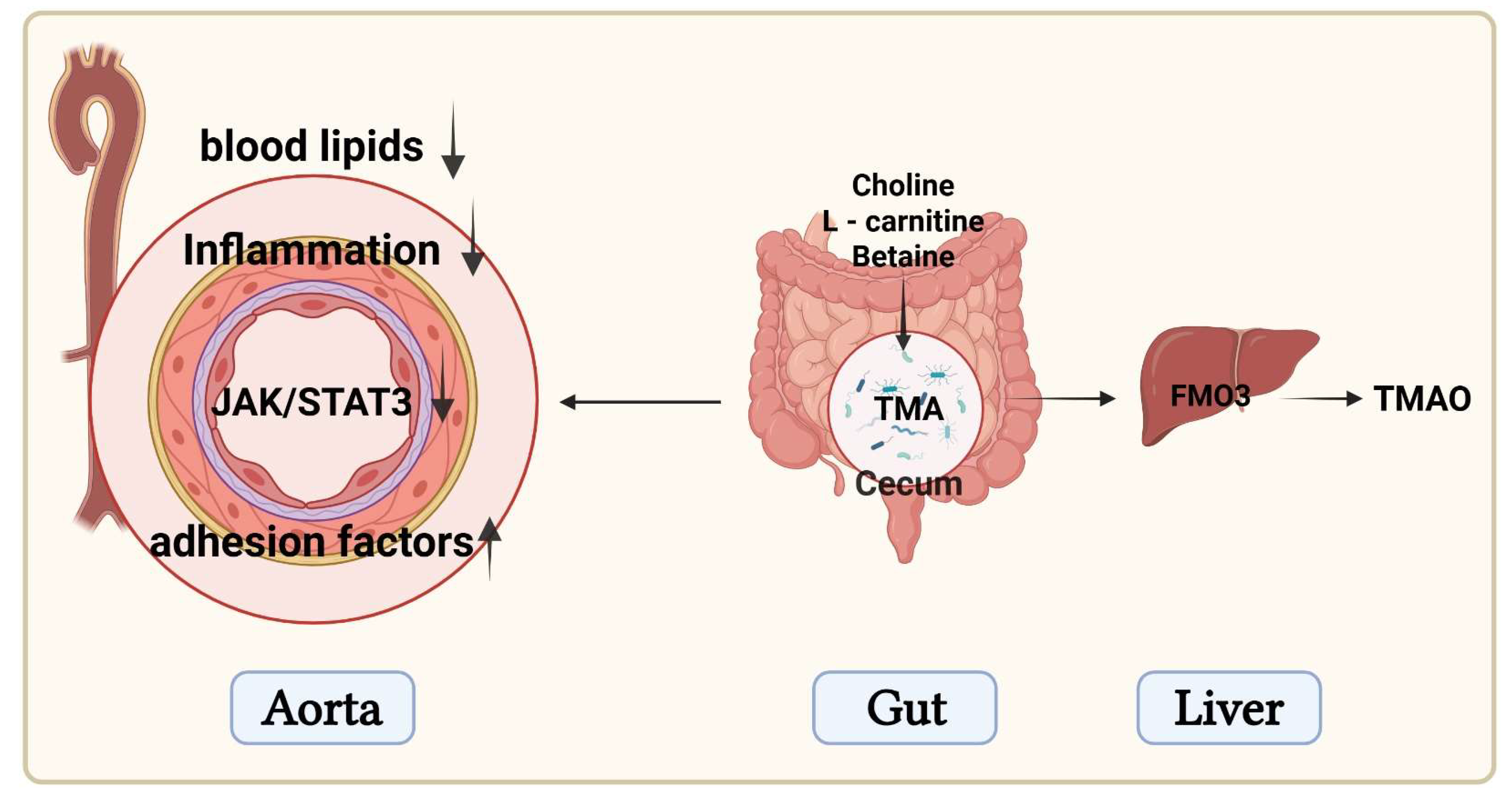
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vinderola, G.; Sanders, M.E.; Salminen, S. The concept of postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: A review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Feng, C.; Kwok, L.Y.; He, Q.; Sun, Z. Stronger gut microbiome modulatory effects by postbiotics than probiotics in a mouse colitis model. NPJ Sci. Food 2022, 6, 53. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Corrigendum: Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 1486. [Google Scholar] [CrossRef]
- Rayasam, G.V.; Tulasi, V.K.; Davis, J.A.; Bansal, V.S. Fatty acid receptors as new therapeutic targets for diabetes. Expert Opin. Ther. Targets 2007, 11, 661–671. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, H.; Li, Y.; Wang, Y.; Zheng, Y.; Liang, J.; Zhang, S.; Liu, M.; Fang, Z. Yin-xing-tong-mai decoction attenuates atherosclerosis via activating PPARγ-LXRα-ABCA1/ABCG1 pathway. Pharmacol. Res. 2021, 169, 105639. [Google Scholar] [CrossRef] [PubMed]
- Daniali, M.; Nikfar, S.; Abdollahi, M. Antibiotic resistance propagation through probiotics. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1207–1215. [Google Scholar] [CrossRef]
- Mosca, A.; Abreu, Y.A.A.T.; Gwee, K.A.; Ianiro, G.; Tack, J.; Nguyen, T.V.H.; Hill, C. The clinical evidence for postbiotics as microbial therapeutics. Gut Microbes 2022, 14, 2117508. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef]
- Oktaviono, Y.H.; Dyah Lamara, A.; Saputra, P.B.T.; Arnindita, J.N.; Pasahari, D.; Saputra, M.E.; Suasti, N.M.A. The roles of trimethylamine-N-oxide in atherosclerosis and its potential therapeutic aspect: A literature review. Biomol. Biomed. 2023, 23, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Y.; Huang, F.Q.; Lao, X.; Lu, Y.; Gao, X.; Alolga, R.N.; Yin, K.; Zhou, X.; Wang, Y.; Liu, B.; et al. Integrated metagenomics identifies a crucial role for trimethylamine-producing Lachnoclostridium in promoting atherosclerosis. NPJ Biofilms Microbiomes 2022, 8, 11. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut microbiota-derived TMAO: A causal factor promoting atherosclerotic cardiovascular disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Bian, X.Y.; Wang, H.X.; Huang, L.; Chen, X.X. Akkermansia muciniphila postbiotic administration mitigates choline-induced plasma Trimethylamine-N-Oxide production in mice. Appl. Biol. Chem. 2024, 67, 52. [Google Scholar] [CrossRef]
- Yadav, S.; Sapra, L.; Srivastava, R.K. Polysaccharides to postbiotics: Nurturing bone health via modulating “gut-immune axis”. Int. J. Biol. Macromol. 2024, 278 Pt 2, 134655. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z. Impact of trimethylamine N-oxide (TMAO) metaorganismal pathway on cardiovascular disease. J. Lab. Precis. Med. 2020, 5, 16. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, Z.; Zhao, Y.; Gao, Y.; Zhao, L.; Li, S. Weizmannia coagulans JA845 improves atherosclerosis induced by vitamin D3 and high-fat diet in rats through modulating lipid metabolism, oxidative stress, and endothelial vascular injury. J. Appl. Microbiol. 2023, 134, lxad165. [Google Scholar] [CrossRef]
- Zhong, B.; Zhao, Y.; Gao, L.; Yang, G.; Gao, Y.; Li, F.; Li, S. Anticancer Effects of Weizmannia coagulans MZY531 postbiotics in CT26 colorectal tumor-bearing mice by regulating apoptosis and autophagy. Life 2024, 14, 1334. [Google Scholar] [CrossRef]
- Jin, L.; Dang, H.; Wu, J.; Yuan, L.; Chen, X.; Yao, J. Supplementation of Weizmannia coagulans BC2000 and ellagic acid inhibits high-fat-induced hypercholesterolemia by promoting liver primary bile acid biosynthesis and intestinal cholesterol excretion in mice. Microorganisms 2023, 11, 264. [Google Scholar] [CrossRef]
- Rafique, N.; Jan, S.Y.; Dar, A.H.; Dash, K.K.; Sarkar, A.; Shams, R.; Pandey, V.K.; Khan, S.A.; Amin, Q.A.; Hussain, S.Z. Promising bioactivities of postbiotics: A comprehensive review. J. Agric. Food Res. 2023, 14, 100708. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, S.; Di, H.; Deng, Z.; Liu, J.; Wang, H. Gut health benefit and application of postbiotics in animal production. J. Anim. Sci. Biotechnol. 2022, 13, 38. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- van den Munckhof, I.C.L.; Kurilshikov, A.; Ter Horst, R.; Riksen, N.P.; Joosten, L.A.B.; Zhernakova, A.; Fu, J.; Keating, S.T.; Netea, M.G.; de Graaf, J.; et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 1719–1734. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Mao, B.; Zhang, Q.; Tang, X.; Yang, B.; Zhao, J.; Cui, S.; Zhang, H. Postbiotics prepared using Lactobacillus paracasei CCFM1224 prevent nonalcoholic fatty liver disease by modulating the gut microbiota and liver metabolism. Int. J. Mol. Sci. 2022, 23, 13522. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe-/- mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]
- Harui, A.; Maruyama, S.; Segawa, Y.; Kurihara, N. Effect of saccharina japonica intake on blood pressure and gut microbiota composition in spontaneously hypertensive rats. Microorganisms 2024, 12, 556. [Google Scholar] [CrossRef]
- Cho, C.E.; Aardema, N.D.J.; Bunnell, M.L.; Larson, D.P.; Aguilar, S.S.; Bergeson, J.R.; Malysheva, O.V.; Caudill, M.A.; Lefevre, M. Effect of choline forms and gut microbiota composition on trimethylamine-N-oxide response in healthy men. Nutrients 2020, 12, 2220. [Google Scholar] [CrossRef]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021, 20, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Z.; Lv, Y.; Tong, L.; Liu, T.; Yi, H.; Zhou, X.; Yu, Z.; Tian, X.; Cui, Q.; et al. Reduction of intestinal trimethylamine by probiotics ameliorated lipid metabolic disorders associated with atherosclerosis. Nutrition 2020, 79–80, 110941. [Google Scholar] [CrossRef]
- Qiu, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018, 9, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, M.; Liu, Y.; Xu, M.; Shi, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Bifidobacterium breve and Bifidobacterium longum attenuate choline-induced plasma trimethylamine N-oxide production by modulating gut microbiota in mice. Nutrients 2022, 14, 1222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Li, P.L.; Lee, S.W.; Wang, Y.; Tan, C.M.; Shang, N. Weizmannia coagulans: An ideal probiotic for gut health. Food Sci. Hum. Wellness 2024, 13, 16–26. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-LETHAL inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- Zhang, S.; Meng, Y.; Zhou, L.; Qiu, L.; Wang, H.; Su, D.; Zhang, B.; Chan, K.M.; Han, J. Targeting epigenetic regulators for inflammation: Mechanisms and intervention therapy. MedComm 2022, 3, e173. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Salari, A.; Kheirkhah, J.; Ghorbani, Z. The effects of probiotics on inflammation, endothelial dysfunction, and atherosclerosis progression: A mechanistic overview. Heart Lung Circ. 2022, 31, e45–e71. [Google Scholar] [CrossRef] [PubMed]
- Hossain, E.; Li, Y.; Anand-Srivastava, M.B. Role of the JAK2/STAT3 pathway in angiotensin II-induced enhanced expression of Giα proteins and hyperproliferation of aortic vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 2021, 99, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, S.; Li, B.; Sun, A.; Zou, Y.; Ge, J. A protective role of ciglitazone in ox-LDL-induced rat microvascular endothelial cells via modulating PPARγ-dependent AMPK/eNOS pathway. J. Cell. Mol. Med. 2015, 19, 92–102. [Google Scholar] [CrossRef]
- Zhai, T.; Wang, P.; Hu, X.; Zheng, L. Probiotics bring new hope for atherosclerosis prevention and treatment. Oxidative Med. Cell. Longev. 2022, 2022, 3900835. [Google Scholar] [CrossRef]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Li, N.; Zhao, Z.; Zhao, Y.; Yang, G.; Zhao, L.; Li, S. Weizmannia coagulans JA845 Postbiotics Alleviate Atherosclerosis via TMAO-Related Gut Microbiota Regulation and JAK/STAT3 Pathway Inhibition. Nutrients 2025, 17, 3027. https://doi.org/10.3390/nu17193027
Ma L, Li N, Zhao Z, Zhao Y, Yang G, Zhao L, Li S. Weizmannia coagulans JA845 Postbiotics Alleviate Atherosclerosis via TMAO-Related Gut Microbiota Regulation and JAK/STAT3 Pathway Inhibition. Nutrients. 2025; 17(19):3027. https://doi.org/10.3390/nu17193027
Chicago/Turabian StyleMa, Liying, Nan Li, Zijian Zhao, Yujuan Zhao, Ge Yang, Lei Zhao, and Shengyu Li. 2025. "Weizmannia coagulans JA845 Postbiotics Alleviate Atherosclerosis via TMAO-Related Gut Microbiota Regulation and JAK/STAT3 Pathway Inhibition" Nutrients 17, no. 19: 3027. https://doi.org/10.3390/nu17193027
APA StyleMa, L., Li, N., Zhao, Z., Zhao, Y., Yang, G., Zhao, L., & Li, S. (2025). Weizmannia coagulans JA845 Postbiotics Alleviate Atherosclerosis via TMAO-Related Gut Microbiota Regulation and JAK/STAT3 Pathway Inhibition. Nutrients, 17(19), 3027. https://doi.org/10.3390/nu17193027





