Difficulties of Eating and Masticating Solid Food in Children with Spinal Muscular Atrophy—Preliminary Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Assessment Tools and Outcome Measures
- It includes regular, everyday foods that are naturally soft in texture and require minimal chewing.
- Foods at this level do not require modifications in particle size but should be easy to chew and swallow safely.
- It is intended for individuals who do not need texture-modified diets but may have mild chewing difficulties or fatigue.
- Foods should be moist, tender, and not hard, tough, crunchy, or dry.
2.2.1. Test of Masticating and Swallowing Solids in Children (TOMASS-C)
- The number of discrete bites required to finish the cracker.
- The number of masticatory cycles (defined as up-and-down jaw movements, excluding rotary jaw and tongue movements used to clear oral residue).
- The number of swallows (observed via the movement of the thyroid cartilage).
- The total duration of eating (measured with a digital stopwatch from the first bite to the final swallow) [25].
2.2.2. Iowa Oral Performance Instrument (IOPI)
2.3. Statistical Analysis
3. Results
3.1. Study Sample
3.2. Objective Measures TOMASS-C
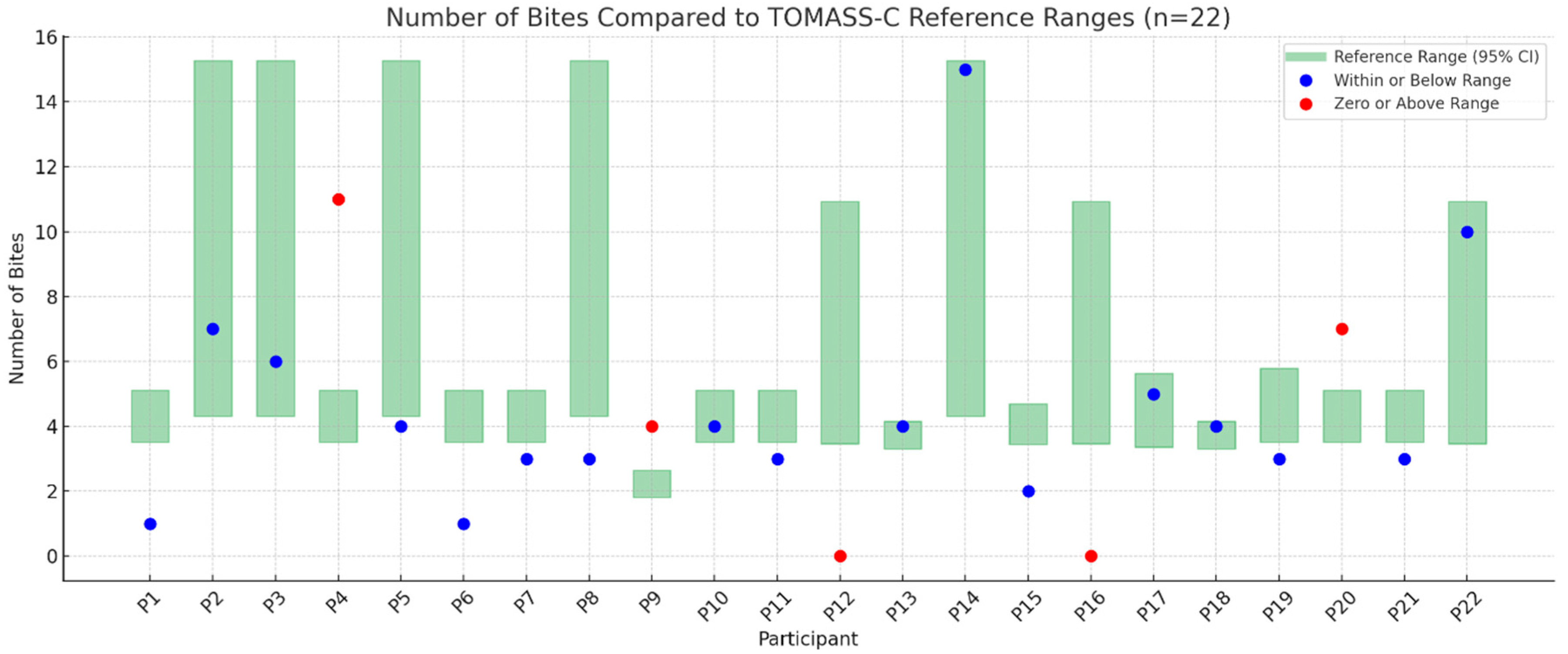
3.3. Number of Bites
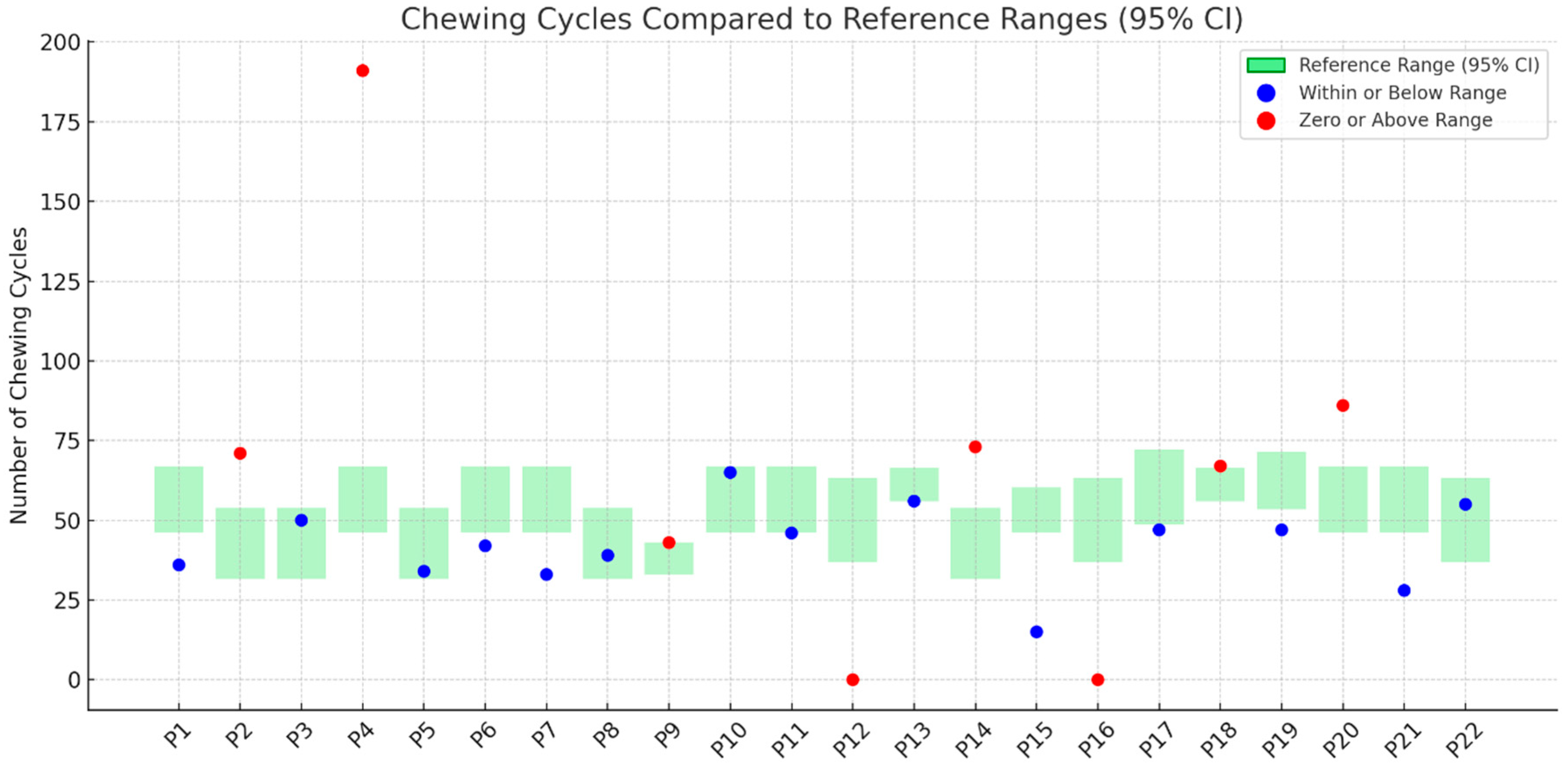
3.4. Number of Chewing Cycles
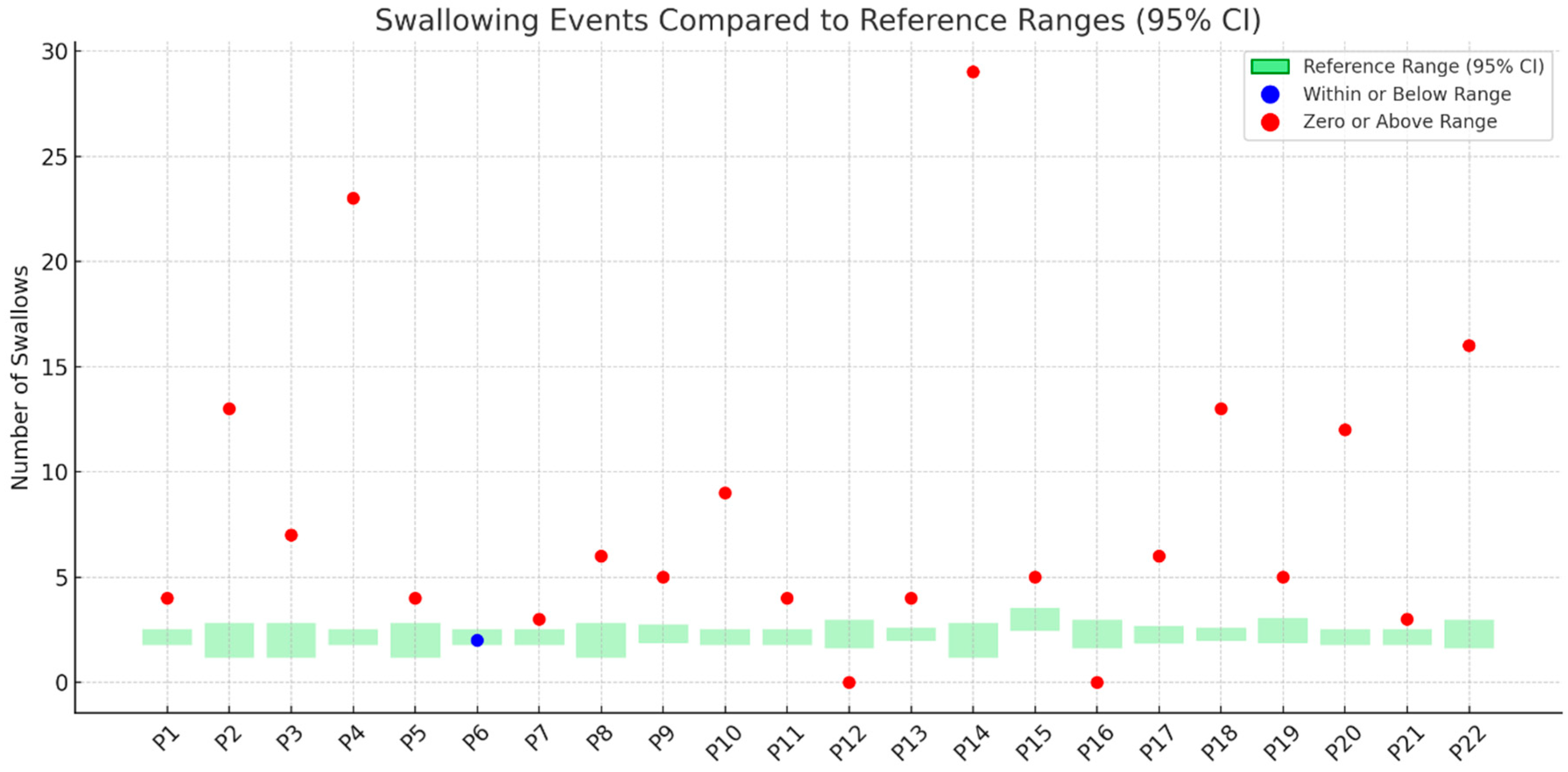
3.5. Number of Swallows
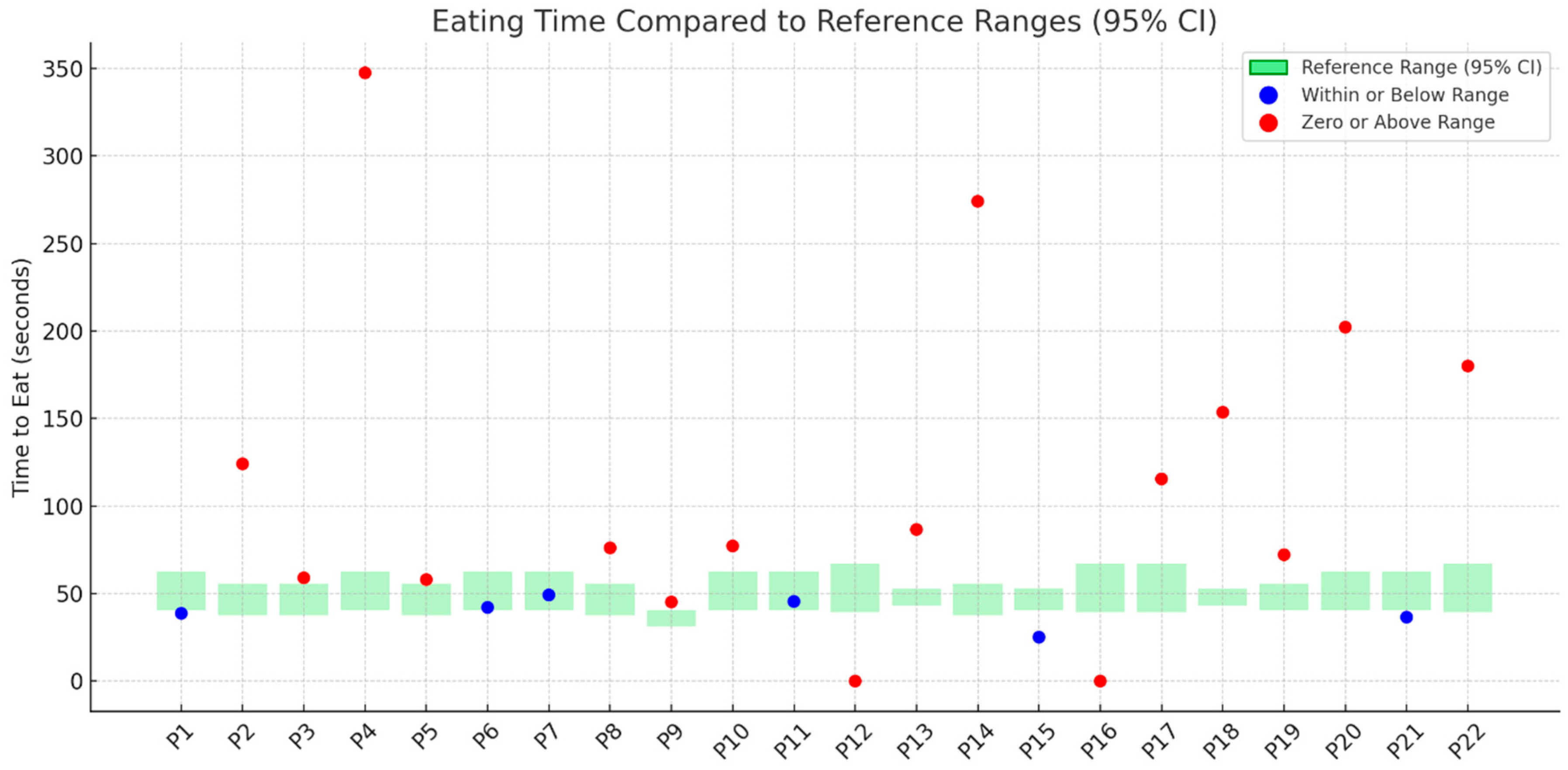
3.6. Total Eating Time
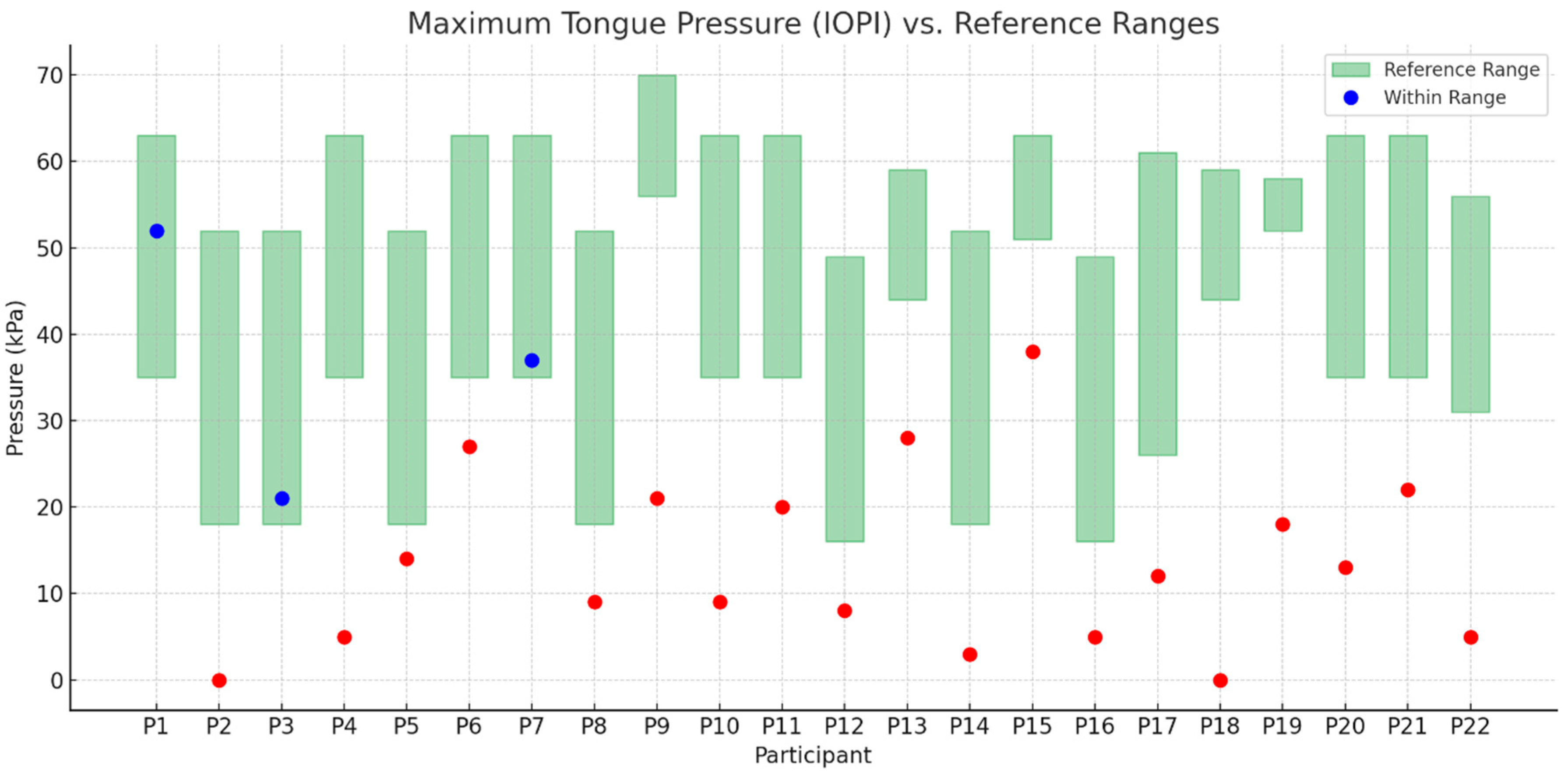
3.7. Objective Measures IOPI
- Blue dots indicate results that fall within the age- and sex-specific reference range (green shaded area).
- Red dots indicate results that fall outside the reference range, suggesting decreased or abnormal tongue muscle strength.
- The green shaded rectangles represent normative intervals based on published reference data.
3.8. Results of Statistical Analysis
- Number of bites versus tongue strength: r = −0.72; p = 0.0003;
- Number of chewing cycles versus tongue strength: r = −0.72; p = 0.0004;
- Number of swallows versus tongue strength: r = −0.83; p < 0.0001;
- Eating time (second) versus tongue strength: r = −0.81; p < 0.0001.
4. Discussion
- Tongue strength in children with SMA, as measured by IOPI, was significantly lower than in their healthy peers.
- Performance in the TOMASS-C deviated from normative values across several domains. Two participants (P12, P16) were unable to complete the test, indicating potential difficulties with task initiation or regulation. In the “number of bites” domain, 23% showed signs of feeding difficulty. For “chewing cycles”, 36% demonstrated abnormal values, with some exceeding the normative range, possibly due to inefficient mastication. Almost all participants (95%) had atypical “number of swallows”, and 73% showed prolonged “total eating time”, indicating delayed oral processing.
- Tongue strength showed a statistically significant relationship with feeding efficiency across all domains measured by TOMASS-C. A reduction in tongue strength increased the likelihood of poorer performance.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef]
- Mercuri, E.; Bertini, E.; Iannaccone, S.T. Childhood spinal muscular atrophy: Controversies and challenges. Lancet Neurol. 2012, 11, 443–452. [Google Scholar] [CrossRef]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. NMD 2018, 28, 103–115. [Google Scholar] [CrossRef]
- Wadman, R.I.; Stam, M.; Gijzen, M.; Lemmink, H.H.; Snoeck, I.N.; Wijngaarde, C.A.; Braun, K.P.J.; Schoenmakers, M.A.G.C.; van den Berg, L.H.; Dooijes, D.; et al. Association of motor milestones, SMN2 copy and outcome in spinal muscular atrophy types 0-4. J. Neurol. Neurosurg. Psychiatry 2017, 88, 365–367. [Google Scholar] [CrossRef]
- McGrattan, K.E.; Graham, R.J.; Mohr, A.H.; Miles, A.; Allen, J.; Ochura, J.; Hernandez, K.; Walsh, K.; Rao, V.; Stevens, M.; et al. Characterization of swallowing biomechanics and function in untreated infants with spinal muscular atrophy: A natural history dataset. J. Neuromuscul. Dis. 2025, 12, 22143602241308762. [Google Scholar] [CrossRef]
- Martí, Y.; Aponte Ribero, V.; Batson, S.; Mitchell, S.; Gorni, K.; Gusset, N.; Oskoui, M.; Servais, L.; Deconinck, N.; McGrattan, K.E.; et al. A Systematic Literature Review of the Natural History of Respiratory, Swallowing, Feeding, and Speech Functions in Spinal Muscular Atrophy (SMA). J. Neuromuscul. Dis. 2024, 11, 889–904. [Google Scholar] [CrossRef]
- Finkel, R.S.; McDermott, M.P.; Kaufmann, P.; Darras, B.T.; Chung, W.K.; Sproule, D.M.; Kang, P.B.; Foley, A.R.; Yang, M.L.; Martens, W.B.; et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology 2014, 83, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-A.; Suh, D.I.; Chae, J.-H.; Shin, H.-I. Trajectory of change in the swallowing status in spinal muscular atrophy type I. Int. J. Pediatr. Otorhinolaryngol. 2020, 130, 109818. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, K.E.; Graham, R.J.; DiDonato, C.J.; Darras, B.T. Dysphagia Phenotypes in Spinal Muscular Atrophy: The Past, Present, and Promise for the Future. Am. J. Speech-Lang. Pathol. 2021, 30, 1008–1022. [Google Scholar] [CrossRef]
- van der Heul, A.M.B.; Wijngaarde, C.A.; Wadman, R.I.; Asselman, F.; van den Aardweg, M.T.A.; Bartels, B.; Cuppen, I.; Gerrits, E.; van den Berg, L.H.; van der Pol, W.L.; et al. Bulbar Problems Self-Reported by Children and Adults with Spinal Muscular Atrophy. J. Neuromuscul. Dis. 2019, 6, 361–368. [Google Scholar] [CrossRef] [PubMed]
- van der Heul, A.M.B.; Cuppen, I.; Wadman, R.I.; Asselman, F.; Schoenmakers, M.A.G.C.; van de Woude, D.R.; Gerrits, E.; van der Pol, W.L.; van den Engel-Hoek, L. Feeding and Swallowing Problems in Infants with Spinal Muscular Atrophy Type 1: An Observational Study. J. Neuromuscul. Dis. 2020, 7, 323–330. [Google Scholar] [CrossRef]
- Messina, S.; Pane, M.; De Rose, P.; Vasta, I.; Sorleti, D.; Aloysius, A.; Sciarra, F.; Mangiola, F.; Kinali, M.; Bertini, E.; et al. Feeding problems and malnutrition in spinal muscular atrophy type II. Neuromuscul. Disord. NMD 2008, 18, 389–393. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Shih, H.-H.; Chen, T.-H.; Kuo, C.-H.; Jong, Y.-J. Prevalence and risk factors for feeding and swallowing difficulties in spinal muscular atrophy types II and III. J. Pediatr. 2012, 160, 447–451.e1. [Google Scholar] [CrossRef] [PubMed]
- Wadman, R.I.; De Amicis, R.; Brusa, C.; Battezzati, A.; Bertoli, S.; Davis, T.; Main, M.; Manzur, A.; Mastella, C.; Munot, P.; et al. Feeding difficulties in children and adolescents with spinal muscular atrophy type 2. Neuromuscul. Disord. NMD 2021, 31, 101–112. [Google Scholar] [CrossRef]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Yamashita, M.; Rigo, F.; Hung, G.; Schneider, E.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study. Lancet 2016, 388, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Baranello, G.; Darras, B.T.; Day, J.W.; Deconinck, N.; Klein, A.; Masson, R.; Mercuri, E.; Rose, K.; El-Khairi, M.; Gerber, M.; et al. Risdiplam in Type 1 Spinal Muscular Atrophy. N. Engl. J. Med. 2021, 384, 915–923. [Google Scholar] [CrossRef]
- Shell, R.D.; McGrattan, K.E.; Hurst-Davis, R.; Young, S.D.; Baranello, G.; Lavrov, A.; O’Brien, E.; Wallach, S.; LaMarca, N.; Reyna, S.P.; et al. Onasemnogene abeparvovec preserves bulbar function in infants with presymptomatic spinal muscular atrophy: A post-hoc analysis of the SPR1NT trial. Neuromuscul. Disord. NMD 2023, 33, 670–676. [Google Scholar] [CrossRef]
- Leon-Astudillo, C.; Brooks, O.; Salabarria, S.M.; Coker, M.; Corti, M.; Lammers, J.; Plowman, E.K.; Byrne, B.J.; Smith, B.K. Longitudinal changes of swallowing safety and efficiency in infants with spinal muscular atrophy who received disease modifying therapies. Pediatr. Pulmonol. 2024, 59, 1364–1371. [Google Scholar] [CrossRef]
- Ruggiero, M.; Giannotta, G.; Pirani, G.; Saponaro, F.; Oliva, M.C.; Ferrante, C.; Trabacca, A. Swallowing function in patients with spinal muscular atrophy before and after the introduction of new gene-based therapies: What has changed? Neurol. Sci. 2025, 46, 1137–1149. [Google Scholar] [CrossRef]
- McGrattan, K.E.; Shell, R.D.; Hurst-Davis, R.; Young, S.D.; O’Brien, E.; Lavrov, A.; Wallach, S.; LaMarca, N.; Reyna, S.P.; Darras, B.T. Patients with Spinal Muscular Atrophy Type 1 Achieve and Maintain Bulbar Function Following Onasemnogene Abeparvovec Treatment. J. Neuromuscul. Dis. 2023, 10, 531–540. [Google Scholar] [CrossRef]
- Giess, D.; Erdos, J.; Wild, C. An updated systematic review on spinal muscular atrophy patients treated with nusinersen, onasemnogene abeparvovec (at least 24 months), risdiplam (at least 12 months) or combination therapies. Eur. J. Paediatr. Neurol. 2024, 51, 84–92. [Google Scholar] [CrossRef]
- Winnicka, E.; Łabuz, A.; Grochowski, T.; Kułaga, Z.; Socha, P. Difficulties of Eating and Masticating Solid Food in Children with Spinal Muscular Atrophy—Preliminary Study. In Proceedings of the 57th Annual Meeting of the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition, Helsinki, Finland, 14–17 May 2025. [Google Scholar]
- Level 7 Regular Easy to Chew for Adults. Available online: https://www.iddsi.org/images/Publications-Resources/PatientHandouts/English/Adults/7_easy_to_chew_adult_consumer_handout_30jan2019.pdf (accessed on 5 July 2025).
- Frank, U.; van den Engel-Hoek, L.; Nogueira, D.; Schindler, A.; Adams, S.; Curry, M.; Huckabee, M.-L. International standardisation of the test of masticating and swallowing solids in children. J. Oral Rehabil. 2019, 46, 161–169. [Google Scholar] [CrossRef]
- Adams, V.; Mathisen, B.; Baines, S.; Lazarus, C.; Callister, R. A systematic review and meta-analysis of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument (IOPI). Dysphagia 2013, 28, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Potter, N.L.; Short, R. Maximal tongue strength in typically developing children and adolescents. Dysphagia 2009, 24, 391–397. [Google Scholar] [CrossRef]
- Colot, C.; Benmechri, S.; Everaert, E.; Muys, S.; Van Himme, L.; Tahon, V.; Salmon, M.; Van Dyck, D.; De Vos, E.; Deconinck, N. Assessing the Swallowing Function in Children with Spinal Muscular Atrophy: An Easily Accessible and Objective Multidimensional Approach. J. Neuromuscul. Dis. 2024, 11, 839–853. [Google Scholar] [CrossRef]
- Trucco, F.; Salmin, F.; Lizio, A.; Coratti, G.; Albamonte, E.; Frisoni, M.C.; Mauro, L.; Carraro, E.; Palazzo, G.; Lops, J.; et al. Assessing Prevalence and Characteristics of Oro-bulbar Involvement in Children and Adults with SMA Type 2 and 3 Using a Multimodal Approach. Dysphagia 2023, 38, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- van der Heul, A.M.B.; van Eijk, R.P.A.; Wadman, R.I.; Asselman, F.; Cuppen, I.; Nievelstein, R.A.J.; Gerrits, E.; van der Pol, W.L.; van den Engel-Hoek, L. Mastication in Patients with Spinal Muscular Atrophy Types 2 and 3 is Characterized by Abnormal Efficiency, Reduced Endurance, and Fatigue. Dysphagia 2022, 37, 715–723. [Google Scholar] [CrossRef]
- van der Heul, A.M.B.; Nievelstein, R.A.J.; van Eijk, R.P.A.; Asselman, F.; Erasmus, C.E.; Cuppen, I.; Bittermann, A.J.N.; Gerrits, E.; van der Pol, W.L.; van den Engel-Hoek, L. Swallowing Problems in Spinal Muscular Atrophy Types 2 and 3: A Clinical, Videofluoroscopic and Ultrasound Study. J. Neuromuscul. Dis. 2023, 10, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Mano, T.; Katsuno, M.; Banno, H.; Suzuki, K.; Suga, N.; Hashizume, A.; Araki, A.; Watanabe, H.; Tanaka, S.; Yamamoto, M.; et al. Tongue pressure as a novel biomarker of spinal and bulbar muscular atrophy. Neurology 2014, 82, 255–262. [Google Scholar] [CrossRef]
- Umemoto, G.; Fujioka, S.; Arahata, H.; Kawazoe, M.; Sakae, N.; Sasagasako, N.; Furuya, H.; Tsuboi, Y. Relationship between tongue pressure and functional oral intake scale diet type in patients with neurological and neuromuscular disorders. Clin. Neurol. Neurosurg. 2020, 198, 106196. [Google Scholar] [CrossRef] [PubMed]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmüller, H. Prevalence, incidence and carrier frequency of 5q–linked spinal muscular atrophy—A literature review. Orphanet J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef]
| Participant | Age (Months) | SMA Type | Treatment | Ventilatory Support | Feeding Mode |
|---|---|---|---|---|---|
| P1 | 87 | 3 | risdiplam, nusinersen | none | oral |
| P2 | 51 | 1 | nusinersen, onasemnogen | NIV 7 h | oral |
| P3 | 60 | 1 | branaplam, onasemnogen | none | oral |
| P4 | 80 | 1 | nusinersen | NIV 9 h | oral |
| P5 | 48 | 1 | nusinersen, onasemnogen | NIV 6 h | oral |
| P6 | 92 | 1 | nusinersen | NIV 8 h | oral |
| P7 | 94 | 1 | nusinersen | none | oral |
| P8 | 54 | 2 | nusinersen | none | oral |
| P9 | 190 | 1 | risdiplam | NIV 5 h | oral |
| P10 | 88 | 1 | risdiplam | none | oral |
| P11 | 76 | 2 | nusinersen | none | oral |
| P12 | 57 | 1 | nusinersen, onasemnogen, risdiplam | NIV 4 h | oral |
| P13 | 116 | 1 | nusinersen | NIV 8 h | oral |
| P14 | 57 | 1 | risdiplam, onasemnogen | none | >50% enteral |
| P15 | 120 | 3 | nusinersen | none | oral |
| P16 | 49 | 1 | nusinersen, onasemnogen | NIV 11 h | oral |
| P17 | 77 | 1 | branaplam, onasemnogen, nusinersen | NIV 12 h | oral |
| P18 | 109 | 1 | nusinersen | none | >50% enteral |
| P19 | 112 | 1 | nusinersen | none | oral |
| P20 | 82 | 1 | nusinersen, onasemnogen | NIV 8–10 h | >50% enteral |
| P21 | 76 | 2 | nusinersen | NIV 8 h | oral |
| P22 | 67 | 1 | nusinersen | none | oral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winnicka, E.; Łabuz, A.; Kułaga, Z.; Grochowski, T.; Socha, P. Difficulties of Eating and Masticating Solid Food in Children with Spinal Muscular Atrophy—Preliminary Study. Nutrients 2025, 17, 2561. https://doi.org/10.3390/nu17152561
Winnicka E, Łabuz A, Kułaga Z, Grochowski T, Socha P. Difficulties of Eating and Masticating Solid Food in Children with Spinal Muscular Atrophy—Preliminary Study. Nutrients. 2025; 17(15):2561. https://doi.org/10.3390/nu17152561
Chicago/Turabian StyleWinnicka, Ewa, Adrianna Łabuz, Zbigniew Kułaga, Tomasz Grochowski, and Piotr Socha. 2025. "Difficulties of Eating and Masticating Solid Food in Children with Spinal Muscular Atrophy—Preliminary Study" Nutrients 17, no. 15: 2561. https://doi.org/10.3390/nu17152561
APA StyleWinnicka, E., Łabuz, A., Kułaga, Z., Grochowski, T., & Socha, P. (2025). Difficulties of Eating and Masticating Solid Food in Children with Spinal Muscular Atrophy—Preliminary Study. Nutrients, 17(15), 2561. https://doi.org/10.3390/nu17152561







