Resveratrol as a Therapeutic Agent in Alzheimer’s Disease: Evidence from Clinical Studies
Abstract
1. Introduction
1.1. Challenges with the Currently Available Therapies
1.2. BBB Limits Drug Delivery
1.3. Need for Precision Medicine
1.4. Role of Natural Compounds in Neuroprotection
1.5. Resveratrol (RSV)
1.6. Pharmacokinetics and Bioavailability
2. Neuroprotective Mechanisms of RSV
2.1. Anti-Inflammatory and Antioxidant Properties
2.2. Modulation of Amyloid-Beta (Aβ) Accumulation
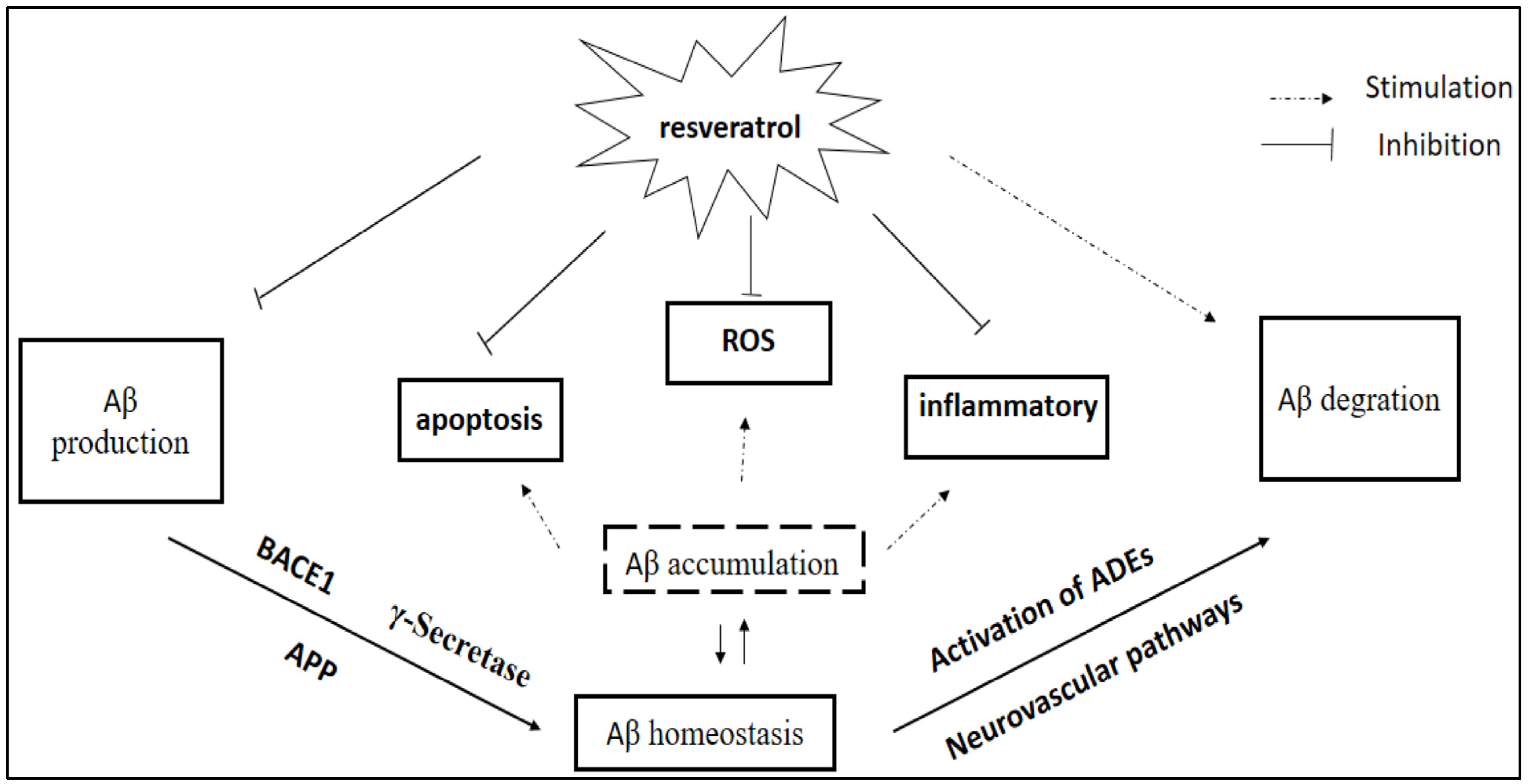
2.3. Reduction in Tau Protein Hyperphosphorylation
2.4. Influence on Key Signaling Pathways
3. RSV in Preclinical and Clinical Studies for AD
4. Future Perspectives and Research Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, M. World Alzheimer Report 2015: The Global Impact of Dementia An AnAlysIs of Prevalence, Incidence, Cost and Trends. Ph.D. Thesis, Alzheimer’s Disease International, London, UK, 2015. [Google Scholar]
- Inouye, K.; Pedrazzani, E.S.; Pavarini, S.C.I. Alzheimer’s disease influence on the perception of quality of life from the elderly people. Rev. Da Esc. Enferm. Da USP 2010, 44, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; Mercken, M.; Strooper, B.D. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Selkoe, D.J. Clearing the brain’s amyloid cobwebs. Neuron 2001, 32, 177–180. [Google Scholar] [CrossRef]
- Harrison, S.L.; Lang, C.; Whitehead, C.; Crotty, M.; Ratcliffe, J.; Wesselingh, S.; Inacio, C. Trends in Prevalence of Dementia for People Accessing Aged Care Services in Australia. J. Gerontol. Ser. A 2020, 75, 318–325. [Google Scholar] [CrossRef]
- Matthews, F.E.; Stephan, B.C.M.; Robinson, L.; Jagger, C.; Barnes, L.E.; Arthur, A.; Brayne, C. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat. Commun. 2016, 7, 11398. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef]
- Zheng, Y.; Bonfili, L.; Wei, T.; Eleuteri, A.M. Understanding the Gut–Brain Axis and Its Therapeutic Implications for Neurodegenerative Disorders. Nutrients 2023, 15, 4631. [Google Scholar] [CrossRef]
- Bekris, L.M.; Yu, C.E.; Bird, T.D.; Tsuang, D.W. Genetics of Alzheimer Disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213. [Google Scholar] [CrossRef] [PubMed]
- Bagaria, J.; Bagyinszky, E.; An, S.S.A. Genetics, Functions, and Clinical Impact of Presenilin-1 (PSEN1) Gene. Int. J. Mol. Sci. 2022, 23, 10970. [Google Scholar] [CrossRef] [PubMed]
- Zekanowski, C.; Styczyńska, M.; Pepłońska, B.; Gabryelewicz, T.; Religa, D.; Ilkowski, J.; Kijanowska-Haładyna, B.; Kotapka-Minc, S.; Mikkelsen, S.; Pfeffer, A.; et al. Mutations in presenilin 1, presenilin 2 and amyloid precursor protein genes in patients with early-onset Alzheimer’s disease in Poland. Exp. Neurol. 2003, 184, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Pinals, R.L.; Tsai, L.H. Building in vitro models of the brain to understand the role of APOE in Alzheimer’s disease. Life Sci. Alliance 2022, 5, e202201542, Erratum in Life Sci. Alliance 2022, 6, e202201845. https://doi.org/10.26508/lsa.202201845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanekiyo, T.; Xu, H.; Bu, G. ApoE and Aβ in Alzheimer’s disease: Accidental encounters or partners? Neuron 2014, 81, 740–754. [Google Scholar] [CrossRef]
- Pires, M.; Rego, A.C. Apoe4 and Alzheimer’s Disease Pathogenesis—Mitochondrial Deregulation and Targeted Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 778. [Google Scholar] [CrossRef]
- Liu, E.; Zhang, Y.; Wang, J.Z. Updates in Alzheimer’s disease: From basic research to diagnosis and therapies. Transl. Neurodegener. 2024, 13, 45. [Google Scholar] [CrossRef]
- Tosto, G.; Reitz, C. Genome-wide Association Studies in Alzheimer’s Disease: A Review. Curr. Neurol. Neurosci. Rep. 2013, 13, 381. [Google Scholar] [CrossRef]
- Shen, L.; Jia, J. An Overview of Genome-Wide Association Studies in Alzheimer’s Disease. Neurosci. Bull. 2016, 32, 183. [Google Scholar] [CrossRef]
- Aisen, P.S.; Cummings, J.; Jack, C.R.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimers Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.D.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.; Kucharska, E.; Garcez, M.L.; Rodrigues, M.S.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells 2021, 10, 2581. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- D’alessandro, M.C.B.; Kanaan, S.; Geller, M.; Praticò, D.; Daher, J.P.L. Mitochondrial Dysfunction in Alzheimer’s Disease. Ageing Res. Rev. 2025, 107, 102713. [Google Scholar] [CrossRef] [PubMed]
- Dhapola, R.; Sharma, P.; Kumari, S.; Bhatti, J.S.; HariKrishnaReddy, D. Environmental Toxins and Alzheimer’s Disease: A Comprehensive Analysis of Pathogenic Mechanisms and Therapeutic Modulation. Mol. Neurobiol. 2024, 61, 3657–3677. [Google Scholar] [CrossRef]
- Suresh, S.; Singh, S.A.; Rushendran, R.; Vellapandian, C.; Prajapati, B. Alzheimer’s disease: The role of extrinsic factors in its development, an investigation of the environmental enigma. Front. Neurol. 2023, 14, 1303111. [Google Scholar] [CrossRef]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43. [Google Scholar] [CrossRef]
- Puranik, N.; Song, M. Therapeutic Role of Heterocyclic Compounds in Neurodegenerative Diseases: Insights from Alzheimer’s and Parkinson’s Diseases. Neurol. Int. 2025, 17, 26. [Google Scholar] [CrossRef]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Iwatsubo, T. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 142–143. [Google Scholar] [CrossRef]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef]
- Robles, A. Pharmacological Treatment of Alzheimer’s Disease: Is. it Progressing Adequately? Open Neurol. J. 2009, 3, 27. [Google Scholar] [CrossRef]
- Hensley, K. Neuroinflammation in Alzheimer’s Disease: Mechanisms, Pathologic Consequences, and Potential for Therapeutic Manipulation. J. Alzheimers Dis. 2010, 21, 1. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.B.; Roberts, N.G.; Perez, J.P.; Connolly, N.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177. [Google Scholar] [CrossRef]
- Li, J.; Zheng, M.; Shimoni, O.; Banks, W.A.; Bush, A.I.; Gamble, J.R.; Shi, B. Development of Novel Therapeutics Targeting the Blood–Brain Barrier: From Barrier to Carrier. Adv. Sci. 2021, 8, 2101090. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Brockmann, R.; Nixon, J.; Love, B.L.; Yunusa, I. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer’s disease: Patient outcomes, healthcare costs, and drug development. Lancet Reg. Health-Am. 2023, 20, 100467. [Google Scholar] [CrossRef]
- Ramanan, V.K.; Armstrong, M.J.; Choudhury, P.; Coerver, K.A.; Hamilton, R.H.; Klein, B.C.; Wolk, D.A.; Wessels, S.R.; Jones, L.K., Jr. Antiamyloid Monoclonal Antibody Therapy for Alzheimer Disease: Emerging Issues in Neurology. Neurology 2023, 101, 842–852. [Google Scholar] [CrossRef]
- Dyer, O. Aduhelm: Biogen abandons Alzheimer’s drug after controversial approval left it unfunded by Medicare. BMJ Br. Med. J. 2024, 384, q281. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, S.; Nam, Y.; Park, Y.H.; Shin, S.M.; Moon, M. Second-generation anti-amyloid monoclonal antibodies for Alzheimer’s disease: Current landscape and future perspectives. Transl. Neurodegener. 2025, 14, 6. [Google Scholar] [CrossRef]
- Pardridge, W.M. Alzheimer’s disease drug development and the problem of the blood-brain barrier. Alzheimer’s Dement. 2009, 5, 427–432. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef]
- Pardridge, W.M. Treatment of Alzheimer’s Disease and Blood-Brain Barrier Drug Delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Han, J.; Wei, W.; Chen, F. Blood-brain barrier dysfunction and Alzheimer’s disease: Associations, pathogenic mechanisms, and therapeutic potential. Front. Aging Neurosci. 2023, 15, 1258640. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Forloni, G. Alzheimer’s disease: From basic science to precision medicine approach. BMJ Neurol. Open 2020, 2, e000079. [Google Scholar] [CrossRef]
- Arafah, A.; Khatoon, S.; Rasool, I.; Khan, A.; Rather, M.A.; Abujabal, K.A.; Faqih, Y.A.H.; Rashid, H.; Rashid, S.M.; Bilal Ahmad, S.; et al. The Future of Precision Medicine in the Cure of Alzheimer’s Disease. Biomedicines 2023, 11, 335. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Albarrati, A.; Albratty, M.; Najmi, A.; Meraya, A.M.; Bungau, S. The road to precision medicine: Eliminating the One Size Fits All approach in Alzheimer’s disease. Biomed. Pharmacother. 2022, 153, 113337. [Google Scholar] [CrossRef]
- Di Meco, A.; Vassar, R. Early detection and personalized medicine: Future strategies against Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2020, 177, 157. [Google Scholar] [CrossRef]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid. Based Complement. Altern. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef]
- Tavan, M.; Hanachi, P.; de la Luz Cádiz-Gurrea, M.; Segura Carretero, A.; Mirjalili, M.H. Natural Phenolic Compounds with Neuroprotective Effects. Neurochem. Res. 2023, 49, 306–326. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.F.; Ramalho, T.C. Future Therapeutic Perspectives into the Alzheimer’s Disease Targeting the Oxidative Stress Hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef]
- Samanta, S.; Chakraborty, S.; Bagchi, D. Pathogenesis of Neurodegenerative Diseases and the Protective Role of Natural Bioactive Components. J. Am. Nutr. Assoc. 2024, 43, 20–32. [Google Scholar] [CrossRef]
- Lim, D.W.; Lee, J.E.; Lee, C.; Kim, Y.T. Natural Products and Their Neuroprotective Effects in Degenerative Brain Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 11223. [Google Scholar] [CrossRef]
- Dziedziński, M.; Kobus-Cisowska, J.; Stachowiak, B. Pinus Species as Prospective Reserves of Bioactive Compounds with Potential Use in Functional Food—Current State of Knowledge. Plants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural basis for antioxidant activity of trans-resveratrol: Ab initio calculations and crystal and molecular structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Nowacka, A.; Śniegocka, M.; Smuczyński, W.; Liss, S.; Ziółkowska, E.; Bożiłow, D.; Śniegocki, M.; Wiciński, M. The Potential Application of Resveratrol and Its Derivatives in Central Nervous System Tumors. Int. J. Mol. Sci. 2024, 25, 13338. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, Y.; Nakata, R.; Fukuhara, K.; Yamashita, H.; Kubodera, H.; Inoue, H. The 4′-Hydroxyl Group of Resveratrol Is Functionally Important for Direct Activation of PPARα. PLoS ONE 2015, 10, e0120865. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Lee, W.-C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 62, 1800066. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Kiselev, K.V. Perspectives for production and application of resveratrol. Appl. Microbiol. Biotechnol. 2011, 90, 417–425. [Google Scholar] [CrossRef]
- Ngo, T.H.; Lee, Y.J.; Choi, H.; Song, K.S.; Lee, K.J.; Nam, J.W. Evaluating the anticancer potential of Polygonum multiflorum root-derived stilbenes against H2452 malignant pleural mesothelioma cells. Fitoterapia 2024, 177, 106135. [Google Scholar] [CrossRef]
- Cha, J.; Yun, J.H.; Choi, J.H.; Lee, J.H.; Choi, B.T.; Shin, H.K. Preclinical Evidence and Underlying Mechanisms of Polygonum multiflorum and Its Chemical Constituents Against Cognitive Impairments and Alzheimer’s Disease. J. Pharmacopunct. 2024, 27, 70. [Google Scholar] [CrossRef]
- Hasan, M.M.; Cha, M.; Bajpai, V.K.; Baek, K.H. Production of a major stilbene phytoalexin, resveratrol in peanut (Arachis hypogaea) and peanut products: A mini review. Rev. Environ. Sci. Biotechnol. 2013, 12, 209–221. [Google Scholar] [CrossRef]
- Sales, J.M.; Resurreccion, A.V.A. Maximising resveratrol and piceid contents in UV and ultrasound treated peanuts. Food Chem. 2009, 117, 674–680. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Gu, T.; Yan, Y.; Guo, J.; Zhang, X.; Pang, H.; Chen, J. The Metabolic Characteristics and Bioavailability of Resveratrol Based on Metabolic Enzymes. Nutr. Rev. 2024, 18, 749–770. [Google Scholar] [CrossRef]
- Resveratrol, D.; Liu, J.; Shi, D.; Morkovin, E.; Litvinov, R.; Koushner, A.; Babkov, D. Resveratrol and Extra Virgin Olive Oil: Protective Agents Against Age-Related Disease. Nutrients 2024, 16, 4258. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Kaddoumi, A. Extra-Virgin Olive Oil in Alzheimer’s Disease: A Comprehensive Review of Cellular, Animal, and Clinical Studies. Int. J. Mol. Sci. 2024, 25, 1914. [Google Scholar] [CrossRef] [PubMed]
- Bartra, C.; Yuan, Y.; Vuraić, K.; Valdés-Quiroz, H.; Garcia-Baucells, P.; Slevin, M.; Pastorello, Y.; Suñol, C.; Sanfeliu, C. Resveratrol Activates Antioxidant Protective Mechanisms in Cellular Models of Alzheimer’s Disease Inflammation. Antioxidants 2024, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Quadros Gomes, B.A.; Bastos Silva, J.P.; Rodrigues Romeiro, C.F.; dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Tamaki, N.; Cristina Orihuela-Campos, R.; Inagaki, Y.; Fukui, M.; Nagata, T.; Ito, H.O. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic. Biol. Med. 2014, 75, 222–229. [Google Scholar] [CrossRef]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and Neuroprotection: Impact and Its Therapeutic Potential in Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 619024. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef]
- Ma, T.; Tan, M.S.; Yu, J.T.; Tan, L. Resveratrol as a Therapeutic Agent for Alzheimer’s Disease. BioMed Res. Int. 2014, 2014, 350516. [Google Scholar] [CrossRef]
- Ge, J.F.; Qiao, J.P.; Qi, C.C.; Wang, C.W.; Zhou, J.N. The binding of resveratrol to monomer and fibril amyloid beta. Neurochem. Int. 2012, 61, 1192–1201. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef]
- Deng, H.; Mi, M.T. Resveratrol Attenuates Aβ25-35 Caused Neurotoxicity by Inducing Autophagy Through the TyrRS-PARP1-SIRT1 Signaling Pathway. Neurochem. Res. 2016, 41, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, N.; Liu, X. Resveratrol and Amyloid-Beta: Mechanistic Insights. Nutrients 2017, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Alfaifi, M.Y. Trans-resveratrol Inhibits Tau Phosphorylation in the Brains of Control and Cadmium Chloride-Treated Rats by Activating PP2A and PI3K/Akt Induced-Inhibition of GSK3β. Neurochem. Res. 2019, 44, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, S.; Matthes, F.; Posey, K.; Kickstein, E.; Weber, S.; Hettich, M.M.; Pfurtscheller, S.; Ehninger, D.; Schneider, R.; Krauß, S. Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Sci. Rep. 2017, 7, 13753. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Rizak, J.D.; Wu, S.; Wang, Z.; He, R.; Su, M.; Qin, D.; Wang, J.; Hu, X. Resveratrol Attenuates Formaldehyde Induced Hyperphosphorylation of Tau Protein and Cytotoxicity in N2a Cells. Front. Neurosci. 2017, 10, 598. [Google Scholar] [CrossRef]
- Surya, K.; Manickam, N.; Jayachandran, K.S.; Kandasamy, M.; Anusuyadevi, M. Resveratrol Mediated Regulation of Hippocampal Neuroregenerative Plasticity via SIRT1 Pathway in Synergy with Wnt Signaling: Neurotherapeutic Implications to Mitigate Memory Loss in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 94, S125–S140. [Google Scholar] [CrossRef]
- Jin, S.; Guan, X.; Min, D. Evidence of Clinical Efficacy and Pharmacological Mechanisms of Resveratrol in the Treatment of Alzheimer’s Disease. Curr. Alzheimer Res. 2023, 20, 588–602. [Google Scholar] [CrossRef]
- Feng, X.; Liang, N.; Zhu, D.; Gao, Q.; Peng, L.; Dong, H.; Yue, Q.; Liu, H.; Bao, L.; Zhang, J.; et al. Resveratrol inhibits β-amyloid-induced neuronal apoptosis through regulation of SIRT1-ROCK1 signaling pathway. PLoS ONE 2013, 8, e59888. [Google Scholar] [CrossRef]
- Pan, L.F.; Wang, X.B.; Xie, S.S.; Li, S.Y.; Kong, L.Y. Multitarget-directed resveratrol derivatives: Anti-cholinesterases, anti-β-amyloid aggregation and monoamine oxidase inhibition properties against Alzheimer’s disease. Medchemcomm 2014, 5, 609–616. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamilanban, T.; Kumarasamy, V.; Sekar, M.; Subramaniyan, V.; Wong, L.S. Design, Synthesis, and Invitro Pharmacological Evaluation of Novel Resveratrol Surrogate Molecules against Alzheimer’s Disease. Chem. Biodivers. 2024, 21, e202401430. [Google Scholar] [CrossRef]
- Martínez, A. Synthesis, in vitro activity, and molecular docking of caffeic acid and resveratrol derivatives against Alzheimer’s disease-related enzymes. Med. Chem. Res. 2024, 33, 1681–1697. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, C.; Zhang, L.; Sui, R. Novel combination of Olesoxime/Resveratrol-encapsulated exosomes to improve cognitive function by targeting amyloid β-induced Alzheimer’s disease: Investigation on in vitro and in vivo model. Inflammopharmacology 2024, 32, 2613–2628. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; Van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Islam, F.; Nafady, M.H.; Islam, M.R.; Saha, S.; Rashid, S.; Akter, A.; Or-Rashid, M.H.; Akhtar, M.F.; Perveen, A.; Md Ashraf, G.; et al. Resveratrol and neuroprotection: An insight into prospective therapeutic approaches against Alzheimer’s disease from bench to bedside. Mol. Neurobiol. 2022, 59, 4384–4404. [Google Scholar] [CrossRef]
- Villaflores, O.B.; Chen, Y.J.; Chen, C.P.; Yeh, J.M.; Wu, T.Y. Effects of curcumin and demethoxycurcumin on amyloid-β precursor and tau proteins through the internal ribosome entry sites: A potential therapeutic for Alzheimer’s disease. Taiwan. J. Obstet. Gynecol. 2012, 51, 554–564. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.Y.; Liu, H.; Lu, Y.F.; Wu, Q.; Liu, J.; Shi, J.S. Resveratrol Produces Neurotrophic Effects on Cultured Dopaminergic Neurons through Prompting Astroglial BDNF and GDNF Release. Evid.-Based Complement. Altern. Med. 2012, 2012, 937605. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.P.; Yang, S.G.; Wang, Y.J.; Zhang, X.; Du, X.T.; Sun, X.X.; Zhao, M.; Huang, L.; Liu, R.T. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 2009, 30, 986–995. [Google Scholar] [CrossRef]
- Albani, D.; Polito, L.; Batelli, S.; De Mauro, S.; Fracasso, C.; Martelli, G.; Colombo, L.; Manzoni, C.; Salmona, M.; Caccia, S.; et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by α-synuclein or amyloid-β (1-42) peptide. J. Neurochem. 2009, 110, 1445–1456. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; de Oliveira, A.C.P.; Gräf, S.; Bhatia, H.S.; Hüll, M.; Muñoz, E.; Fiebich, B.L. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J. Neuroinflammation 2007, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.L.; Ganaraja, B.; Suresh, P.K.; Joy, T.; Ullal, S.D.; Manjrekar, P.A.; Murlimanju, B.V.; Sharma, B.G.; Massand, A.; Agrawal, A. Outcome of resveratrol and resveratrol with donepezil combination on the β-amyloid plaques and neurofibrillary tangles in Alzheimer’s disease. 3 Biotech 2024, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Manandhar, S.; Harish Kumar, B.; Famurewa, A.C.; Gurram, P.C.; Suggala, R.S.; Sankhe, R.; Mudgal, J.; Pai, K.S.R. Oxyresveratrol-β-cyclodextrin mitigates streptozotocin-induced Alzheimer’s model cognitive impairment, histone deacetylase activity in rats: In silico & in vivo studies. Sci. Rep. 2024, 14, 9897. [Google Scholar] [CrossRef]
- Shamsher, E.; Khan, R.S.; Davis, B.M.; Dine, K.; Luong, V.; Cordeiro, M.F.; Shindler, K.S. Intranasal Resveratrol Nanoparticles Enhance Neuroprotection in a Model of Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 4047. [Google Scholar] [CrossRef]
- Buglio, D.S.; Marton, L.T.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Buchaim, R.L.; Goulart, R.A.; Rubira, C.J.; Barbalho, S.M. The Role of Resveratrol in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. J. Med. Food 2022, 25, 797–806. [Google Scholar] [CrossRef]
- Freyssin, A.; Rioux Bilan, A.; Fauconneau, B.; Galineau, L.; Serrière, S.; Tauber, C.; Perrin, F.; Guillard, J.; Chalon, S.; Page, G. Trans ε-Viniferin Decreases Amyloid Deposits With Greater Efficiency Than Resveratrol in an Alzheimer’s Mouse Model. Front. Neurosci. 2022, 15, 803927. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, G.W.; Liang, Z.M.; Sheng, S.Y.; Shi, Y.S.; Peng, L.; Wang, Y.P.; Wang, F.; Zhang, X.M. Resveratrol improves cognition and decreases amyloid plaque formation in Tg6799 mice. Mol. Med. Rep. 2019, 19, 3783–3790. [Google Scholar] [CrossRef]
- Corpas, R.; Griñán-Ferré, C.; Rodríguez-Farré, E.; Pallàs, M.; Sanfeliu, C. Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Mol. Neurobiol. 2018, 56, 1502–1516. [Google Scholar] [CrossRef]
- Puranik, N.; Yadav, D.; Song, M. Advancements in the Application of Nanomedicine in Alzheimer’s Disease: A Therapeutic Perspective. Int. J. Mol. Sci. 2023, 24, 14044. [Google Scholar] [CrossRef]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability is the Problem, What is the Solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef] [PubMed]
- Tosatti, J.A.G.; Fontes, A.F.D.S.; Caramelli, P.; Gomes, K.B. Effects of Resveratrol Supplementation on the Cognitive Function of Patients with Alzheimer’s Disease: A Systematic Review of Randomized Controlled Trials. Drugs Aging 2022, 39, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflammation 2017, 14, 1. [Google Scholar] [CrossRef]
- Zhu, C.W.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 609–616. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol brain delivery for neurological disorders prevention and treatment. Front. Pharmacol. 2018, 9, 404110. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. The uses of resveratrol for neurological diseases treatment and insights for nanotechnology based-drug delivery systems. Int. J. Pharm. 2020, 589, 119832. [Google Scholar] [CrossRef]
- Ortega, A.; Chernicki, B.; Ou, G.; Parmar, M.S. From Lab Bench to Hope: Emerging Gene Therapies in Clinical Trials for Alzheimer’s Disease. Mol. Neurobiol. 2024, 62, 1112–1135. [Google Scholar] [CrossRef]
- Paul, D.; Agrawal, R.; Singh, S. Alzheimer’s disease and clinical trials. J. Basic. Clin. Physiol. Pharmacol. 2024, 35, 31–44. [Google Scholar] [CrossRef]
- Nasr, M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016, 23, 1444–1452. [Google Scholar] [CrossRef]
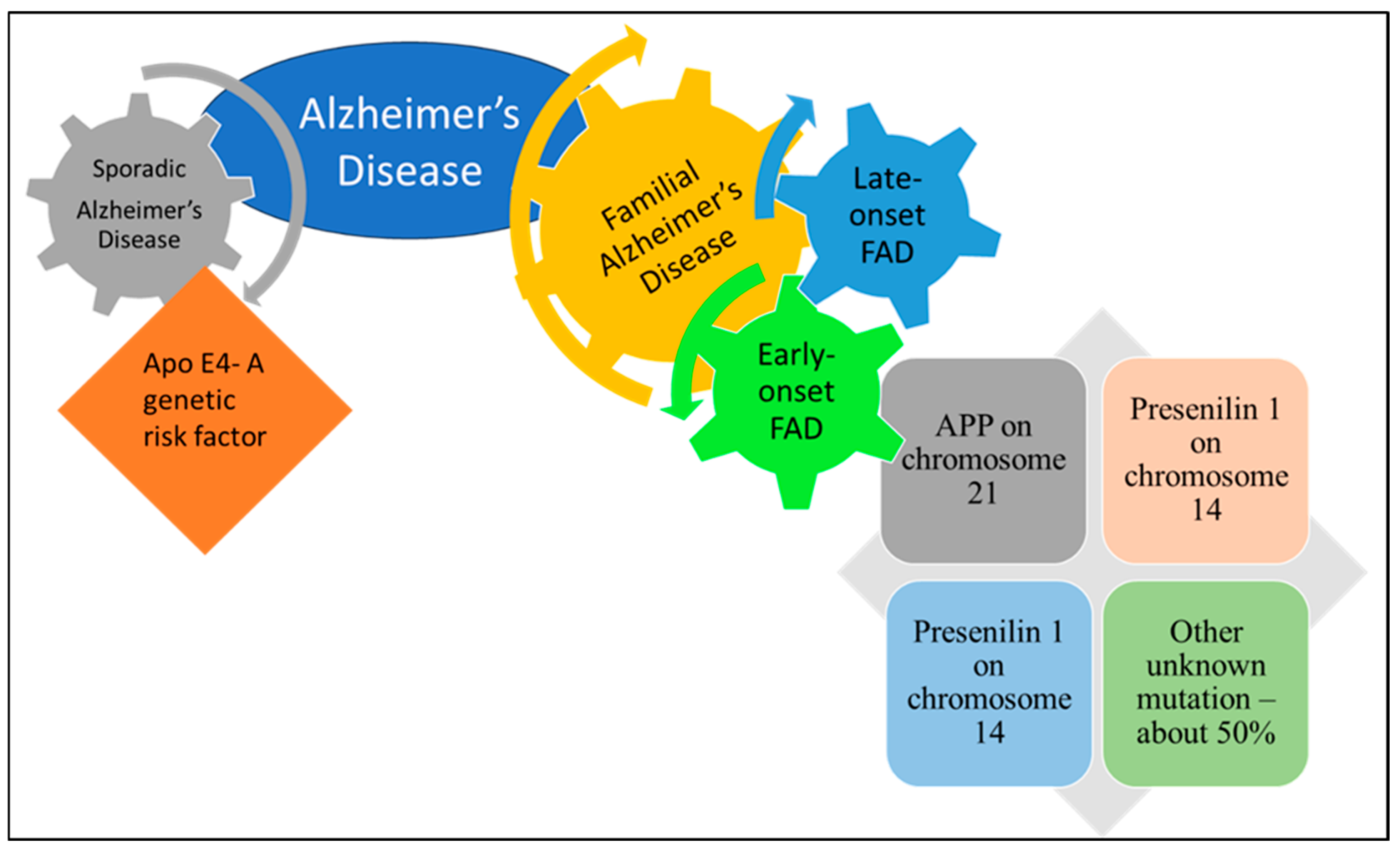
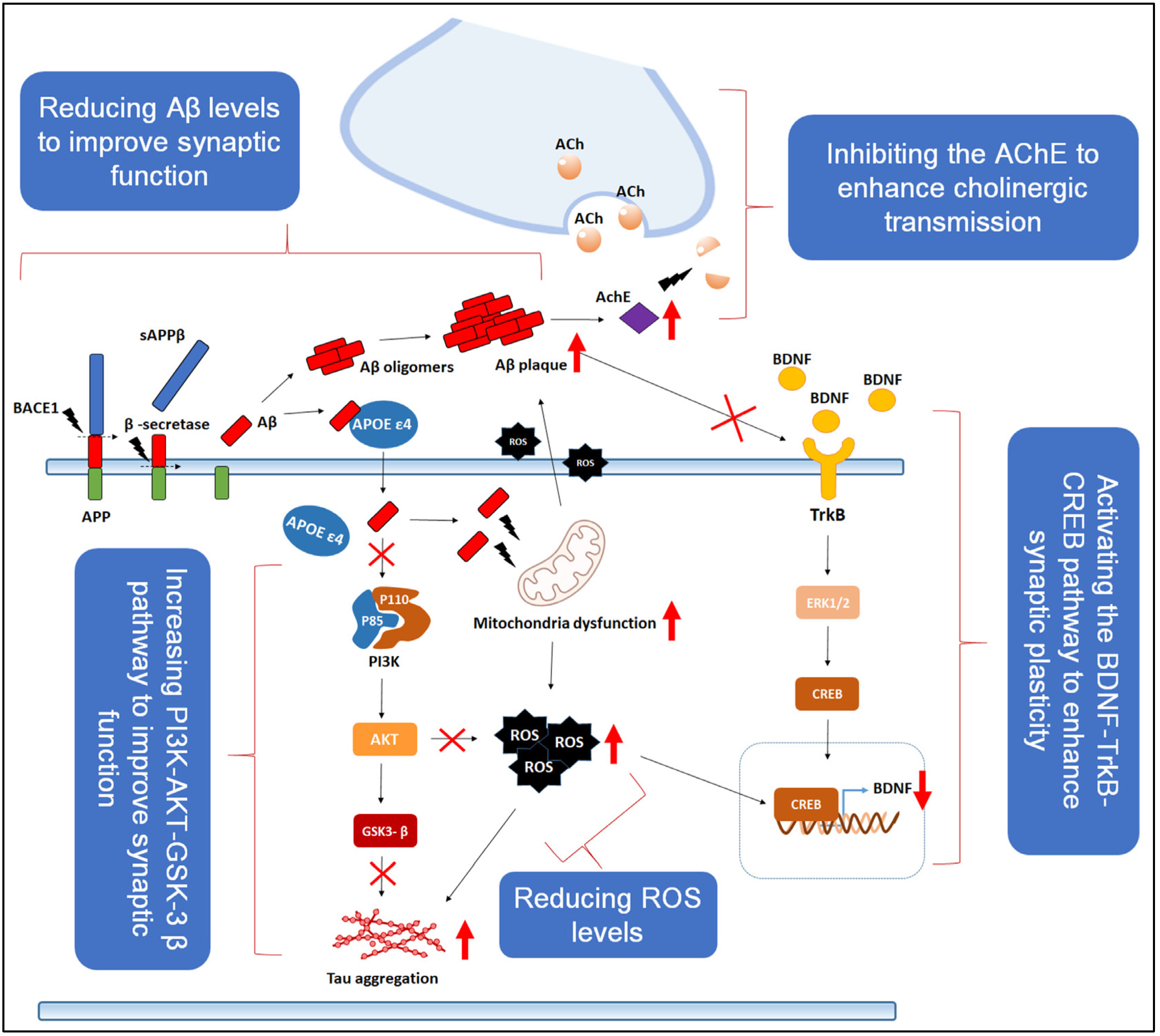
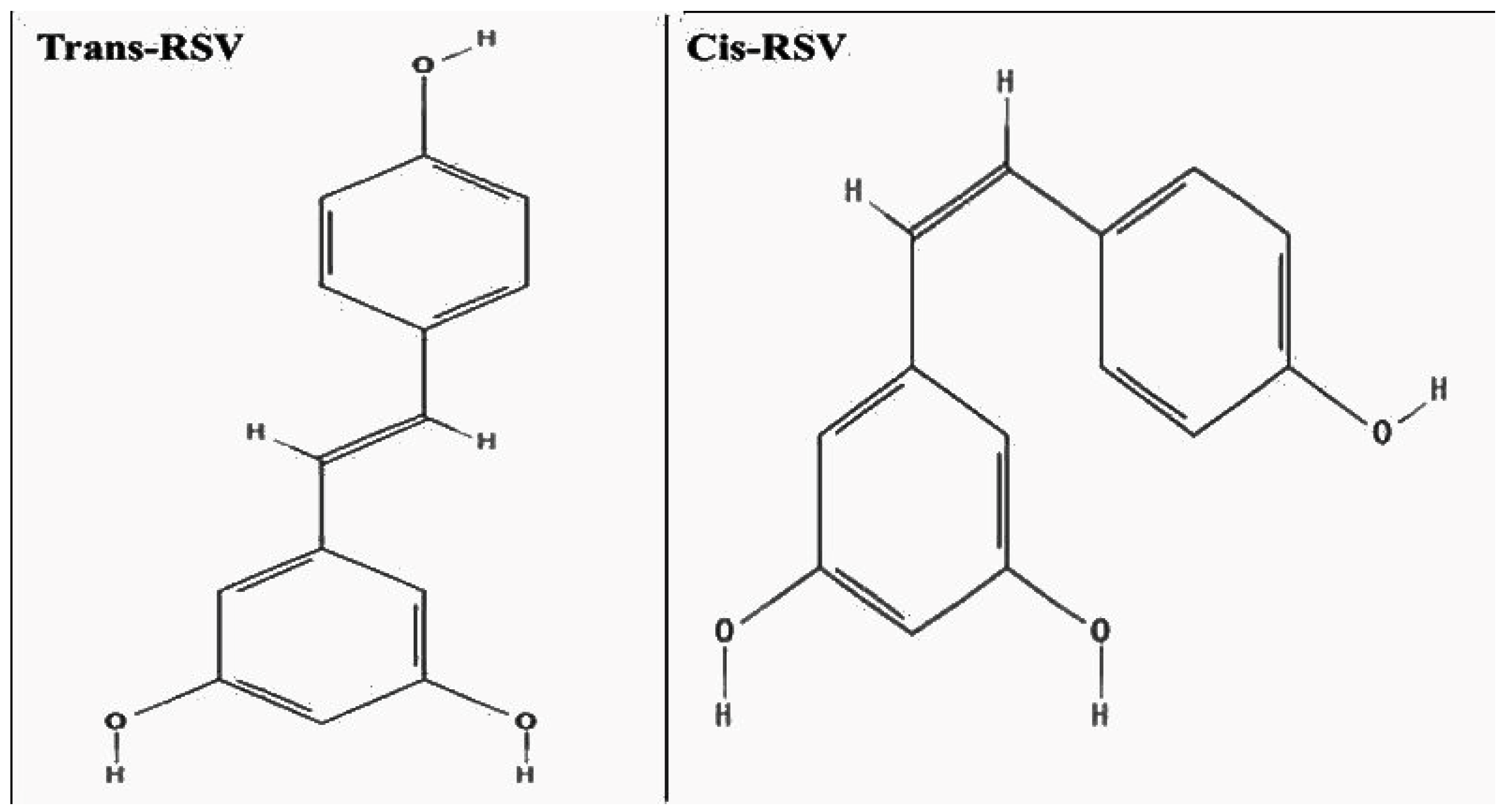

| Category | Risk Factor | Mechanism/Pathophysiology |
|---|---|---|
| Amyloid Pathology | Amyloid-β (Aβ) accumulation | Overproduction or impaired clearance of Aβ peptides, leading to plaque formation and synaptotoxicity. |
| Tau Pathology | Hyperphosphorylation of tau | Formation of neurofibrillary tangles; disrupted microtubule stability and axonal transport. |
| Genetic Factors | APOE ε4 allele | Impairs Aβ clearance, promotes lipid dysregulation and neuroinflammation. |
| APP, PSEN1, PSEN2 mutations | Increase Aβ42 production via altered γ-secretase activity. | |
| Neuroinflammation | Chronic glial activation | Microglia and astrocytes release proinflammatory cytokines (e.g., IL-1β, TNF-α); NLRP3 inflammasome activation. |
| Oxidative Stress | ROS overproduction | Mitochondrial damage and lipid, protein, DNA oxidation contribute to neuronal death. |
| Mitochondrial Dysfunction | Impaired ATP production | Reduced energy metabolism, increased ROS, and cytochrome c release. |
| Synaptic Dysfunction | Aβ oligomers | Disrupt synaptic transmission, inhibit LTP, and cause early synapse loss. |
| Proteostasis Impairment | UPS and autophagy dysfunction | Accumulation of misfolded proteins; reduced degradation of Aβ and tau aggregates. |
| BBB Dysfunction | Reduced clearance and barrier integrity | Impaired Aβ efflux, increased neurotoxin and immune cell entry into CNS. |
| Lipid Metabolism Dysregulation | Altered cholesterol transport | Affects APP processing and tau phosphorylation; APOE isoform-specific effects. |
| Insulin Resistance | “Type 3 diabetes” | Impaired PI3K/Akt signaling; decreased glucose uptake and neuroprotection. |
| Excitotoxicity | Excess glutamate/NMDA activation | Calcium overload, mitochondrial dysfunction, and neuronal death. |
| Calcium Dyshomeostasis | Disrupted Ca2+ signaling | Affects mitochondrial integrity, activates cell death pathways. |
| Epigenetic Changes | DNA methylation, histone modifications | Alters gene expression relevant to inflammation, metabolism, and synaptic function. |
| Vascular Dysfunction | Cerebral hypoperfusion | Reduced oxygen/nutrient delivery; promotes white matter lesions and Aβ retention. |
| Gut–Brain Axis Disruption | Microbiota imbalance | Increases systemic and CNS inflammation; affects BBB and amyloid pathology. |
| RSV Source | Cell Model | Key Findings | References |
|---|---|---|---|
| Synthetic | H19–7 hippocampal neuronal cells | Resveratrol protected against β-amyloid-induced oxidative damage and preserved memory-associated proteins | [97] |
| Synthetic | murine neuroblastoma (N2A) cell model | Demonstrated the anti-Alzheimer effects of resveratrol and curcuminoids, highlighting inhibition of Aβ aggregation | [98] |
| Natural | BV2 microglial cells | Resveratrol inhibited LPS and mCRP-induced COX-2 expression, reduced proinflammatory cytokine release, and upregulated antioxidant enzymes, suggesting neuroprotective effects against AD-related inflammation | [75] |
| Synthetic | SH-SY5Y neuroblastoma cells | Resveratrol activated AMPK signaling and modulated amyloid-β peptide metabolism | [99] |
| Synthetic | SH-SY5Y neuroblastoma cells | Olesoxime and resveratrol co-encapsulated in exosomes suppressed Aβ1–42 aggregation, protected against Aβ-induced cytotoxicity, and enhanced antioxidant defenses, indicating potential therapeutic synergy in AD models | [95] |
| Synthetic | Dopaminergic neurons | Resveratrol promoted the astroglial release of BDNF and GDNF, offering neurotrophic support | [100] |
| Synthetic | SH-SY5Y neuroblastoma cells | Inhibited β-amyloid oligomeric cytotoxicity without preventing oligomer formation | [101] |
| Synthetic | SK-N-BE neuroblastoma cells | Showed antioxidant effects and protection against α-synuclein and Aβ42 toxicity | [102] |
| Natural | Primary rat microglia | Resveratrol reduced prostaglandin E2 production and free radical formation in activated microglia | [103] |
| Source | Animal Model | Key Findings | References |
|---|---|---|---|
| Synthetic | Colchicine-induced AD in Wistar rats | Resveratrol (RS) at 10 mg/kg, both alone and combined with donepezil (DPZ), significantly reduced β-amyloid plaques and neurofibrillary tangles in the hippocampus. Prophylactic administration of RS exhibited neuroprotective effects, with the combination therapy yielding the most pronounced benefits | [104] |
| Synthetic | Streptozotocin-induced AD in rats | Oxyresveratrol-β-cyclodextrin complex improved cognitive function and reduced histone deacetylase activity in the hippocampus and frontal cortex. The treatment also decreased malondialdehyde levels, indicating reduced oxidative stress | [105] |
| Synthetic | 3xTg-AD mice | Intranasal administration of resveratrol nanoparticles protected mice against retinal and brain neurodegeneration. Treatment reduced amyloid-beta and phosphorylated Tau deposition in the brain, suggesting the potential for noninvasive delivery | [106] |
| Synthetic | APP/PS1 transgenic mice | Exosomes co-encapsulating olesoxime and resveratrol suppressed amyloid-beta aggregation, enhanced antioxidant defenses, and improved spatial learning and memory in AD mice | [95] |
| Natural | Review of the various animal models | A systematic review indicated that resveratrol supplementation improved cognitive function and reduced neuroinflammation in AD models. The benefits were attributed to the antioxidant properties and modulation of signaling pathways | [107] |
| Synthetic | APPswePS1dE9 transgenic mice | Trans-ε-viniferin, a resveratrol dimer, decreased amyloid deposits more effectively than resveratrol. Treatment also partially improved spatial memory decline, highlighting its therapeutic potential | [108] |
| Natural | Tg6799 mice | Resveratrol administration (60 mg/kg daily for 60 days) reduced amyloid plaque formation, decreased Aβ42 levels, and improved spatial memory performance | [109] |
| Natural | 3xTg-AD mice | Dietary resveratrol (100 mg/kg from 2 to 12 months of age) prevented memory loss, reduced Aβ and Tau pathologies, and enhanced proteostasis mechanisms | [110] |
| Synthetic | Human subjects with mild to moderate AD | High-dose resveratrol (up to 2 g orally daily) was safe and penetrated the blood–brain barrier, with observed reductions in cerebrospinal fluid Aβ40 levels | [96] |
| Clinical Trial | Interventions | Type of Study | Number of AD Patients | Clinical Phase Status | Related Publication |
|---|---|---|---|---|---|
| NCT01504854 | Resveratrol | Double-blind, placebo-controlled trial | AD 120 patients | Phase 2 completed | [116] |
| NCT00743743 | Longevinex brand resveratrol supplement | Randomized, double-blind, placebo-controlled clinical trial | AD 50 patients | Phase 3 withdrawn | NA |
| NCT00678431 | Resveratrol with Glucose and Malate | Randomized, double-blind, placebo-controlled trial | AD 16 patients | Phase 3 completed | [117] |
| NCT02502253 | Resveratrol, extract from grape seed | Randomized | Mild Cognitive Impairment (MCI) and Prediabetes | Phase 1 completed | NA |
| NCT06470061 | Resveratrol, Quercetin, and Curcumin (RQC) | Randomized | AD and Retinal Amyloid-β | Not yet recruiting | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puranik, N.; Kumari, M.; Tiwari, S.; Dhakal, T.; Song, M. Resveratrol as a Therapeutic Agent in Alzheimer’s Disease: Evidence from Clinical Studies. Nutrients 2025, 17, 2557. https://doi.org/10.3390/nu17152557
Puranik N, Kumari M, Tiwari S, Dhakal T, Song M. Resveratrol as a Therapeutic Agent in Alzheimer’s Disease: Evidence from Clinical Studies. Nutrients. 2025; 17(15):2557. https://doi.org/10.3390/nu17152557
Chicago/Turabian StylePuranik, Nidhi, Meenakshi Kumari, Shraddha Tiwari, Thakur Dhakal, and Minseok Song. 2025. "Resveratrol as a Therapeutic Agent in Alzheimer’s Disease: Evidence from Clinical Studies" Nutrients 17, no. 15: 2557. https://doi.org/10.3390/nu17152557
APA StylePuranik, N., Kumari, M., Tiwari, S., Dhakal, T., & Song, M. (2025). Resveratrol as a Therapeutic Agent in Alzheimer’s Disease: Evidence from Clinical Studies. Nutrients, 17(15), 2557. https://doi.org/10.3390/nu17152557








