Neural Correlates Underlying General and Food-Related Working Memory in Females with Overweight/Obesity
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. Hunger and Desire to Eat
2.3.2. Working Memory Tasks (Two-Back Tasks) (Figure 1)

2.3.3. General Two-Back Task (Figure 1A)
2.3.4. Food-Related Two-Back Task (Figure 1B)
2.4. Behavioral Analysis
2.5. EEG Recording and Analysis
3. Results
3.1. Hunger and Desire to Eat
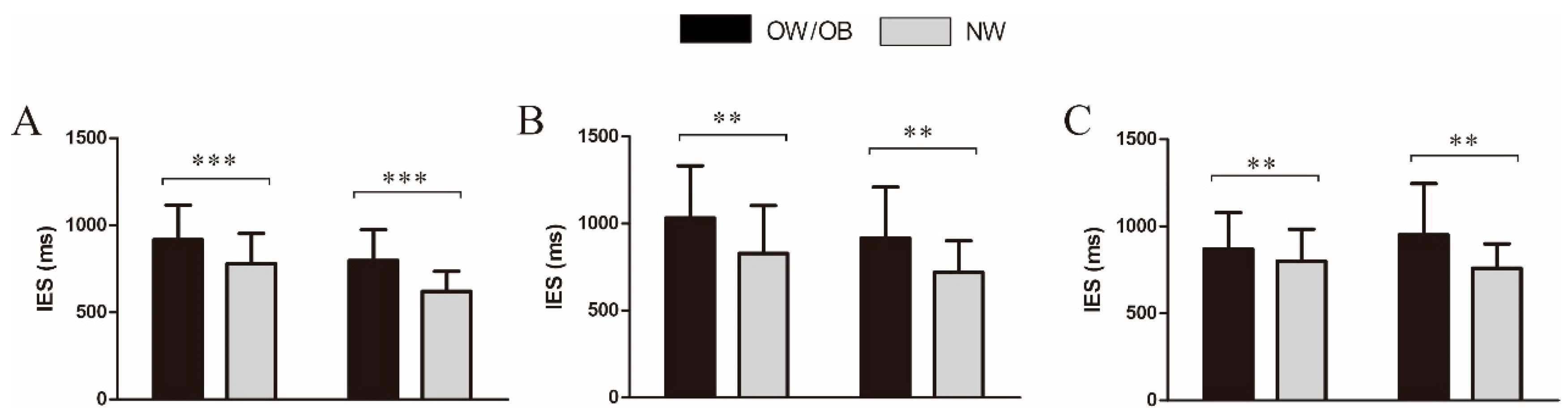
3.2. Behavioral Results
3.2.1. General Two-Back Task
3.2.2. Food-Related Two-Back Task
3.3. Neural Correlate Results
3.3.1. General Two-Back Task (Figure 3)
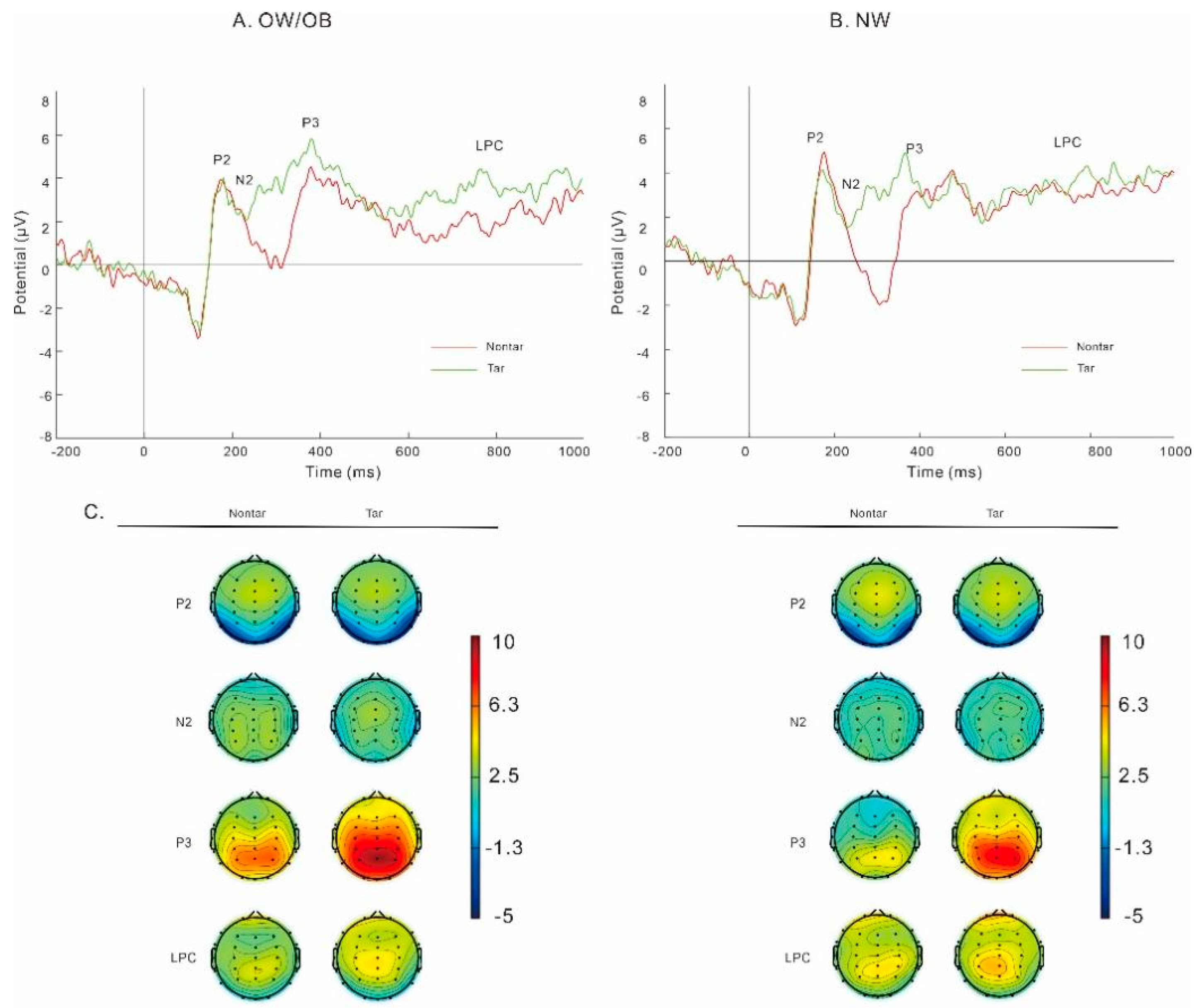

3.3.2. Food-Related Two-Back Task (Figure 5)
P2
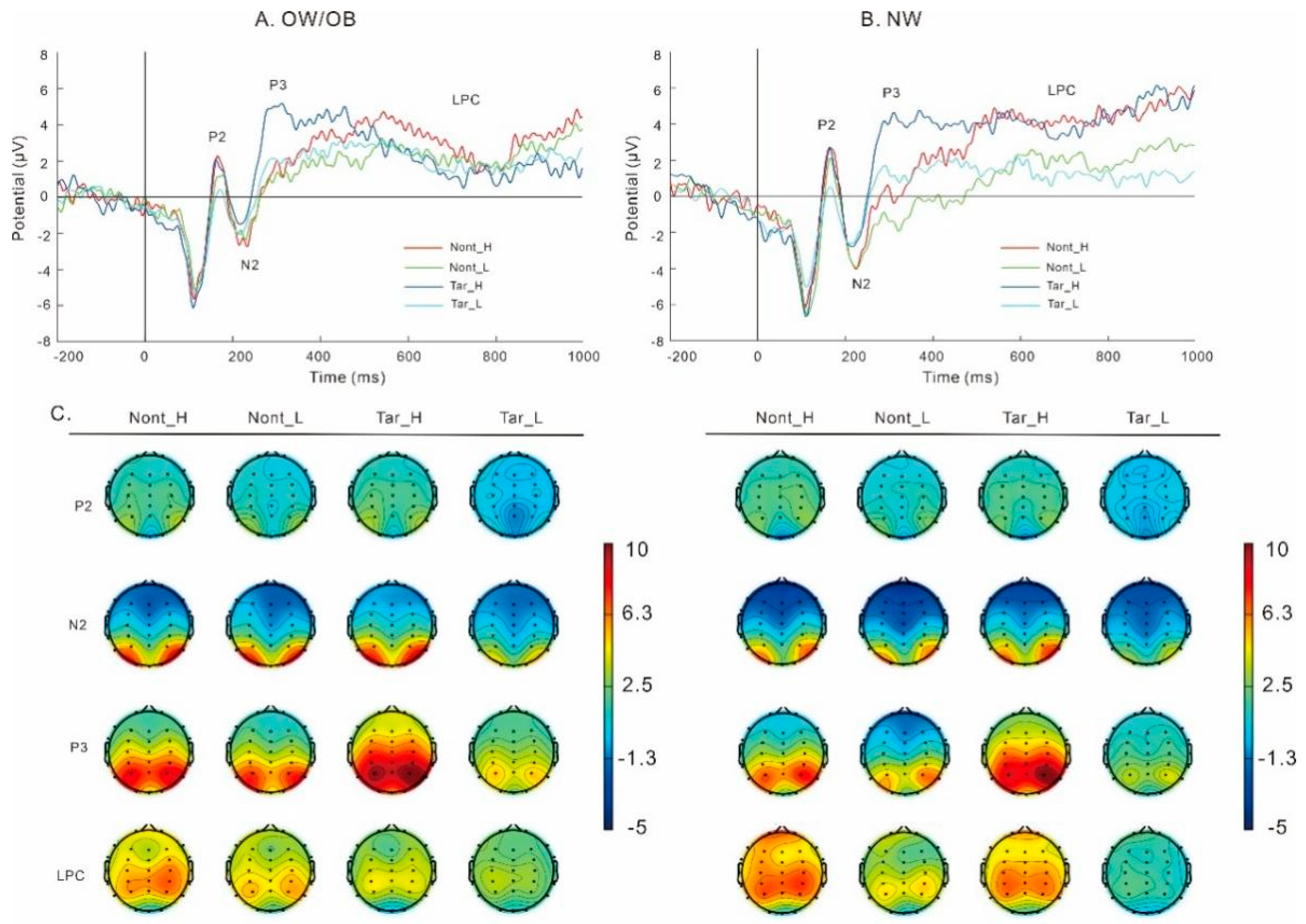
N2
P3
LPC
Theta
Alpha
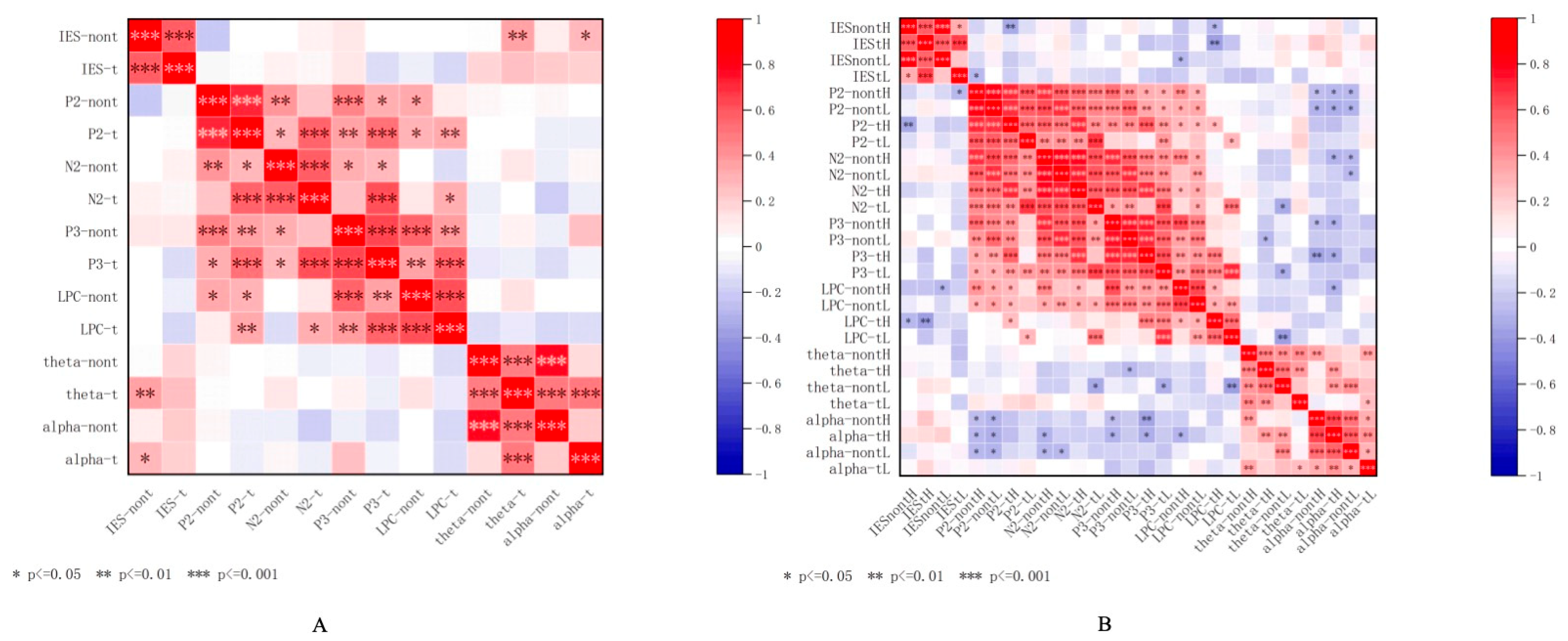
Correlation Between Behavioral and EEG Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Zhang, M.; Zhao, Z.; Huang, Z.; Deng, Q.; Li, Y.; Pan, A.; Li, C.; Chen, Z.; Zhou, M.; et al. Geographic Variation in Prevalence of Adult Obesity in China: Results from the 2013–2014 National Chronic Disease and Risk Factor Surveillance. Ann. Intern. Med. 2020, 172, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Jan, C.; Ma, Y.; Dong, B.; Zou, Z.; Yang, Y.; Xu, R.; Song, Y.; Ma, J.; Sawyer, S.M.; et al. Economic development and the nutritional status of Chinese school-aged children and adolescents from 1995 to 2014: An analysis of five successive national surveys. Lancet Diabetes Endocrinol. 2019, 7, 288–299. [Google Scholar] [CrossRef]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef]
- Alarcón, G.; Ray, S.; Nagel, B.J. Lower Working Memory Performance in Overweight and Obese Adolescents Is Mediated by White Matter Microstructure. J. Int. Neuropsychol. Soc. 2016, 22, 281–292. [Google Scholar] [CrossRef]
- Wu, N.; Chen, Y.; Yang, J.; Li, F. Childhood Obesity and Academic Performance: The Role of Working Memory. Front. Psychol. 2017, 8, 611. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Mamrot, P.; Hanć, T. The association of the executive functions with overweight and obesity indicators in children and adolescents: A literature review. Neurosci. Biobehav. Rev. 2019, 107, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Skirde, A.K.; Freund, R.; Vögele, C.; Kübler, A. High-calorie food-cues impair working memory performance in high and low food cravers. Appetite 2012, 59, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, A.B.; O’Brien, S.; Lavender, J.M.; Pearson, C.M.; Le Grange, D.; Hunter, S.J. Executive functioning in a racially diverse sample of children who are overweight and at risk for eating disorders. Appetite 2018, 124, 43–49. [Google Scholar] [CrossRef]
- Allom, V.; Mullan, B. Individual differences in executive function predict distinct eating behaviours. Appetite 2014, 80, 123–130. [Google Scholar] [CrossRef]
- Nyaradi, A.; Foster, J.K.; Hickling, S.; Li, J.; Ambrosini, G.L.; Jacques, A.; Oddy, W.H. Prospective associations between dietary patterns and cognitive performance during adolescence. J. Child Psychol. Psychiatry 2014, 55, 1017–1024. [Google Scholar] [CrossRef]
- Whitelock, V.; Nouwen, A.; Akker, O.v.D.; Higgs, S. The role of working memory sub-components in food choice and dieting success. Appetite 2018, 124, 24–32. [Google Scholar] [CrossRef]
- Blanchet, S.; Gagnon, G.; Bastien, C. Event-related potential study of dynamic neural mechanisms of semantic organizational strategies in verbal learning. Brain Res. 2007, 1170, 59–70. [Google Scholar] [CrossRef]
- Lenartowicz, A.; Escobedo-Quiroz, R.; Cohen, J.D. Updating of context in working memory: An event-related potential study. Cogn. Affect. Behav. Neurosci. 2010, 10, 298–315. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Zhao, J.; Zhang, L.; Chen, H. Neurocognitive Correlates of Food-Related Response Inhibition in Overweight/Obese Adults. Brain Topogr. 2020, 33, 101–111. [Google Scholar] [CrossRef]
- Nijs, I.M.; Franken, I.H.; Muris, P. Food-related Stroop interference in obese and normal-weight individuals: Behavioral and electrophysiological indices. Eat. Behav. 2010, 11, 258–265. [Google Scholar] [CrossRef]
- Liu, Y.; Quan, H.; Song, S.; Zhang, X.; Yang, C.; Chen, H. Decreased Conflict Control in Overweight Chinese Females: Behavioral and Event-Related Potentials Evidence. Nutrients 2019, 11, 1450. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Zhang, X.; Gao, X.; Xu, W.; Chen, H. Overweight adults are more impulsive than normal weight adults: Evidence from ERPs during a chocolate-related delayed discounting task. Neuropsychologia 2019, 133, 107181. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Lundqvist, M.; Bastos, A.M. Working Memory 2.0. Neuron 2018, 100, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Tesche, C.D. Frontal theta activity in humans increases with memory load in a working memory task. Eur. J. Neurosci. 2002, 15, 1395–1399. [Google Scholar] [CrossRef]
- Lundqvist, M.; Herman, P.; Lansner, A. Theta and gamma power increases and alpha/beta power decreases with memory load in an attractor network model. J. Cogn. Neurosci. 2011, 23, 3008–3020. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Zhou, Y.; Yang, R.; Han, B.; Zhao, Y.; Pang, Y.; Yuan, H.; Chen, H. High-Calorie Food-Cues Impair Conflict Control: EEG Evidence from a Food-Related Stroop Task. Nutrients 2022, 14, 4593. [Google Scholar] [CrossRef]
- Hume, D.J.; Howells, F.M.; Rauch, H.L.; Kroff, J.; Lambert, E.V. Electrophysiological indices of visual food cue-reactivity. Differences in obese, overweight and normal weight women. Appetite 2015, 85, 126–137. [Google Scholar] [CrossRef]
- Gameiro, F.; Perea, M.; Ladera, V.; Rosa, B.; García, R. Executive functioning in obese individuals waiting for clinical treatment. Psicothema 2017, 1, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bonnefond, M.; Jensen, O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr. Biol. 2012, 22, 1969–1974. [Google Scholar] [CrossRef]
- Palva, S.; Palva, J.M. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007, 30, 150–158. [Google Scholar] [CrossRef]
- Spitzer, B.; Fleck, S.; Blankenburg, F. Parametric alpha- and beta-band signatures of supramodal numerosity information in human working memory. J. Neurosci. 2014, 34, 4293–4302. [Google Scholar] [CrossRef]
- Babiloni, C.; Marzano, N.; Lizio, R.; Valenzano, A.; Triggiani, A.I.; Petito, A.; Bellomo, A.; Lecce, B.; Mundi, C.; Soricelli, A.; et al. Resting state cortical electroencephalographic rhythms in subjects with normal and abnormal body weight. NeuroImage 2011, 58, 698–707. [Google Scholar] [CrossRef]
- van der Laan, L.N.; Papies, E.; Hooge, I.; Smeets, P. Goal-directed visual attention drives health goal priming: An eye-tracking experiment. Health Psychol. 2017, 36, 82–90. [Google Scholar] [CrossRef] [PubMed]
- García-García, I.; Jurado, M.Á.; Garolera, M.; Marqués-Iturria, I.; Horstmann, A.; Segura, B.; Pueyo, R.; Sender-Palacios, M.J.; Vernet-Vernet, M.; Villringer, A.; et al. Functional network centrality in obesity: A resting-state and task fMRI study. Psychiatry Res. 2015, 233, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Moerel, D.; Psihoyos, J.; Carlson, T.A. The Time-Course of Food Representation in the Human Brain. J. Neurosci. 2024, 44, e1101232024. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Xu, Z.; Yan, H.; Tie, B.; Zhao, W.; Jing, Y.; Pang, Y.; Liu, X.; Zhao, J.; Liu, Y. Individuals’ Food Preferences can be Influenced by the Music Styles: An ERP Study. Brain Topogr. 2025, 38, 22. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.S.; Yurgelun-Todd, D.A. Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. NeuroReport 2005, 16, 859–863. [Google Scholar] [CrossRef]
- Hallam, J.; Boswell, R.G.; DeVito, E.E.; Kober, H. Gender-related Differences in Food Craving and Obesity. Yale J. Biol. Med. 2016, 89, 161–173. [Google Scholar]
- Ren, M.; Xu, J.; Li, Y.; Wang, M.; Georgiev, G.; Shen, L.; Zhao, J.; Cao, Z.; Zhang, S.; Wang, W.; et al. Neural signatures for the n-back task with different loads: An event-related potential study. Biol. Psychol. 2023, 177, 108485. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, Y.; Chen, H.; Liu, Y. Inhibition ability of food cues between successful and unsuccessful restrained eaters: A two-choice oddball task. PLoS ONE 2015, 10, e0120522. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Jackson, T.; Wang, J.; Yang, R.; Chen, H. Effects of negative mood state on event-related potentials of restrained eating subgroups during an inhibitory control task. Behav. Brain Res. 2020, 377, 112249. [Google Scholar] [CrossRef]
- Bruyer, R.; Brysbaert, M. Combining speed and accuracy in cognitive psychology: Is the inverse efficiency score (IES) a better dependent variable than the mean reaction time (RT) and the percentage of errors (PE)? Psychol. Belg. 2011, 51, 5–13. [Google Scholar] [CrossRef]
- Lin, L.; Leung, A.W.; Wu, J.; Zhang, L. Individual differences under acute stress: Higher cortisol responders performs better on N-back task in young men. Int. J. Psychophysiol. 2020, 150, 20–28. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Braboszcz, C.; Delorme, A. Lost in thoughts: Neural markers of low alertness during mind wandering. NeuroImage 2011, 54, 3040–3047. [Google Scholar] [CrossRef]
- Carretié, L.; Martín-Loeches, M.; Hinojosa, J.A.; Mercado, F. Emotion and attention interaction studied through event-related potentials. J. Cogn. Neurosci. 2001, 13, 1109–1128. [Google Scholar] [CrossRef]
- Polich, J.; Kok, A. Cognitive and biological determinants of P300: An integrative review. Biol. Psychol. 1995, 41, 103–146. [Google Scholar] [CrossRef]
- Blechert, J.; Feige, B.; Hajcak, G.; Tuschen-Caffier, B. To eat or not to eat? Availability of food modulates the electrocortical response to food pictures in restrained eaters. Appetite 2010, 54, 262–268. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Stoerig, P.; Falkenstein, M. ERP—Correlates of response selection in a response conflict paradigm. Brain Res. 2008, 1189, 127–134. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Hajcak, G.; MacNamara, A.; Foti, D.; Ferri, J.; Keil, A. The dynamic allocation of attention to emotion: Simultaneous and independent evidence from the late positive potential and steady state visual evoked potentials. Biol. Psychol. 2013, 92, 447–455. [Google Scholar] [CrossRef]
- Hajcak, G.; Nieuwenhuis, S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn. Affect. Behav. Neurosci. 2006, 6, 291–297. [Google Scholar] [CrossRef]
- Chen, S.; Jia, Y.; Woltering, S. Neural differences of inhibitory control between adolescents with obesity and their peers. Int. J. Obes. 2018, 42, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Stingl, K.T.; Kullmann, S.; Ketterer, C.; Heni, M.; Häring, H.-U.; Fritsche, A.; Preissl, H. Neuronal correlates of reduced memory performance in overweight subjects. NeuroImage 2012, 60, 362–369. [Google Scholar] [CrossRef]
- Zacks, R.T.; Hasher, L. Aging and Long-Term Memory: Deficits Are Not Inevitable. In Lifespan Cognition: Mechanisms of Change; Oxford University Press: New York, NY, USA, 2006; pp. 162–177. [Google Scholar]
- Hasher, L.; Zacks, R.T. Working Memory, Comprehension, and Aging: A Review and a New View. Psychol. Learn. Motiv. 1988, 22, 193–225. [Google Scholar]
- Yeung, N.; Botvinick, M.M.; Cohen, J.D. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol. Rev. 2004, 111, 931–959. [Google Scholar] [CrossRef]
- Kaunitz, L.N.; Kamienkowski, J.E.; Varatharajah, A.; Sigman, M.; Quiroga, R.Q.; Ison, M.J. Looking for a face in the crowd: Fixation-related potentials in an eye-movement visual search task. NeuroImage 2014, 89, 297–305. [Google Scholar] [CrossRef]
- Maayan, L.; Hoogendoorn, C.; Sweat, V.; Convit, A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity 2011, 19, 1382–1387. [Google Scholar] [CrossRef]
- Guxens, M.; Mendez, M.A.; Julvez, J.; Plana, E.; Forns, J.; Basagaña, X.; Torrent, M.; Sunyer, J. Cognitive function and overweight in preschool children. Am. J. Epidemiology 2009, 170, 438–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Favieri, F.; Forte, G.; Casagrande, M. The Executive Functions in Overweight and Obesity: A Systematic Review of Neuropsychological Cross-Sectional and Longitudinal Studies. Front. Psychol. 2019, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.; Yurgelun-Todd, D.A. Sex differences in cerebral responses to images of high versus low-calorie food. NeuroReport 2010, 21, 354–358. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Salzberg, A.K.; Endly, D.-C.; Bessesen, D.H.; Tregellas, J.R. Sex-based differences in the behavioral and neuronal responses to food. Physiol. Behav. 2010, 99, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Gibson, S. Waist-to-height ratio as an indicator of ‘early health risk’: Simpler and more predictive than using a ‘matrix’ based on BMI and waist circumference. BMJ Open 2016, 6, e010159. [Google Scholar] [CrossRef] [PubMed]

| OW/OB (M ± SD; N = 25) | NW (M ± SD; N = 26) | |||
|---|---|---|---|---|
| Nontar | Tar | Nontar | Tar | |
| IES | 917.49 (198.61) | 797.69 (176.39) | 779.95 (173.40) | 620.39 (115.19) |
| P2 | 3.08 (2.95) | 2.98 (4.05) | 3.73 (3.09) | 3.41 (3.59) |
| N2 | 2.03 (2.98) | 2.50 (3.28) | 1.68 (3.61) | 1.94 (4.43) |
| P3 | 2.43 (4.73) | 4.71 (4.74) | 0.69 (3.96) | 3.69 (5.88) |
| LPC | 1.97 (5.64) | 3.14 (4.57) | 2.94 (3.67) | 3.29 (4.47) |
| theta | 0.66 (0.74) | 0.48 (0.55) | 0.44 (0.62) | 0.32 (0.52) |
| alpha | 0.06 (0.38) | −0.06 (0.66) | −0.10 (0.59) | −0.15 (0.59) |
| OW/OB (M ± SD; N = 26) | NW (M ± SD; N = 28) | |||||||
|---|---|---|---|---|---|---|---|---|
| NontarH | TarH | NontarL | TarL | NontarH | TarH | NontarL | TarL | |
| IES | 1032.32 (554.19) | 915.52 (293.65) | 869.51 (210.27) | 951.41 (294.52) | 828.29 (274.64) | 719.17 (181.00) | 799.63 (183.15) | 759.23 (140.20) |
| P2 | 1.79 (5.01) | 2.07 (5.78) | 0.84 (4.19) | −0.56 (3.46) | 0.29 (4.94) | 0.31 (5.92) | −0.04 (4.38) | −0.48 (3.34) |
| N2 | −1.07 (5.33) | 0.07 (4.87) | −1.16 (4.31) | −1.64 (3.88) | −3.88 (5.28) | −3.09 (5.93) | −3.92 (5.32) | −2.29 (3.82) |
| P3 | 2.44 (5.55) | 4.67 (5.05) | 0.98 (4.05) | 1.24 (4.34) | −0.19 (5.77) | 2.87 (6.69) | −1.59 (5.35) | 1.01 (3.79) |
| LPC | 3.06 (5.45) | 2.09 (4.69) | 1.43 (3.35) | 0.78 (4.29) | 4.15 (4.99) | 4.45 (5.38) | 1.73 (4.18) | 1.50 (3.98) |
| theta | 0.45 (0.61) | 0.37 (0.76) | 0.26 (0.55) | 0.38 (0.57) | 0.75 (0.86) | 1.02 (1.13) | 0.67 (1.10) | 0.45 (0.56) |
| alpha | 0.02 (0.37) | −0.01 (0.35) | −0.09 (0.46) | 0.03 (0.24) | −0.61 (1.04) | −0.44 (0.94) | −0.44 (0.88) | −0.08 (0.72) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Jing, Y.; Zhao, J.; Liu, X.; Zhao, W.; Liu, Y.; Chen, H. Neural Correlates Underlying General and Food-Related Working Memory in Females with Overweight/Obesity. Nutrients 2025, 17, 2552. https://doi.org/10.3390/nu17152552
Pang Y, Jing Y, Zhao J, Liu X, Zhao W, Liu Y, Chen H. Neural Correlates Underlying General and Food-Related Working Memory in Females with Overweight/Obesity. Nutrients. 2025; 17(15):2552. https://doi.org/10.3390/nu17152552
Chicago/Turabian StylePang, Yazhi, Yuanluo Jing, Jia Zhao, Xiaolin Liu, Wen Zhao, Yong Liu, and Hong Chen. 2025. "Neural Correlates Underlying General and Food-Related Working Memory in Females with Overweight/Obesity" Nutrients 17, no. 15: 2552. https://doi.org/10.3390/nu17152552
APA StylePang, Y., Jing, Y., Zhao, J., Liu, X., Zhao, W., Liu, Y., & Chen, H. (2025). Neural Correlates Underlying General and Food-Related Working Memory in Females with Overweight/Obesity. Nutrients, 17(15), 2552. https://doi.org/10.3390/nu17152552








