Relationship Between Body Composition and Biomarkers in Adult Females with Breast Cancer: 1-Year Follow-Up Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Clinical and Anthropometric Data
2.4. Body Composition Measurement
2.5. Nutritional Ultrasonography
2.6. Assessment of Grip Strength
2.7. Diagnosis of Sarcopenic Obesity
2.8. Biomarkers
2.9. Statistical Analysis
3. Results
3.1. Baseline Description
3.2. Body Composition and Muscle Strength Evolution: Sarcopenic Obesity Prevalence
3.3. Biomarkers Evolution
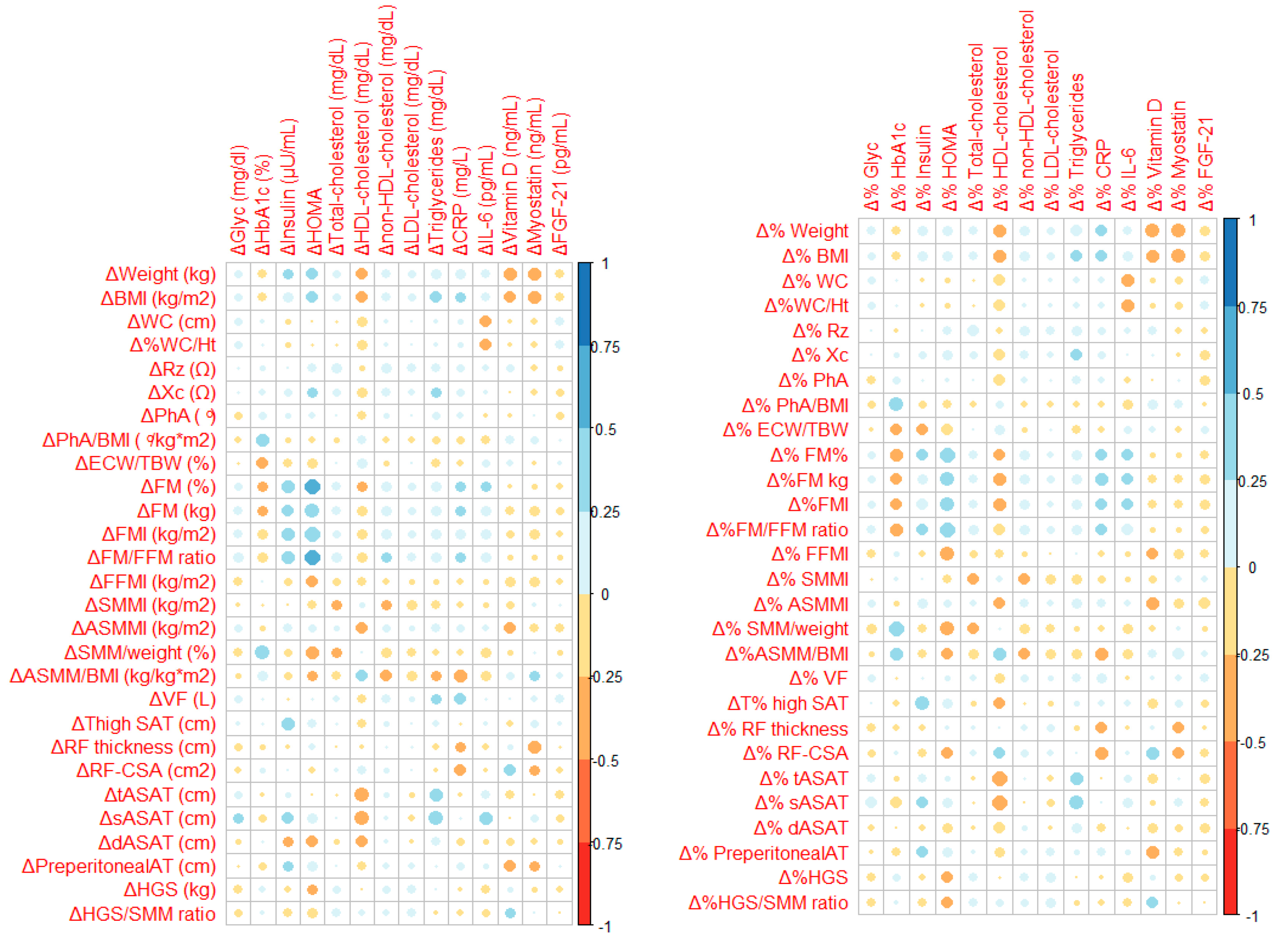
3.4. Association Between Body Composition Changes and Biomarkers Evolution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASMM | appendicular skeletal muscle mass |
| AT | adipose tissue |
| BC | body composition |
| BIA | bioelectrical impedance analysis |
| BMI | body mass index |
| ChT | chemotherapy |
| CT | computed tomography |
| DXA | dual-energy X-ray absorptiometry |
| dASAT | deep abdominal subcutaneous adipose tissue |
| ECW | extracellular water |
| EASO | European Association for the Study of Obesity |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| FGF21 | fibroblast growth factor 21 |
| FFM | fat-free mass |
| FM | fat mass |
| GDF8 | growth differentiation factor 8 |
| HGS | handgrip strength |
| HT | hormone therapy |
| IL6 | interleukin 6 |
| PhA | phase angle |
| RF | rectus femoris |
| RF-CS | rectus femoris cross-sectional area |
| RT | radiation therapy |
| Rz | resistance |
| SAT | subcutaneous adipose tissue |
| SMM | skeletal muscle mass |
| sASAT | superficial abdominal subcutaneous adipose tissue |
| SEEDO | Spanish Society for the Study of Obesity |
| SO | sarcopenic obesity |
| TBW | total body water |
| T2DM | type 2 diabetes mellitus |
| TNF-α | tumor necrosis factor-alpha |
| tASAT | total abdominal subcutaneous adipose tissue |
| US | ultrasound |
| VAT | visceral adipose tissue |
| VF | visceral fat |
| WC | waist circumference |
| WHtR | waist-to-height ratio |
| Xc | reactance |
References
- Xia, C.; Liu, Y.; Yong, W.; Qing, X. Global Evolution of Breast Cancer Incidence in Childbearing-Age Women Aged 15–49 Years: A 30-Year Analysis. J. Cancer Res. Clin. Oncol. 2025, 151, 75. [Google Scholar] [CrossRef]
- Wojtyla, C.; Bertuccio, P.; Wojtyla, A.; La Vecchia, C. European Trends in Breast Cancer Mortality, 1980–2017 and Predictions to 2025. Eur. J. Cancer 2021, 152, 4–17. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Valente, S.; Roesch, E. Breast Cancer Survivorship. J. Surg. Oncol. 2024, 130, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Dehesh, T.; Fadaghi, S.; Seyedi, M.; Abolhadi, E.; Ilaghi, M.; Shams, P.; Ajam, F.; Mosleh-Shirazi, M.A.; Dehesh, P. The Relation between Obesity and Breast Cancer Risk in Women by Considering Menstruation Status and Geographical Variations: A Systematic Review and Meta-Analysis. BMC Women’s Health 2023, 23, 392. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Vieira, R.; Abar, L.; Aune, D.; Balducci, K.; Cariolou, M.; Greenwood, D.C.; Markozannes, G.; Nanu, N.; Becerra-Tomás, N.; et al. Postdiagnosis Body Fatness, Weight Change and Breast Cancer Prognosis: Global Cancer Update Program (CUP Global) Systematic Literature Review and Meta-Analysis. Int. J. Cancer 2023, 152, 572–599. [Google Scholar] [CrossRef]
- Karra, P.; Winn, M.; Pauleck, S.; Bulsiewicz-Jacobsen, A.; Peterson, L.; Coletta, A.; Doherty, J.; Ulrich, C.M.; Summers, S.A.; Gunter, M.; et al. Metabolic Dysfunction and Obesity-Related Cancer: Beyond Obesity and Metabolic Syndrome. Obesity 2022, 30, 1323–1334. [Google Scholar] [CrossRef]
- Pang, Y.; Wei, Y.; Kartsonaki, C. Associations of Adiposity and Weight Change with Recurrence and Survival in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Breast Cancer 2022, 29, 575–588. [Google Scholar] [CrossRef]
- Bradshaw, P.T.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Albers, K.B.; Chen, W.Y.; Caan, B.J. Adipose Tissue Distribution and Survival Among Women with Nonmetastatic Breast Cancer. Obesity 2019, 27, 997–1004. [Google Scholar] [CrossRef]
- Poltronieri, T.S.; Pérsico, R.S.; Viana, L.V. Body Adipose Tissue Depots and Treatment Outcomes for Women with Breast Cancer: A Systematic Review. Clin. Nutr. 2024, 43, 1033–1042. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, J.H.; Song, S.W.; Jung, W.S.; Lee, Y.A.; Kim, H.N. Relationship between Deep Subcutaneous Abdominal Adipose Tissue and Metabolic Syndrome: A Case Control Study. Diabetol. Metab. Syndr. 2016, 8, 10. [Google Scholar] [CrossRef]
- Walker, G.E.; Verti, B.; Marzullo, P.; Savia, G.; Mencarelli, M.; Zurleni, F.; Liuzzi, A.; Di Blasio, A.M. Deep Subcutaneous Adipose Tissue: A Distinct Abdominal Adipose Depot. Obesity 2007, 15, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and Adverse Breast Cancer Risk and Outcome: Mechanistic Insights and Strategies for Intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, E.M.C.; Kroenke, C.H.; Caan, B.J. The Obesity Paradox in Cancer: How Important Is Muscle? Annu. Rev. Nutr. 2025, 38, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.; Barchiesi, G.; Resuli, B.; Verrico, M.; Speranza, I.; Cristofani, L.; Pediconi, F.; Tomao, F.; Botticelli, A.; Santini, D. Sarcopenia in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 596. [Google Scholar] [CrossRef]
- Gortan Cappellari, G.; Brasacchio, C.; Laudisio, D.; Lubrano, C.; Pivari, F.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Sarcopenic Obesity: What about in the Cancer Setting? Nutrition 2022, 98, 111624. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Clin. Nutr. 2022, 41, 990–1000. [Google Scholar] [CrossRef]
- BonDurant, L.D.; Potthoff, M.J. Fibroblast Growth Factor 21: A Versatile Regulator of Metabolic Homeostasis. Annu. Rev. Nutr. 2018, 38, 173–196. [Google Scholar] [CrossRef]
- Akyol, M.; Alacacioglu, A.; Demir, L.; Kucukzeybek, Y.; Yildiz, Y.; Gumus, Z.; Kara, M.; Salman, T.; Varol, U.; Taskaynatan, H.; et al. The Alterations of Serum FGF-21 Levels, Metabolic and Body Composition in Early Breast Cancer Patients Receiving Adjuvant Endocrine Therapy. Cancer Biomark. 2017, 18, 441–449. [Google Scholar] [CrossRef]
- Ranuncolo, S.M.; Armanasco, E.; Nuñez, M.; Yuan, L.; Makhkamov, S.; De Lorenzo, M.S. Role of the Serum Levels of the Inter-Organs Messenger Fibroblast Growth Factor 21 (FGF21) in the Diagnosis and Prognosis of Breast Cancer Patients. Cell Commun. Signal 2025, 23, 37. [Google Scholar] [CrossRef]
- Lee, S.J. Myostatin: A Skeletal Muscle Chalone. Annu. Rev. Physiol. 2023, 85, 269–291. [Google Scholar] [CrossRef]
- Wallner, C.; Drysch, M.; Becerikli, M.; Jaurich, H.; Wagner, J.M.; Dittfeld, S.; Nagler, J.; Harati, K.; Dadras, M.; Philippou, S.; et al. Interaction with the GDF8/11 Pathway Reveals Treatment Options for Adenocarcinoma of the Breast. Breast 2018, 37, 134–141. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Battaglini, C.L.; Williams, G.R. Bioelectrical Impedance Analysis for the Assessment of Sarcopenia in Patients with Cancer: A Systematic Review. Oncologist 2020, 25, 170–182. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.J.; Plaar, K.B.S.; Izaola-Jauregui, O.; Primo-Martín, D.; Gómez-Hoyos, E.; Torres-Torres, B.; De Luis-Román, D.A. Muscular Ultrasonography in Morphofunctional Assessment of Patients with Oncological Pathology at Risk of Malnutrition. Nutrients 2022, 14, 1573. [Google Scholar] [CrossRef] [PubMed]
- Cuatrecasas, G.; de Cabo, F.; Coves, M.J.; Patrascioiu, I.; Aguilar, G.; March, S.; Balfegó, M.; Bretxa, C.; Calbo, M.; Cuatrecasas, G.; et al. Ultrasound Measures of Abdominal Fat Layers Correlate with Metabolic Syndrome Features in Patients with Obesity. Obes. Sci. Pract. 2020, 6, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of Skeletal Muscle Mass by Bioelectrical Analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Yu, S.C.Y.; Powell, A.; Khow, K.S.F.; Visvanathan, R. The Performance of Five Bioelectrical Impedance Analysis Prediction Equations against Dual X-Ray Absorptiometry in Estimating Appendicular Skeletal Muscle Mass in an Adult Australian Population. Nutrients 2016, 8, 189. [Google Scholar] [CrossRef]
- Barbat-Artigas, S.; Filion, M.E.; Plouffe, S.; Aubertin-Leheudre, M. Muscle Quality as a Potential Explanation of the Metabolically Healthy but Obese and Sarcopenic Obese Paradoxes. Metab. Syndr. Relat. Disord. 2012, 10, 117–122. [Google Scholar] [CrossRef]
- Jung, W.; Park, S.H.; Park, Y.M.M.; Song, Y.M.; Park, J.H.; Yu, J.; Cho, I.Y.; Kim, B.S.; Han, K.; Shin, D.W. Weight Change and Cardiovascular Disease Incidence in Breast Cancer Survivors: A Nationwide Cohort Study. Breast Cancer Res. Treat. 2025, 210, 583–593. [Google Scholar] [CrossRef]
- Makari-Judson, G.; Viskochil, R.; Katz, D.; Barham, R.; Mertens, W.C. Insulin Resistance and Weight Gain in Women Treated for Early Stage Breast Cancer. Breast Cancer Res. Treat. 2022, 194, 423–431. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Ogunsina, K.; Braithwaite, D.; Akinyemiju, T. Metabolic Syndrome and Risk of Breast Cancer Mortality by Menopause, Obesity, and Subtype. Breast Cancer Res. Treat. 2019, 174, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.; Mo, F.K.F.; Pang, E.; Suen, J.J.S.; Koh, J.; Loong, H.H.F.; Yip, C.C.H.; Ng, R.Y.W.; Yip, C.H.W.; Tang, N.L.S.; et al. Profiles of Lipids, Blood Pressure and Weight Changes among Premenopausal Chinese Breast Cancer Patients after Adjuvant Chemotherapy. BMC Women’s Health 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Sheean, P.M.; Hoskins, K.; Stolley, M. Body Composition Changes in Females Treated for Breast Cancer: A Review of the Evidence. Breast Cancer Res. Treat. 2012, 135, 663–680. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.; Delmar, C.; Bendtsen, M.D.; Bosaeus, I.; Carus, A.; Falkmer, U.; Groenkjaer, M. Changes in Weight and Body Composition among Women with Breast Cancer during and after Adjuvant Treatment. Cancer Nurs. 2017, 40, 369–376. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Silva, C.; Galofré, J.C.; Escalada, J.; Santos, S.; Millán, D.; Vila, N.; Ibãez, P.; Gil, M.J.; Valentí, V.; et al. Body Mass Index Classification Misses Subjects with Increased Cardiometabolic Risk Factors Related to Elevated Adiposity. Int. J. Obes. 2012, 36, 286–294. [Google Scholar] [CrossRef]
- Aslani, A.; Smith, R.C.; Allen, B.J.; Pavlakis, N.; Levi, J.A. Changes in Body Composition During Breast Cancer Chemotherapy with the CMF-Regimen. Breast Cancer Res. Treat. 1999, 57, 285–290. [Google Scholar] [CrossRef]
- Chumlea, W.C.; Schubert, C.M.; Sun, S.S.; Demerath, E.; Towne, B.; Siervogel, R.M. A Review of Body Water Status and the Effects of Age and Body Fatness in Children and Adults. J. Nutr. Health Aging 2007, 11, 111–118. [Google Scholar]
- Blas, X.; Guigni, A.; Callahan, D.M.; Tourville, T.W.; Miller, M.S.; Fiske, B.; Voigt, T.; Korwin-Mihavics, B.; Anathy, V.; Dittus, K.; et al. Skeletal Muscle Atrophy and Dysfunction in Breast Cancer Patients: Role for Chemotherapy-Derived Oxidant Stress. Am. J. Physiol. Cell Physiol. 2018, 315, 744–756. [Google Scholar] [CrossRef]
- da Silva, B.R.; Rufato, S.; Mialich, M.S.; Cruz, L.P.; Gozzo, T.; Jordão, A.A. Phase Angle Is Related to Oxidative Stress and Antioxidant Biomarkers in Breast Cancer Patients Undergoing Chemotherapy. PLoS ONE 2023, 18, e0283235. [Google Scholar] [CrossRef]
- Ginzac, A.; Thivat, É.; Mouret-Reynier, M.A.; Dubray-Longeras, P.; Van Praagh, I.; Passildas, J.; Abrial, C.; Kwiatkowski, F.; Boirie, Y.; Duclos, M.; et al. Weight Evolution During Endocrine Therapy for Breast Cancer in Postmenopausal Patients: Effect of Initial Fat Mass Percentage and Previous Adjuvant Treatments. Clin. Breast Cancer 2018, 18, e1093–e1102. [Google Scholar] [CrossRef]
- Lee, K.; Sami, N.; Sweeney, F.C.; Dieli-Conwright, C.M. Body Composition with Dual-Energy X-Ray Absorptiometry and Bioelectrical Impedance Analysis in Breast Cancer Survivors. Nutr. Clin. Pract. 2019, 34, 421–427. [Google Scholar] [CrossRef]
- Guinan, E.M.; Connolly, E.M.; Healy, L.A.; Carroll, P.A.; Kennedy, M.J.; Hussey, J. The Development of the Metabolic Syndrome and Insulin Resistance after Adjuvant Treatment for Breast Cancer. Cancer Nurs. 2014, 37, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ramos da Silva, B.; Rufato, S.; Mialich, M.S.; Cruz, L.P.; Gozzo, T.; Jordao, A.A. Metabolic Syndrome and Unfavorable Outcomes on Body Composition and in Visceral Adiposities Indexes among Early Breast Cancer Women Post-Chemotherapy. Clin. Nutr. ESPEN 2021, 44, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.Y.; Seo, Y.G.; Cho, M.H.; Kim, M.J.; Choi, H.C. Weight Change and Associated Factors in Long-Term Breast Cancer Survivors. PLoS ONE 2016, 11, e0159098. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical Phase Angle and Impedance Vector Analysis-Clinical Relevance and Applicability of Impedance Parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Morlino, D.; Cioffi, I.; Marra, M.; Di Vincenzo, O.; Scalfi, L.; Pasanisi, F. Bioelectrical Phase Angle in Patients with Breast Cancer: A Systematic Review. Cancers 2022, 14, 2002. [Google Scholar] [CrossRef]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsch, J.F. Bioelectrical Impedance Phase Angle as a Prognostic Indicator in Breast Cancer. BMC Cancer 2008, 8, 249. [Google Scholar] [CrossRef]
- da Silva, B.R.; Gonzalez, M.C.; Cereda, E.; Prado, C.M. Exploring the Potential Role of Phase Angle as a Marker of Oxidative Stress: A Narrative Review. Nutrition 2022, 93, 111493. [Google Scholar] [CrossRef]
- da Costa Pereira, J.P.; de Rebouças, A.S.; Prado, C.M.; Gonzalez, M.C.; Cabral, P.C.; da Diniz, A.S.; Trussardi Fayh, A.P.; Silva, F.M. Phase Angle as a Marker of Muscle Quality: A Systematic Review and Meta-Analysis. Clin. Nutr. 2024, 43, 308–326. [Google Scholar] [CrossRef]
- Trestini, I.; Caldart, A.; Cintoni, M.; Sperduti, I.; Drudi, A.; Aluffi, G.; Fiorio, E.; Parolin, V.; Zambonin, V.; Zanelli, S.; et al. Predictive and Prognostic Effect of Computed Tomography–Derived Body Composition Analysis during Neoadjuvant Chemotherapy for Operable and Locally Advanced Breast Cancer. Nutrition 2023, 105, 111858. [Google Scholar] [CrossRef]
- Pedersini, R.; Schivardi, G.; Laini, L.; Zamparini, M.; Bonalumi, A.; di Mauro, P.; Bosio, S.; Amoroso, V.; Villa, N.; Alberti, A.; et al. Changes in Body Composition in Early Breast Cancer Patients Treated with Aromatase Inhibitors. J. Endocrinol. Investig. 2024, 47, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Hurwitz, S.; Shapiro, C.L.; Leboff, M.S. Premature Ovarian Failure and Body Composition Changes with Adjuvant Chemotherapy for Breast Cancer. Menopause 2011, 18, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Torri, L.; Lambertini, M.; De Giorgis, S.; Calabrese, M.; Tagliafico, A.S. Muscle Mass Loss after Neoadjuvant Chemotherapy in Breast Cancer: Estimation on Breast Magnetic Resonance Imaging Using Pectoralis Muscle Area. Eur. Radiol. 2020, 30, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Godinho-Mota, J.C.M.; Mota, J.F.; Gonçalves, L.V.; Soares, L.R.; Schincaglia, R.M.; Prado, C.M.; Martins, K.A.; Freitas-Junior, R. Chemotherapy Negatively Impacts Body Composition, Physical Function and Metabolic Profile in Patients with Breast Cancer. Clin. Nutr. 2021, 40, 3421–3428. [Google Scholar] [CrossRef]
- Mallard, J.; Hucteau, E.; Hureau, T.J.; Pagano, A.F. Skeletal Muscle Deconditioning in Breast Cancer Patients Undergoing Chemotherapy: Current Knowledge and Insights From Other Cancers. Front. Cell Dev. Biol. 2021, 9, 719643. [Google Scholar] [CrossRef]
- Kadakia, K.C.; Symanowski, J.T.; Aktas, A.; Szafranski, M.L.; Salo, J.C.; Meadors, P.L.; Walsh, D. Malnutrition Risk at Solid Tumor Diagnosis: The Malnutrition Screening Tool in a Large US Cancer Institute. Support. Care Cancer 2022, 30, 2237–2244. [Google Scholar] [CrossRef]
- Rier, H.N.; Jager, A.; Sleijfer, S.; van Rosmalen, J.; Kock, M.C.J.M.; Levin, M.D. Changes in Body Composition and Muscle Attenuation during Taxane-Based Chemotherapy in Patients with Metastatic Breast Cancer. Breast Cancer Res. Treat. 2018, 168, 95–105. [Google Scholar] [CrossRef]
- Mijwel, S.; Cardinale, D.A.; Norrbom, J.; Chapman, M.; Ivarsson, N.; Wengström, Y.; Sundberg, C.J.; Rundqvist, H. Exercise Training during Chemotherapy Preserves Skeletal Muscle Fiber Area, Capillarization, and Mitochondrial Content in Patients with Breast Cancer. FASEB J. 2018, 32, 5495–5505. [Google Scholar] [CrossRef]
- Weinberg, M.S.; Shachar, S.S.; Muss, H.B.; Deal, A.M.; Popuri, K.; Yu, H.; Nyrop, K.A.; Alston, S.M.; Williams, G.R. Beyond Sarcopenia: Characterization and Integration of Skeletal Muscle Quantity and Radiodensity in a Curable Breast Cancer Population. Breast J. 2018, 24, 278–284. [Google Scholar] [CrossRef]
- Barbat-Artigas, S.; Rolland, Y.; Zamboni, M.; Aubertin-Leheudre, M. How to Assess Functional Status: A New Muscle Quality Index. J. Nutr. Health Aging 2012, 16, 67–77. [Google Scholar] [CrossRef]
- Guan, Z.; Minnetti, M.; Heymsfield, S.B.; Poggiogalle, E.; Prado, C.M.; Sim, M.; Stephan, B.C.; Wells, J.C.; Donini, L.M.; Siervo, M. Beyond Traditional Body Composition Metrics: Load-Capacity Indices Emerge as Predictors of Cardiometabolic Outcomes—A Systematic Review and Meta-Analysis. Adv. Nutr. 2025, 16, 100364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Chen, H.W.; Lin, Y.; Li, F.R.; Zhong, Q.; Huang, Y.N.; Wu, X.B. Total and Regional Fat/Muscle Mass Ratio and Risks of Incident Cardiovascular Disease and Mortality. J. Am. Heart Assoc. 2023, 12, e030101. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.J.; Chang, Y.; Kang, J.G.; Kim, J.; Jung, H.S.; Yun, K.E.; Kim, C.W.; Shin, H.; Ryu, S. Low Relative Muscle Mass and Left Ventricular Diastolic Dysfunction in Middle-Aged Adults. Int. J. Cardiol. 2018, 255, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, Y.; Zhang, H.; Chen, C.; Wang, Y.; Zhang, J.; Xia, F.; Benedict, C.; Tan, X.; Lu, Y. Total and Regional Fat-to-Muscle Mass Ratio Measured by Bioelectrical Impedance and Risk of Incident Type 2 Diabetes. J. Cachexia Sarcopenia Muscle 2021, 12, 2154–2162. [Google Scholar] [CrossRef]
- Benavides-Rodríguez, L.; García-Hermoso, A.; Rodrigues-Bezerra, D.; Izquierdo, M.; Correa-Bautista, J.E.; Ramírez-Vélez, R. Relationship between Handgrip Strength and Muscle Mass in Female Survivors of Breast Cancer: A Mediation Analysis. Nutrients 2017, 9, 695. [Google Scholar] [CrossRef]
- Marriott, C.J.C.; Beaumont, L.F.; Farncombe, T.H.; Cranston, A.N.; Athale, U.H.; Yakemchuk, V.N.; Webber, C.E.; Barr, R.D. Body Composition in Long-Term Survivors of Acute Lymphoblastic Leukemia Diagnosed in Childhood and Adolescence: A Focus on Sarcopenic Obesity. Cancer 2018, 124, 1225–1231. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Wong, L.; Waliany, S.; Bernstein, L.; Salehian, B.; Mortimer, J.E. An Observational Study to Examine Changes in Metabolic Syndrome Components in Patients with Breast Cancer Receiving Neoadjuvant or Adjuvant Chemotherapy. Cancer 2016, 122, 2646–2653. [Google Scholar] [CrossRef]
- Martinez, P.; Grant, W.B. Vitamin D: What Role in Obesity-Related Cancer? Semin. Cancer Biol. 2025, 112, 135–149. [Google Scholar] [CrossRef]
- Allen, D.L.; Hittel, D.S.; McPherron, A.C. Expression and Function of Myostatin in Obesity, Diabetes, and Exercise Adaptation. Med. Sci. Sports Exerc. 2011, 43, 1828–1835. [Google Scholar] [CrossRef]
- Morlino, D.; Marra, M.; Cioffi, I.; Santarpia, L.; De Placido, P.; Giuliano, M.; De Angelis, C.; Carrano, S.; Verrazzo, A.; Buono, G.; et al. Prevalence of Sarcopenia in Women with Breast. Cancer Nutr. 2022, 14, 1839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Total (n = 61) | Premenopausal Status (n = 16) | Menopausal Status (n = 45) | p-Value | |
|---|---|---|---|---|
| Age (years) | 58.3 (10.0) | 47.2 (3.0) | 62.3 (8.6) | <0.001 |
| Tumor subtype | ||||
| In situ | 13 (21.3) | 3 (18.8) | 10 (22.2) | |
| Luminal A and B | 41 (67.2) | 12 (75.0) | 29 (64.4) | |
| Her 2+ (Luminal B HER2+ and HER2+) | 4 (6.6) | 1 (6.3) | 3 (6.6) | 0.962 |
| Triple negative | 1 (1.6) | 0 (0.0) | 1 (2.2) | |
| Unknown | 2 (3.3) | 0 (0.0) | 2 (4.4) | |
| Treatment (after surgery) | ||||
| ChT | 14 (23.0) | 4 (25.0) | 10 (22.2) | 1.000 |
| RT | 45 (73.8) | 14 (87.5) | 31 (68.9) | 0.196 |
| HT | 52 (85.3) | 14 (87.5) | 38 (84.4) | 1.000 |
| Tamoxifen | 22 (36.1) | 12 (75.0) | 10 (22.2) | 0.014 |
| Letrozole | 22 (36.1) | 2 (12.5) | 20 (44.4) | |
| Anastrozole | 8 (13.1) | 0 (0.0) | 8 (17.8) | |
| Smoker | ||||
| Never | 53 (86.9) | 15 (93.8) | 38 (84.4) | |
| Currently smoke | 5 (8.2) | 1 (6.3) | 4 (8.9) | 0.526 |
| Ex-smoker | 3 (4.9) | 0 (0.0) | 3 (6.7) | |
| Hypertension/dyslipidaemia | 23 (37.7) | 4 (25.0) | 19 (42.2) | 0.222 |
| Diabetes mellitus | 3 (4.9) | 1 (6.3) | 2 (4.4) | 0.082 |
| Basal | One Year | Absolute Change | Relative Change (%) | p-Value | |
|---|---|---|---|---|---|
| Weight (kg) | 62.6 (12.3) | 63.9 (11.7) | 1.0 (−0.7; 3.9) | 1.4 (−1.2; 6.0) | 0.021 |
| BMI (kg/m2) | 23.9 (4.6) | 25.3 (4.4) | 0.4 (−0.3; 1.5) | 1.4 (−1.3; 6.0) | 0.014 |
| WC (cm) | 87.7 (12.2) | 89.7 (13.6) | 0.6 (−3.0; 8.0) | 0.7 (−3.3; 8.4) | 0.083 |
| WHtR (cm/cm) | 0.55 (0.08) | 0.56 (0.09) | 0.00 (−0.02; 0.05) | 0.73 (−3.30; 8.43) | 0.090 |
| Rz (ohm) | 692.56 (81.02) | 686.36 (81.22) | −9.48 (−32.16; 21.54) | −1.27 (−4.52; 3.40) | 0.271 |
| Xc (ohm) | 56.13 (7.89) | 53.88 (7.89) | −2.21 (−5.24; 0.85) | −3.83 (−8.39; 1.66) | 0.001 |
| PhA (o) | 4.70 (4.15; 5.05) | 4.60 (4.25; 4.90) | −0.10 (−0.30; 0.05) | −2.08 (−6.00; 0.98) | 0.002 |
| PhA/BMI (o/kg∗m2) | 0.19 (0.04) | 0.18 (0.03) | −0.01 (0.02) | −4.90 (8.07) | <0.001 |
| ECW/TBW (%) | 46.23 (45.27; 47.90) | 47.19 (45.89; 48.50) | 0.65 (0.03; 1.48) | 1.36 (0.06; 3.21) | <0.001 |
| FM (kg) | 25.02 (8.69) | 26.22 (8.36) | 1.03 (−0.85; 3.56) | 3.58 (−2.68; 15.22) | 0.017 |
| FM (%) | 38.92 (6.99) | 40.14 (6.64) | 0.90 (−1.16; 3.02) | 2.20 (−2.39; 7.72) | 0.015 |
| FMI (kg/m2) | 9.53 (6.68; 12.86) | 9.99 (7.82; 12.42) | 0.43 (−0.28; 1.41) | 3.62 (−2.54; 15.34) | 0.006 |
| FM/FFM ratio | 0.63 (0.50; 0.81) | 0.67 (0.55; 0.84) | 0.02 (−0.03; 0.09) | 4.37 (−3.52; 12.75) | 0.018 |
| FFMI (kg/m2) | 14.99 (2.08) | 14.96 (1.63) | 0.18 (−0.27; 0.40) | 1.16 (−0.27; 2.99) | 0.848 |
| SMMI (kg/m2) | 6.22 (0.72) | 6.13 (0.76) | −0.08 (−0.33; 011) | −1.15 (−5.16; 1.83) | 0.035 |
| ASMMI (kg/m2) | 5.44 (5.09; 6.02) | 5.48 (5.16; 5.78) | 0.04 (−0.11; 017) | 0.75 (−2.06; 3.18) | 0.215 |
| ASMM/BMI (kg/kg∗m2) | 0.57 (0.07) | 0.56 (0.07) | −0.01 (−0.02; 0.00) | −1.64 (−3.92; 0.60) | 0.002 |
| SMM/weight (%) | 26.45 (2.83) | 25.69 (3.04) | −0.75 (−1.54; −0.06) | −2.90 (−5.70; −0.24) | <0.001 |
| VF (L) | 1.55 (1.20; 2.42) | 1.67 (1.17; 2.41) | 0.05 (−0.29; 0.41) | 2.95 (−16.69; 22.02) | 0.549 |
| HGS (kg) | 23.4 (4.5) | 22.3 (5.7) | −1.0 (3.0) | −3.9 (12.5) | 0.110 |
| HGS/SMM (kg/kg) | 1.45 (4.5) | 1.39 (0.28) | −0.06 (0.21) | −3.02 (14.66) | 0.037 |
| Basal (n = 58) | One Year (n = 55) | Absolute Change | Relative Change (%) | p-Value | |
|---|---|---|---|---|---|
| Abdominal measurements | |||||
| tASAT (cm) | 1.61 (0.67) | 1.80 (0.67) | 0.10 (−0.08; 0.44) | 7.03 (−5.20; 27.10) | 0.011 |
| sASAT (cm) | 0.71 (0.30) | 0.80 (0.33) | 0.01 (−0.09; 0.19) | 1.24 (−15.42; 35.00) | 0.097 |
| dASAT (cm) | 0.90 (0.44) | 1.02 (0.43) | 0.15 (−0.06; 0.31) | 14.44 (−7.43; 48.65) | 0.020 |
| Preperitoneal AT (cm) | 0.59 (0.36; 0.82) | 0.70 (0.51; 0.97) | 0.09 (−0.09; 0.29) | 15.19 (−14.12; 63.38) | 0.031 |
| Thigh and RF measurements | |||||
| Thigh SAT (cm) | 1.04 (0.36) | 1.29 (0.43) | 0.25 (0.11; 0.37) | 24.16 (0.14; 39.15) | <0.001 |
| Thickness RF (cm) | 1.04 (0.91; 1.26) | 1.18 (1.01; 1.40) | 0.12 (0.01; 0.29) | 11.05 (1.10; 30.62) | <0.001 |
| RF-CSA (cm2) | 3.10 (0.81) | 3.45 (0.96) | 0.36 (0.76) | 14.09 (25.25) | 0.001 |
| Basal | One Year | Absolute Change | Relative Change (%) | p-Value | |
|---|---|---|---|---|---|
| Glycemia (mg/dL) | 96.0 (89.0; 102.0) | 96.5 (91.0; 103.8) | 1.5 (−7.8; 8.0) | 1.7 (−7.3; 8.9) | 0.548 (n = 60) |
| HbA1c (%) | 5.6 (5.4; 5.8) | 5.6 (5.4; 5.8) | 0.0 (−0.1; 0.2) | 0.0 (−1.7; 3.4) | 0.076 (n = 57) |
| Insulin (µUI/mL) | 7.4 (4.1; 15.8) | 7.9 (3.6; 15.6) | 0.2 (−4.7; 5.4) | 0.6 (−43.3; 80.3) | 0.797 (n = 49) |
| HOMA-IR | 1.8 (1.2; 3.9) | 2.4 (1.2; 4.9) | 2.4 (1.2; 4.9) | 6.6 (−38.0; 99.7) | 0.436 (n = 37) |
| Total cholesterol (mg/dL) | 208.8 (38.1) | 200.6 (40.6) | −7.6 (35.1) | −2.6 (±17.5) | 0.109 (n = 56) |
| HDL-cholesterol (mg/dL) | 67.4 (14.0) | 66.1 (33.9) | −1.0 (7.7) | −0.5 (±11.2) | 0.334 (n = 55) |
| Non-HDL-cholesterol (mg/dL) | 140.5 (34.0) | 133.5 (35.6) | −9.0 (−27.0; 12.0) | −7.0 (−18.7; 7.9) | 0.159 (n = 55) |
| LDL-cholesterol (mg/dL) | 118.9 (36.4) | 112 (38.5) | −9.0 (−25.0; 10.0) | −9.0 (−25.0; 10.0) | 0.116 (n = 55) |
| Triglycerides (mg/dL) | 92.0 (72.0; 129.5) | 92.0 (74.0; 136.0) | 7.0 (−26.0; 31.8) | 7.9 (−28.4; 31.4) | 0.701 (n = 56) |
| CRP (mg/L) | 2.80 (2.80; 3.20) | 2.80 (2.85; 3.00) | 0.00 (−0.10; 0.00) | 0.00 (−3.45; 0.00) | 0.172 (n = 51) |
| IL-6 (pg/mL) | 1.80 (1.40; 3.50) | 1.40 (1.40; 2.70) | 0.00 (−1.10; 0.50) | 0.00 (−44.00; 21.74) | 0.397 (n = 51) |
| Vitamin D (ng/mL) | 24.0 (14.0; 33.5) | 25.0 (17.0; 38.0) | 1.0 (−4.5; 7.5) | 3.8 (−23.1; 46.2) | 0.226 (n = 53) |
| Myostatin (ng/mL) | 33.03 (27.95; 39.02) | 33.07 (29.67; 37.84) | 0.60 (−3.50; 3.54) | 1.60 (−8.23; 11.79) | 0.968 (n = 41) |
| FGF-21 (pg/mL) | 68.57 (38.75; 144.19) | 92.79 (48.22; 179.20) | 14.89 (−39.77; 76.93) | 32.13 (−32.69; 100.68) | 0.226 (n = 40) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larrad-Sáinz, A.; Hernández Núñez, M.G.; Barabash Bustelo, A.; Gil Prados, I.; Valerio, J.; Espadas Gil, J.L.; Olivares Crespo, M.E.; Herrera de la Muela, M.; Bernaldo Madrid, B.; Serrano García, I.; et al. Relationship Between Body Composition and Biomarkers in Adult Females with Breast Cancer: 1-Year Follow-Up Prospective Study. Nutrients 2025, 17, 2487. https://doi.org/10.3390/nu17152487
Larrad-Sáinz A, Hernández Núñez MG, Barabash Bustelo A, Gil Prados I, Valerio J, Espadas Gil JL, Olivares Crespo ME, Herrera de la Muela M, Bernaldo Madrid B, Serrano García I, et al. Relationship Between Body Composition and Biomarkers in Adult Females with Breast Cancer: 1-Year Follow-Up Prospective Study. Nutrients. 2025; 17(15):2487. https://doi.org/10.3390/nu17152487
Chicago/Turabian StyleLarrad-Sáinz, Angélica, María Gemma Hernández Núñez, Ana Barabash Bustelo, Inés Gil Prados, Johanna Valerio, José Luis Espadas Gil, María Eugenia Olivares Crespo, María Herrera de la Muela, Blanca Bernaldo Madrid, Irene Serrano García, and et al. 2025. "Relationship Between Body Composition and Biomarkers in Adult Females with Breast Cancer: 1-Year Follow-Up Prospective Study" Nutrients 17, no. 15: 2487. https://doi.org/10.3390/nu17152487
APA StyleLarrad-Sáinz, A., Hernández Núñez, M. G., Barabash Bustelo, A., Gil Prados, I., Valerio, J., Espadas Gil, J. L., Olivares Crespo, M. E., Herrera de la Muela, M., Bernaldo Madrid, B., Serrano García, I., Cristóbal García, I., Rubio-Herrera, M. Á., Calle-Pascual, A. L., Brenes Sánchez, J. M., & Matía-Martín, P. (2025). Relationship Between Body Composition and Biomarkers in Adult Females with Breast Cancer: 1-Year Follow-Up Prospective Study. Nutrients, 17(15), 2487. https://doi.org/10.3390/nu17152487






