Effect of Polyphenol-Rich Interventions on Gut Microbiota and Inflammatory or Oxidative Stress Markers in Adults Who Are Overweight or Obese: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Search Strategy
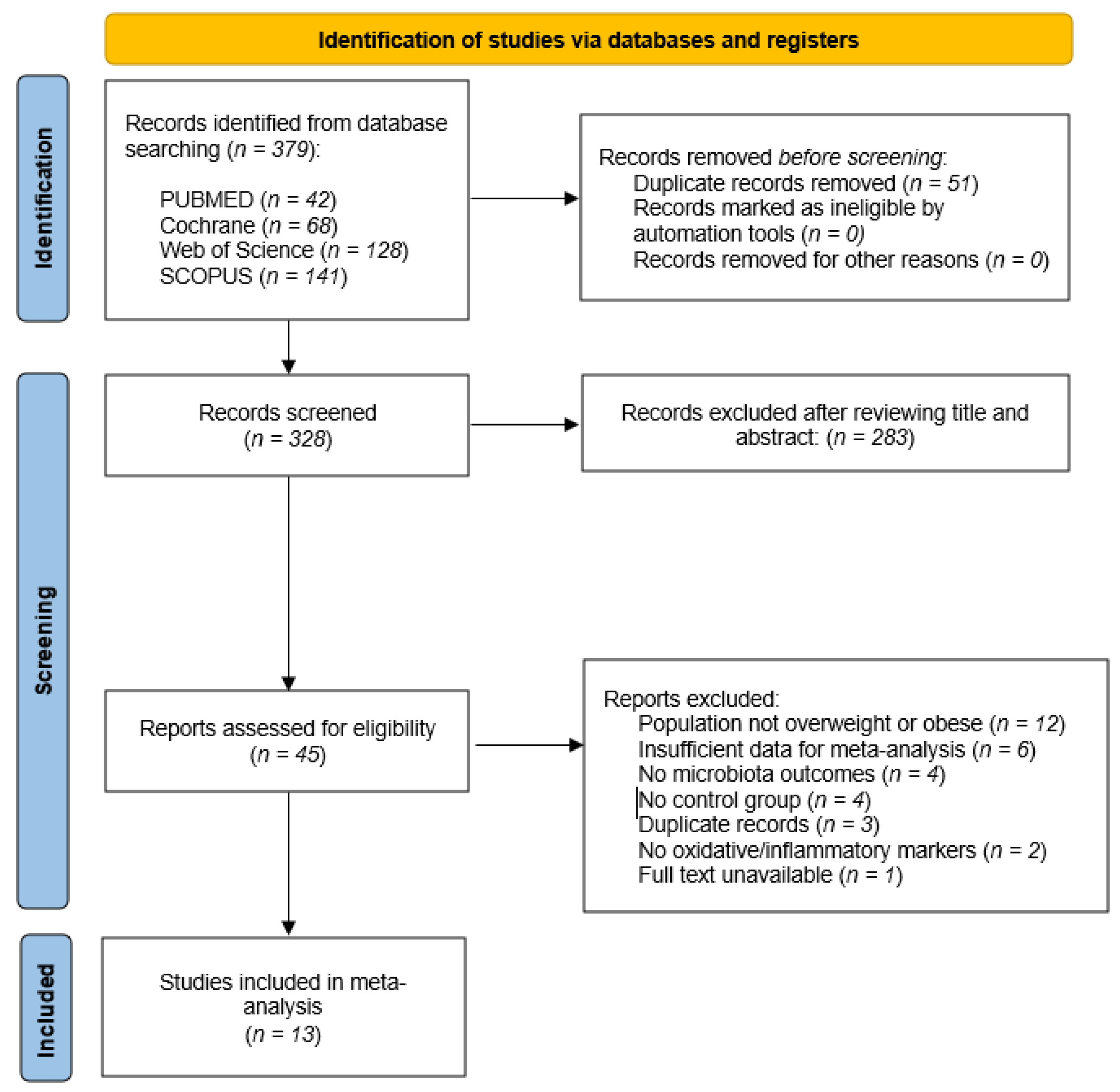
2.4. Study Selection
2.5. Data Extraction
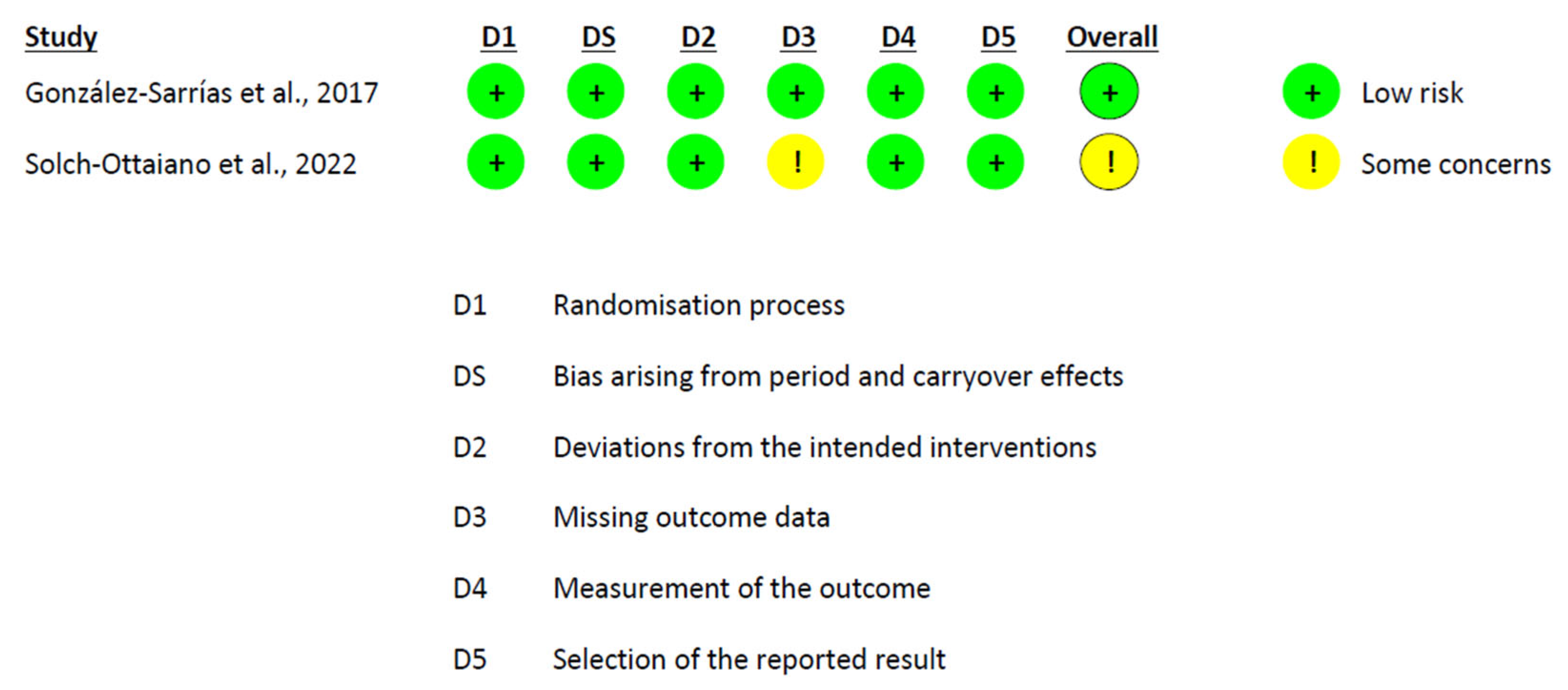
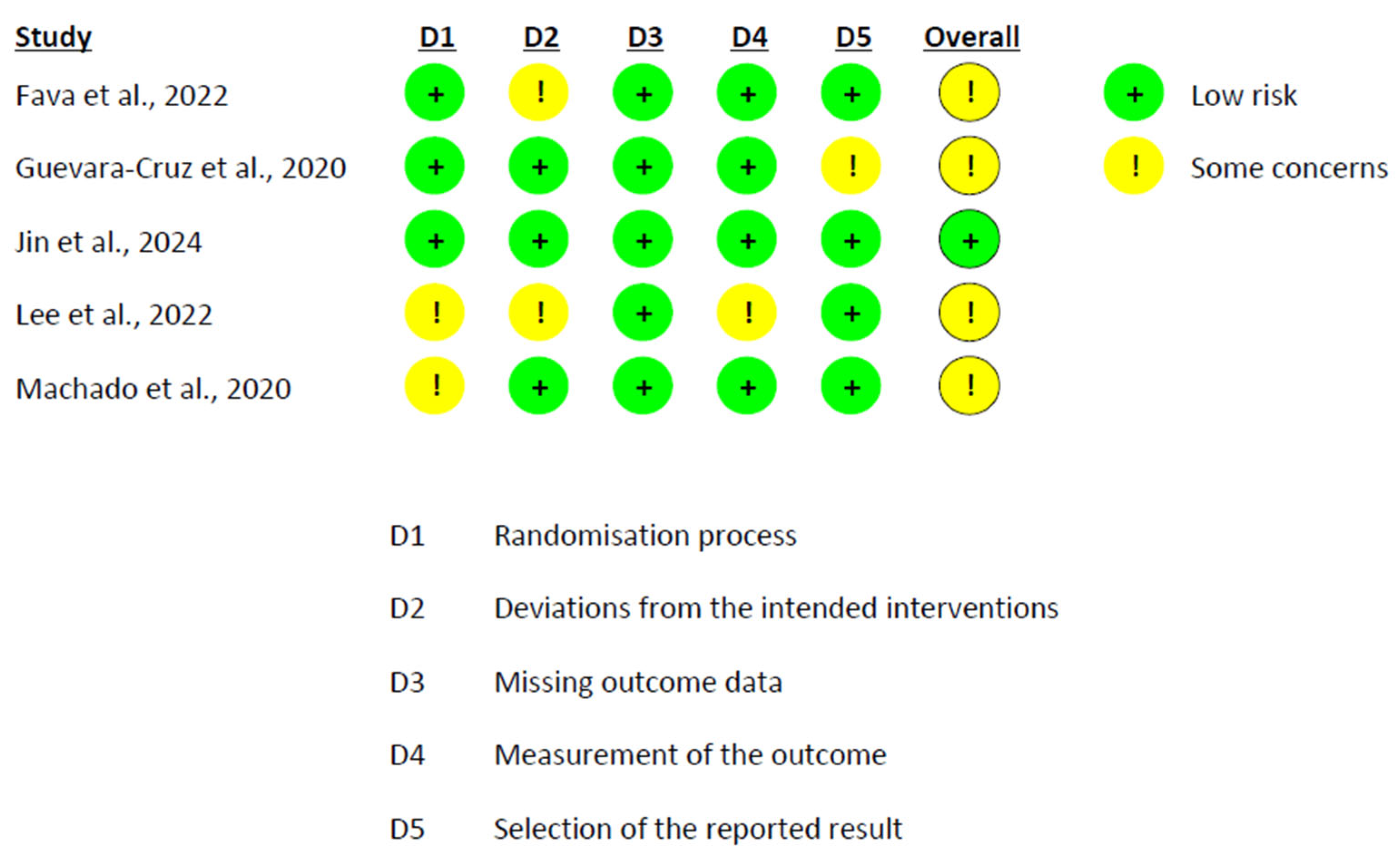
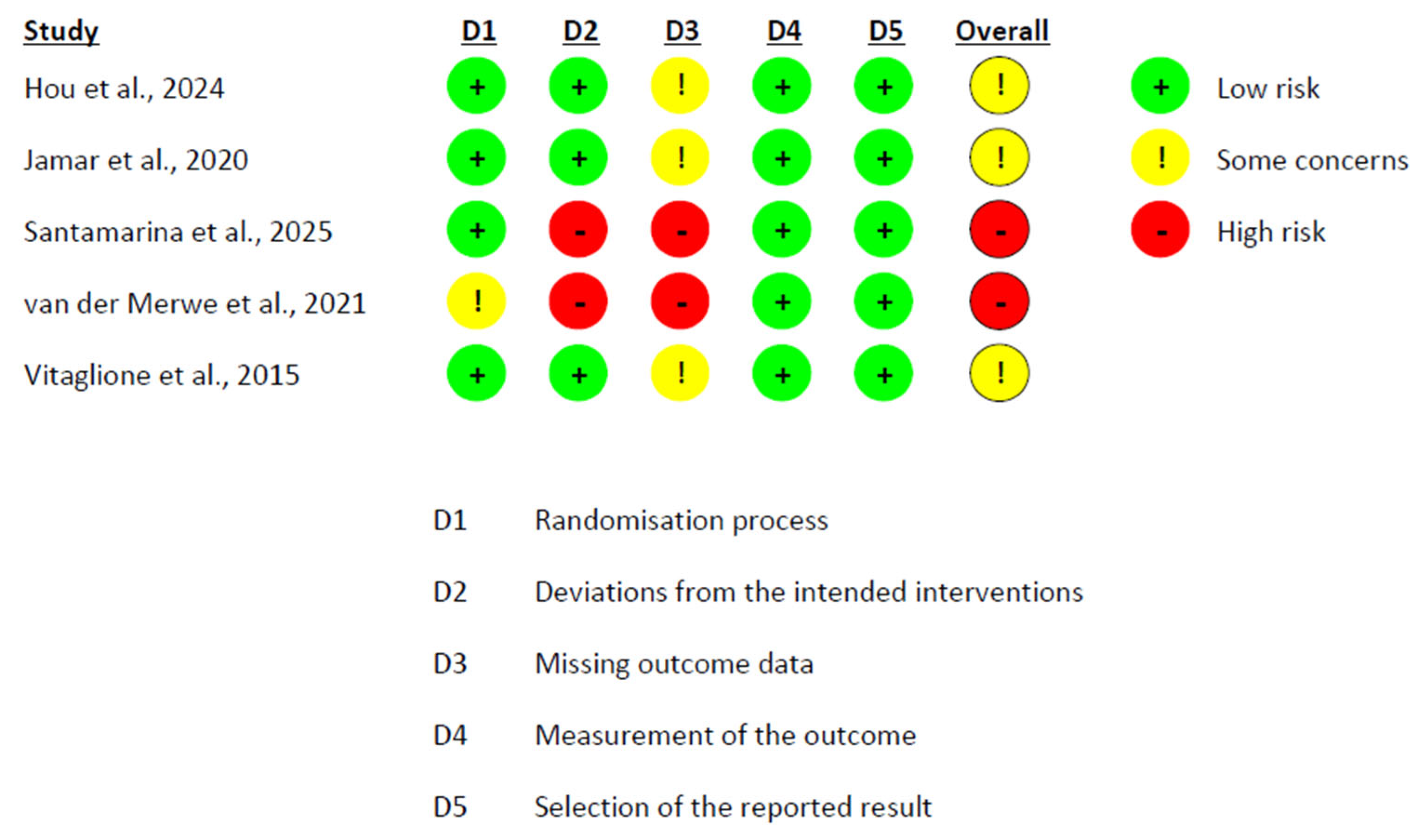
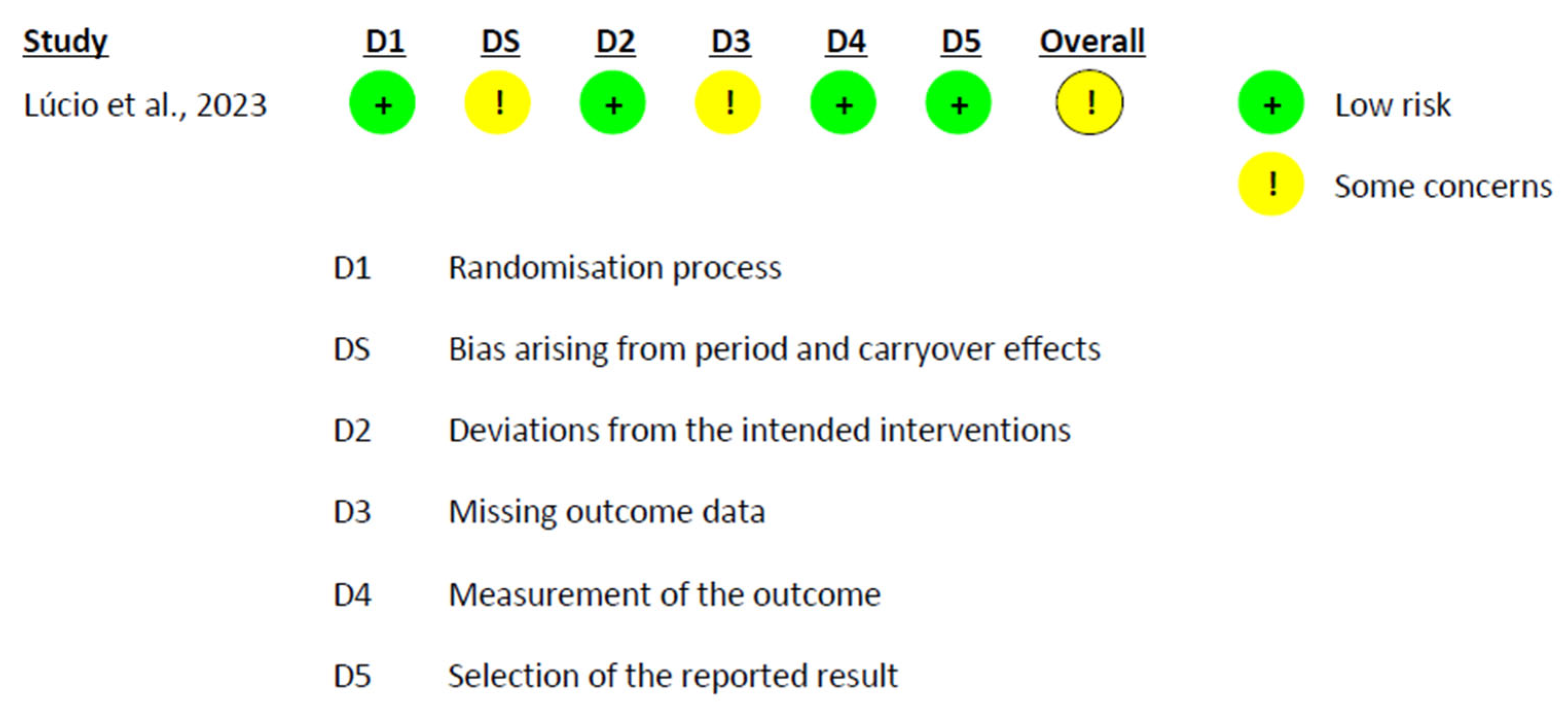
2.6. Risk of Bias Assessment
2.7. Data Synthesis
3. Results
3.1. Study Characteristics
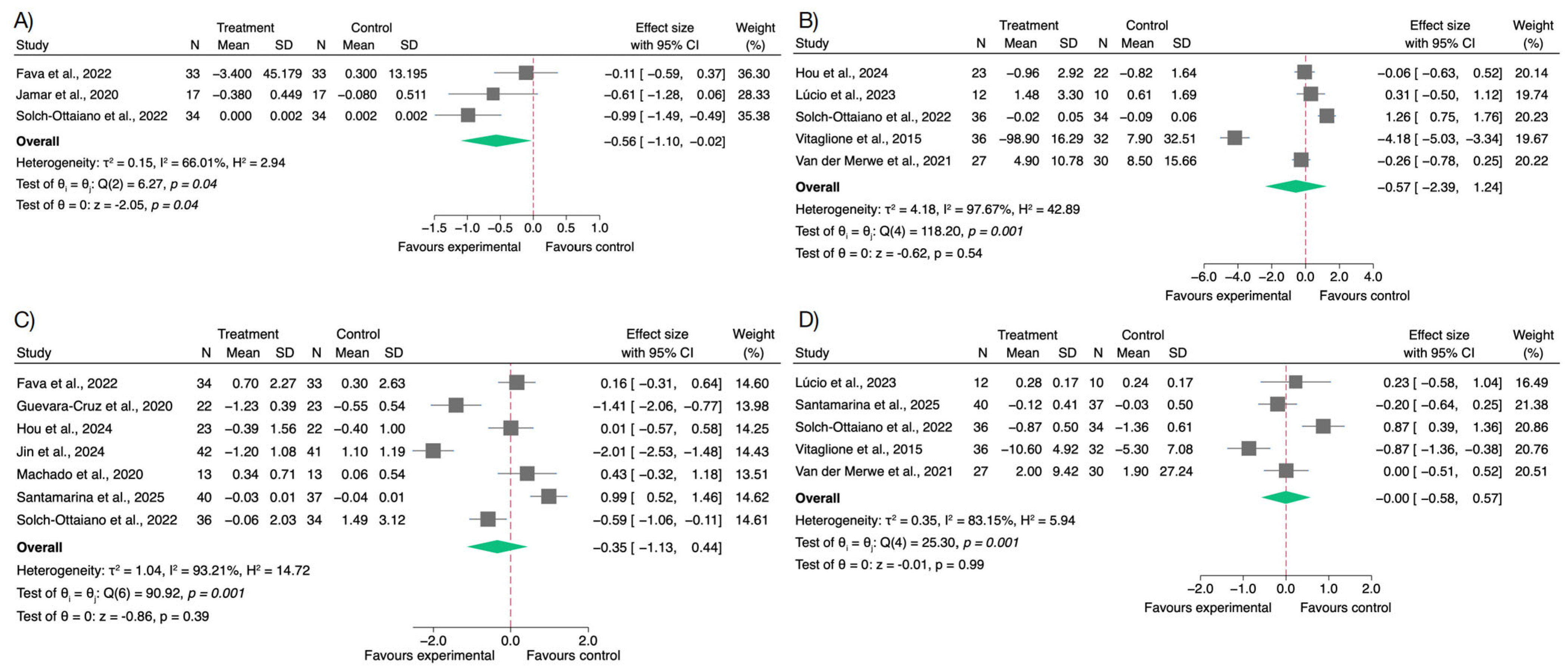
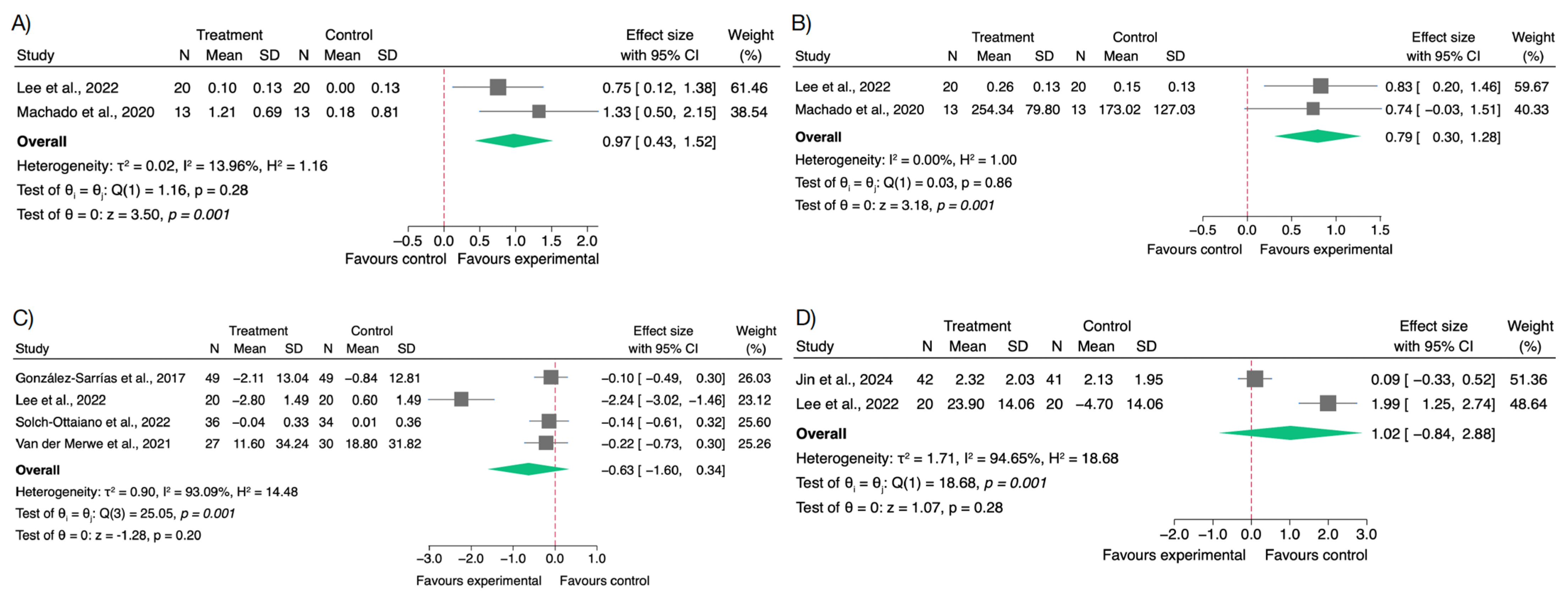
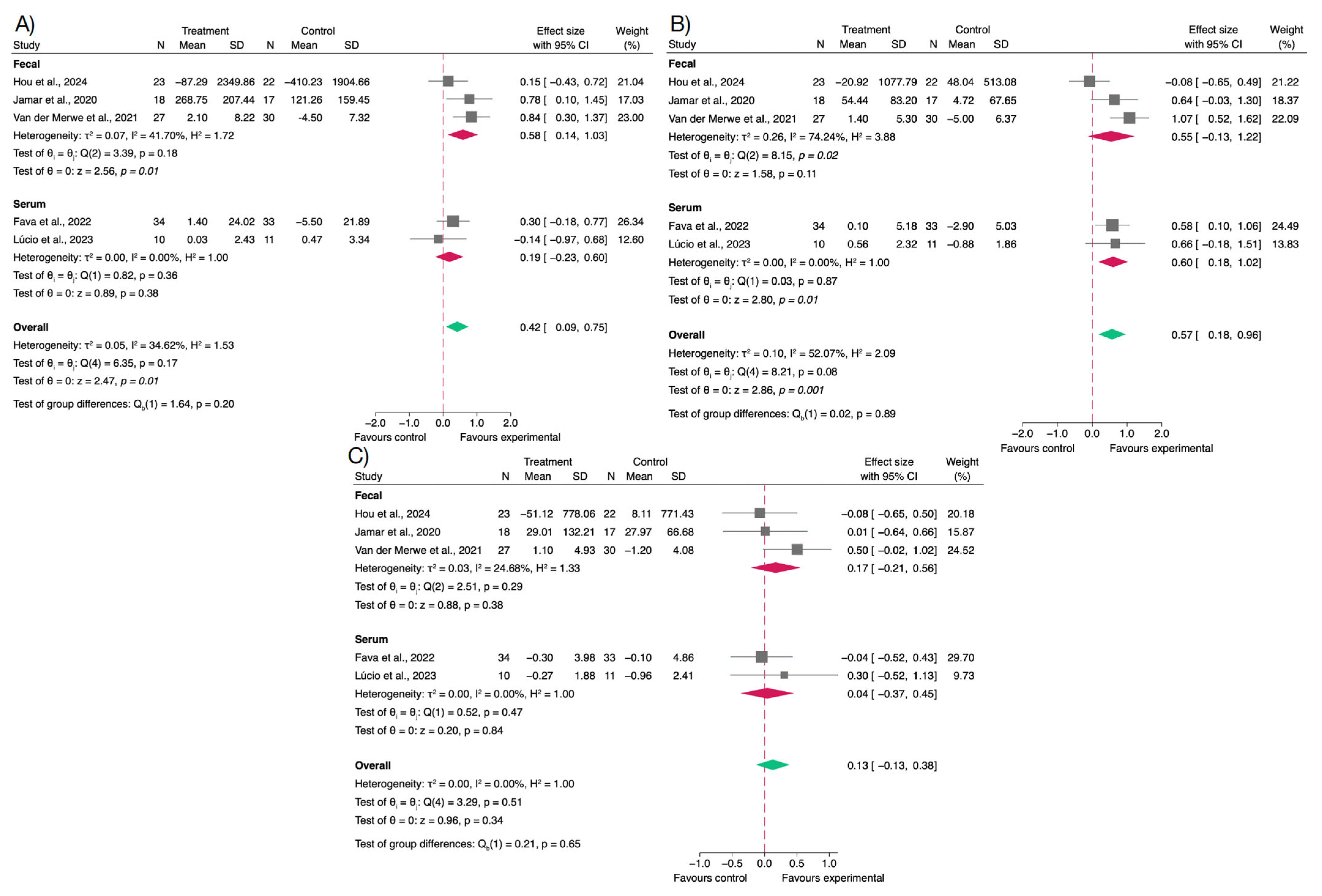
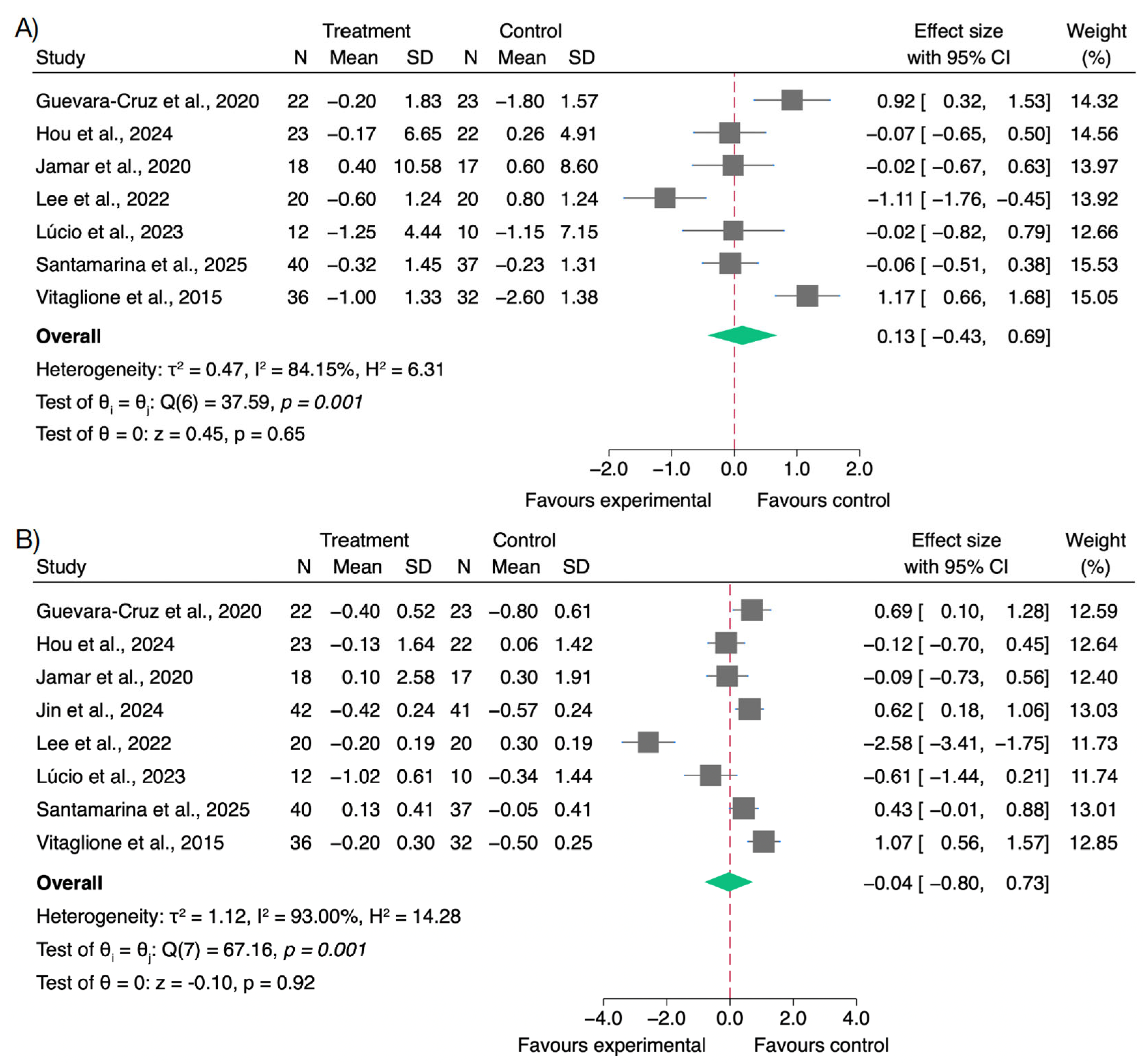
3.2. Effects on Inflammatory Biomarkers
3.3. Effects on Oxidative Stress and Antioxidant Biomarkers
3.4. Effects on Gut Microbiota and Short-Chain Fatty Acids
3.5. Effects on Body Weight and BMI
3.6. Risk of Bias Assessment
4. Discussion
4.1. Modulation of Metabolic Endotoxemia and Inflammatory Status
4.2. Oxidative Stress Biomarkers and Antioxidant Response
4.3. Effects on SCFAs Production
4.4. Effects of Polyphenol Supplementation on Body Weight and BMI
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CAT | Catalase |
| CRP | C-reactive Protein |
| GC–MS | Gas Chromatography–Mass Spectrometry |
| GPx | Glutathione Peroxidase |
| HPLC | High-Performance Liquid Chromatography |
| IL-6 | Interleukin 6 |
| ITT | Intention-to-Treat |
| LPS | Lipopolysaccharide |
| MeSH | Medical Subject Headings |
| MDA | Malondialdehyde |
| PP | Per-protocol |
| qPCR | Quantitative Polymerase Chain Reaction |
| RCTs | Randomized Controlled Trials |
| SCFA | Short-Chain Fatty Acids |
| SCFAs | Short-Chain Fatty Acids |
| SOD | Soperoxide Dismutase |
| TNF-α | Tumor Necrosis Factor Alpha |
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 June 2025).
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut Microbiota: A New Path to Treat Obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and Their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Bäckhed, F.; Cani, P.D. Targeting Gut Microbiota in Obesity: Effects of Prebiotics and Probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M. Novel Opportunities for Next-Generation Probiotics Targeting Metabolic Syndrome. Curr. Opin. Biotechnol. 2015, 32, 21–27. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell Longev. 2009, 2, 270. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping Faecal Gut Microbiota Composition by the Intake of Trans-Resveratrol and Quercetin in High-Fat Sucrose Diet-Fed Rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P.E. The Neuroprotective Potential of Flavonoids: A Multiplicity of Effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Chen, F.; Dai, D. The Regulation of Host Intestinal Microbiota by Polyphenols in the Development and Prevention of Chronic Kidney Disease. Front. Immunol. 2020, 10, 507762. [Google Scholar] [CrossRef]
- Mao, T.; Zhang, Y.; Kaushik, R.; Mohan, M.S. Effects of Polyphenols on Gut Microbiota and Inflammatory Markers in Individuals with Overweight or Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2024, 1–18. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The Impact of Patient, Intervention, Comparison, Outcome (Pico) as a Search Strategy Tool on Literature Search Quality: A Systematic Review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook (accessed on 24 June 2025).
- Automeris WebPlotDigitizer: Computer Vision Assisted Data Extraction from Charts. Available online: https://automeris.io/ (accessed on 24 June 2025).
- Kim, H.; Barnes, R.; Fang, C.; Talcott, S.; Mertens-Talcott, S. Intestinal Microbiota and Host Metabolism Respond Differentially in Lean and Obese Individuals Following Six-Week Consumption of Galloyl Derivatives from Mango (Mangifera Indica L.) Pulp. FASEB J. 2017, 31, 166–168. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Mosele, J.I.; Pizarro, N.; Farràs, M.; de la Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castañer, O.; Solà, R.; Fernandez-Castillejo, S.; et al. Effect of Virgin Olive Oil and Thyme Phenolic Compounds on Blood Lipid Profile: Implications of Human Gut Microbiota. Eur. J. Nutr. 2017, 56, 119–131. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Iglesias, D.E.; Kang, J.; Wang, Z.; Gray, R.; Mastaloudis, A.; Kay, C.D.; Hester, S.N.; Wood, S.M.; et al. A Randomized Placebo-Controlled Cross-over Study on the Effects of Anthocyanins on Inflammatory and Metabolic Responses to a High-Fat Meal in Healthy Subjects. Redox Biol. 2022, 51, 102273. [Google Scholar] [CrossRef]
- Tian, Z.; Li, K.; Fan, D.; Zhao, Y.; Gao, X.; Ma, X.; Xu, L.; Shi, Y.; Ya, F.; Zou, J.; et al. Dose-Dependent Effects of Anthocyanin Supplementation on Platelet Function in Subjects with Dyslipidemia: A Randomized Clinical Trial. EBioMedicine 2021, 70, 103533. [Google Scholar] [CrossRef] [PubMed]
- Lear, R.; O’leary, M.; Andersen, L.O.; Holt, C.C.; Stensvold, C.R.; van der Giezen, M.; Bowtell, J.L. Tart Cherry Concentrate Does Not Alter the Gut Microbiome, Glycaemic Control or Systemic Inflammation in a Middle-Aged Population. Nutrients 2019, 11, 1063. [Google Scholar] [CrossRef]
- Teixeira, K.T.R.; Moreira, L.D.S.G.; Borges, N.A.; Brum, I.; de Paiva, B.R.; Alvarenga, L.; Nakao, L.S.; Leal, V.D.O.; Carraro-Eduardo, J.C.; Rodrigues, S.D.; et al. Effect of Cranberry Supplementation on Toxins Produced by the Gut Microbiota in Chronic Kidney Disease Patients: A Pilot Randomized Placebo-Controlled Trial. Clin. Nutr. ESPEN 2022, 47, 63–69. [Google Scholar] [CrossRef]
- Pushpass, R.A.G.; Alzoufairi, S.; Mancini, A.; Quilter, K.; Fava, F.; Delaiti, S.; Vrhovsek, U.; Christensen, C.; Joyce, S.A.; Tuohy, K.M.; et al. Chronic Consumption of Probiotics, Oats, and Apples Has Differential Effects on Postprandial Bile Acid Profile and Cardiometabolic Disease Risk Markers Compared with an Isocaloric Control (Cornflakes): A Randomized Trial. Am. J. Clin. Nutr. 2023, 117, 252–265. [Google Scholar] [CrossRef]
- Dionísio, A.P.; da Silva, M.d.F.G.; Carioca, A.A.F.; Adriano, L.S.; de Abreu, F.A.P.; Wurlitzer, N.J.; Pinto, C.d.O.; Pontes, D.F. Effect of Yacon Syrup on Blood Lipid, Glucose and Metabolic Endotoxemia in Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Food Sci. Technol. 2019, 40, 194–201. [Google Scholar] [CrossRef]
- Sierra, J.A.; Escobar, J.S.; Corrales-Agudelo, V.; Lara-Guzmán, O.J.; Velásquez-Mejía, E.P.; Henao-Rojas, J.C.; Caro-Quintero, A.; Vaillant, F.; Muñoz-Durango, K. Consumption of Golden Berries (Physalis peruviana L.) Might Reduce Biomarkers of Oxidative Stress and Alter Gut Permeability in Men without Changing Inflammation Status or the Gut Microbiota. Food Res. Int. 2022, 162, 111949. [Google Scholar] [CrossRef]
- Barnes, R.C.; Kim, H.; Fang, C.; Bennett, W.; Nemec, M.; Sirven, M.A.; Suchodolski, J.S.; Deutz, N.; Britton, R.A.; Mertens-Talcott, S.U.; et al. Body Mass Index as a Determinant of Systemic Exposure to Gallotannin Metabolites during 6-Week Consumption of Mango (Mangifera indica L.) and Modulation of Intestinal Microbiota in Lean and Obese Individuals. Mol. Nutr. Food Res. 2019, 63, 1800512. [Google Scholar] [CrossRef] [PubMed]
- Groh, I.A.M.; Riva, A.; Braun, D.; Sutherland, H.G.; Williams, O.; Bakuradze, T.; Pahlke, G.; Richling, E.; Haupt, L.M.; Griffiths, L.R.; et al. Long-Term Consumption of Anthocyanin-Rich Fruit Juice: Impact on Gut Microbiota and Antioxidant Markers in Lymphocytes of Healthy Males. Antioxidants 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Jensen, R.I.; Németh-Balogh, M.; Paulovicsová, B.; Bergström, A.; Wilcks, A.; Licht, T.R.; et al. Intake of Whole Apples or Clear Apple Juice Has Contrasting Effects on Plasma Lipids in Healthy Volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Tassotti, M.; Martini, D.; Rosi, A.; Brighenti, F.; Del Rio, D. The Pocket-4-Life Project, Bioavailability and Beneficial Properties of the Bioactive Compounds of Espresso Coffee and Cocoa-Based Confectionery Containing Coffee: Study Protocol for a Randomized Cross-over Trial. Trials 2017, 18, 1–11. [Google Scholar] [CrossRef]
- Barone Lumaga, R.; Tagliamonte, S.; De Rosa, T.; Valentino, V.; Ercolini, D.; Vitaglione, P. Consumption of a Sourdough-Leavened Croissant Enriched with a Blend of Fibers Influences Fasting Blood Glucose in a Randomized Controlled Trial in Healthy Subjects. J. Nutr. 2024, 154, 2976–2987. [Google Scholar] [CrossRef]
- Gargari, G.; Taverniti, V.; Del Bo’, C.; Bernardi, S.; Hidalgo-Liberona, N.; Meroño, T.; Andres-Lacueva, C.; Kroon, P.A.; Cherubini, A.; Riso, P.; et al. Higher Bacterial DNAemia Can Affect the Impact of a Polyphenol-Rich Dietary Pattern on Biomarkers of Intestinal Permeability and Cardiovascular Risk in Older Subjects. Eur. J. Nutr. 2022, 61, 1209–1220. [Google Scholar] [CrossRef]
- Rehman, A.; Tyree, S.M.; Fehlbaum, S.; DunnGalvin, G.; Panagos, C.G.; Guy, B.; Patel, S.; Dinan, T.G.; Duttaroy, A.K.; Duss, R.; et al. A Water-Soluble Tomato Extract Rich in Secondary Plant Metabolites Lowers Trimethylamine-n-Oxide and Modulates Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled Cross-over Study in Overweight and Obese Adults. J. Nutr. 2023, 153, 96–105. [Google Scholar] [CrossRef]
- Hornero-Ramirez, H.; Morisette, A.; Marcotte, B.; Penhoat, A.; Lecomte, B.; Panthu, B.; Lessard Lord, J.; Thirion, F.; Van-Den-Berghe, L.; Blond, E.; et al. Multifunctional Dietary Approach Reduces Intestinal Inflammation in Relation with Changes in Gut Microbiota Composition in Subjects at Cardiometabolic Risk: The SINFONI Project. Gut Microbes 2025, 17, 2438823. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Iglesias-Aguirre, C.E.; Meoro, A.; Selma, M.V.; Espín, J.C. Pharmacological Therapy Determines the Gut Microbiota Modulation by a Pomegranate Extract Nutraceutical in Metabolic Syndrome: A Randomized Clinical Trial. Mol. Nutr. Food Res. 2021, 65, 2001048. [Google Scholar] [CrossRef]
- Hodges, J.K.; Zhu, J.; Yu, Z.; Vodovotz, Y.; Brock, G.; Sasaki, G.Y.; Dey, P.; Bruno, R.S. Intestinal-Level Anti-Inflammatory Bioactivities of Catechin-Rich Green Tea: Rationale, Design, and Methods of a Double-Blind, Randomized, Placebo-Controlled Crossover Trial in Metabolic Syndrome and Healthy Adults. Contemp. Clin. Trials Commun. 2020, 17, 100495. [Google Scholar] [CrossRef]
- Paquette, M.; Medina Larqué, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and Cranberry Polyphenols Improve Insulin Sensitivity in Insulin-Resistant, Non-Diabetic Adults: A Parallel, Double-Blind, Controlled and Randomised Clinical Trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef]
- Dahlberg, C.J.; Ou, J.J.; Babish, J.G.; Lamb, J.J.; Eliason, S.; Brabazon, H.; Gao, W.; Kaadige, M.R.; Tripp, M.L. A 13-Week Low Glycemic Load Diet and Lifestyle Modification Program Combining Low Glycemic Load Protein Shakes and Targeted Nutraceuticals Improved Weight Loss and Cardio-Metabolic Risk Factors. Can. J. Physiol. Pharmacol. 2017, 95, 1414–1425. [Google Scholar] [CrossRef]
- Most, J.; Timmers, S.; Warnke, I.; Jocken, J.W.E.; Van Boekschoten, M.; De Groot, P.; Bendik, I.; Schrauwen, P.; Goossens, G.H.; Blaak, E.E. Combined Epigallocatechin-3-Gallate and Resveratrol Supplementation for 12 Wk Increases Mitochondrial Capacity and Fat Oxidation, but Not Insulin Sensitivity, in Obese Humans: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2016, 104, 215–227. [Google Scholar] [CrossRef] [PubMed]
- de Santana, A.A.; de Castro Tobaruela, E.; dos Santos, K.G.; Sparvoli, L.G.; do Amaral, C.K.; Magnoni, C.D.; Taddei, C.R.; dos Santos, R.V.T.; Hassimotto, N.M.A.; Lajolo, F.M. ‘Pera’ Orange and ‘Moro’ Blood Orange Juice Improves Oxidative Stress and Inflammatory Response Biomarkers and Modulates the Gut Microbiota of Individuals with Insulin Resistance and Different Obesity Classes. Obesities 2022, 2, 389–412. [Google Scholar] [CrossRef]
- Wilson, R.; Willis, J.; Gearry, R.B.; Hughes, A.; Lawley, B.; Skidmore, P.; Frampton, C.; Fleming, E.; Anderson, A.; Jones, L.; et al. Sungold Kiwifruit Supplementation of Individuals with Prediabetes Alters Gut Microbiota and Improves Vitamin C Status, Anthropometric and Clinical Markers. Nutrients 2018, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Martina, A.; Rosi, A.; Veronesi, L.; Antonini, M.; Mennella, I.; Vitaglione, P.; Grioni, S.; Brighenti, F.; Zavaroni, I.; et al. Glucose- A Nd Lipid-Related Biomarkers Are Affected in Healthy Obese or Hyperglycemic Adults Consuming a Whole-Grain Pasta Enriched in Prebiotics and Probiotics: A 12-Week Randomized Controlled Trial. J. Nutr. 2019, 149, 1714–1723. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red Wine Polyphenols Modulate Fecal Microbiota and Reduce Markers of the Metabolic Syndrome in Obese Patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef]
- Most, J.; Penders, J.; Lucchesi, M.; Goossens, G.H.; Blaak, E.E. Gut Microbiota Composition in Relation to the Metabolic Response to 12-Week Combined Polyphenol Supplementation in Overweight Men and Women. Eur. J. Clin. Nutr. 2017, 71, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; Jamar, G.; Mennitti, L.V.; Cesar, H.d.C.; Vasconcelos, J.R.; Oyama, L.M.; de Rosso, V.V.; Pisani, L.P. Obesity-Related Inflammatory Modulation by Juçara Berry (Euterpe edulis Mart.) Supplementation in Brazilian Adults: A Double-Blind Randomized Controlled Trial. Eur. J. Nutr. 2020, 59, 1693–1705. [Google Scholar] [CrossRef]
- Selma, M.V.; González-Sarrías, A.; Salas-Salvadó, J.; Andrés-Lacueva, C.; Alasalvar, C.; Örem, A.; Tomás-Barberán, F.A.; Espín, J.C. The Gut Microbiota Metabolism of Pomegranate or Walnut Ellagitannins Yields Two Urolithin-Metabotypes That Correlate with Cardiometabolic Risk Biomarkers: Comparison between Normoweight, Overweight-Obesity and Metabolic Syndrome. Clin. Nutr. 2018, 37, 897–905. [Google Scholar] [CrossRef]
- Qiu, L.; Gao, C.; Wang, H.; Ren, Y.; Li, J.; Li, M.; Du, X.; Li, W.; Zhang, J. Effects of Dietary Polyphenol Curcumin Supplementation on Metabolic, Inflammatory, and Oxidative Stress Indices in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2023, 14, 1216708. [Google Scholar] [CrossRef]
- Teets, C.; Ghanem, N.; Ma, G.; Minj, J.; Perkins-Veazie, P.; Johnson, S.A.; Etter, A.J.; Carbonero, F.G.; Solverson, P.M. A One-Week Elderberry Juice Intervention Augments the Fecal Microbiota and Suggests Improvement in Glucose Tolerance and Fat Oxidation in a Randomized Controlled Trial. Nutrients 2024, 16, 3555. [Google Scholar] [CrossRef]
- Fava, F.; Ulaszewska, M.M.; Scholz, M.; Stanstrup, J.; Nissen, L.; Mattivi, F.; Vermeiren, J.; Bosscher, D.; Pedrolli, C.; Tuohy, K.M. Impact of Wheat Aleurone on Biomarkers of Cardiovascular Disease, Gut Microbiota and Metabolites in Adults with High Body Mass Index: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Eur. J. Nutr. 2022, 61, 2651–2671. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Godinez-Salas, E.T.; Sanchez-Tapia, M.; Torres-Villalobos, G.; Pichardo-Ontiveros, E.; Guizar-Heredia, R.; Arteaga-Sanchez, L.; Gamba, G.; Mojica-Espinosa, R.; Schcolnik-Cabrera, A.; et al. Genistein Stimulates Insulin Sensitivity through Gut Microbiota Reshaping and Skeletal Muscle AMPK Activation in Obese Subjects. BMJ Open Diabetes Res. Care 2020, 8, 948. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Örem, A.; Zafrilla, P.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Clustering According to Urolithin Metabotype Explains the Interindividual Variability in the Improvement of Cardiovascular Risk Biomarkers in Overweight-Obese Individuals Consuming Pomegranate: A Randomized Clinical Trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef]
- Hou, C.; Shi, H.; Xiao, J.; Song, X.; Luo, Z.; Ma, X.; Shi, L.; Wei, H.; Li, J. Pomegranate Juice Supplemented with Inulin Modulates Gut Microbiota and Promotes the Production of Microbiota-Associated Metabolites in Overweight/Obese Individuals: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Agric. Food Chem. 2024, 72, 14663–14677. [Google Scholar] [CrossRef] [PubMed]
- Jamar, G.; Santamarina, A.B.; Casagrande, B.P.; Estadella, D.; de Rosso, V.V.; Wagner, R.; Fagundes, M.B.; Pisani, L.P. Prebiotic Potencial of Juçara Berry on Changes in Gut Bacteria and Acetate of Individuals with Obesity. Eur. J. Nutr. 2020, 59, 3767–3778. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, X.; Chen, K.; Chen, Y.; Zhou, L.; Zeng, Y.; Zhou, Y.; Pan, Z.; Wang, D.; Li, Z.; et al. Silymarin Decreases Liver Stiffness Associated with Gut Microbiota in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Lipids Health Dis. 2024, 23, 239. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kang, S.; Kang, N.; Yu, J.; Park, T.; Lee, S.; Kwon, O. Effect of Ecklonia Cava Polyphenol on Adiposity Reduction Is Associated with Gut Microbiota Composition in Subjects with Abdominal Obesity: A Secondary Analysis. J. Funct. Foods 2022, 99, 105333. [Google Scholar] [CrossRef]
- Lúcio, H.; Anunciação, P.; da Silva, B.; da Silva, A.; Queiroz, V.; de Carvalho, C.; Pinheiro-Sant’Ana, H.; Martino, H. Consumption of Extruded Sorghum SC319 Improved Gut Microbiota at Genus Level and Reduced Anthropometric Markers in Men with Overweight: A Randomized Controlled Clinical Trial. Nutrients 2023, 15, 3786. [Google Scholar] [CrossRef]
- Machado, A.M.; da Silva, N.B.M.; de Freitas, R.M.P.; de Freitas, M.B.D.; Chaves, J.B.P.; Oliveira, L.L.; Martino, H.S.D.; Alfenas, R.d.C.G. Effects of Yacon Flour Associated with an Energy Restricted Diet on Intestinal Permeability, Fecal Short Chain Fatty Acids, Oxidative Stress and Inflammation Markers Levels in Adults with Obesity or Overweight: A Randomized, Double Blind, Placebo Controlled Clinical Trial. Arch. Endocrinol. Metab. 2020, 64, 597–607. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Filho, V.N.; de Freitas, J.A.; Franco, L.A.M.; Martins, R.C.; Fonseca, J.V.; Orellana Turri, J.A.; Hufnagel, M.T.; Demarque, D.P.; da Silva, B.F.R.B.; et al. Nutraceutical Blends Promote Weight Loss, Inflammation Reduction, and Better Sleep: The Role of Faecalibacterium Prausnitzii in Overweight Adults—A Double-Blind Trial. Mol. Nutr. Food Res. 2025, e202400806. [Google Scholar] [CrossRef]
- Solch-Ottaiano, R.J.; Judkins, T.C.; Matott, S.H.; McDermott, C.E.; Nieves, C.; Wang, Y.; Colee, J.; Tagliamonte, M.S.; Dissanayake, U.; Mai, V.; et al. High Polyphenolic Cranberry Beverage Alters Specific Fecal Microbiota but Not Gut Permeability Following Aspirin Challenge in Healthy Obese Adults: A Randomized, Double-Blind, Crossover Trial. J. Funct. Foods 2022, 99, 105332. [Google Scholar] [CrossRef]
- van der Merwe, M.; Moore, D.; Hill, J.L.; Keating, F.H.; Buddington, R.K.; Bloomer, R.J.; Wang, A.; Bowman, D.D. The Impact of a Dried Fruit and Vegetable Supplement and Fiber Rich Shake on Gut and Health Parameters in Female Healthcare Workers: A Placebo-controlled, Double-blind, Randomized Clinical Trial. Microorganisms 2021, 9, 843. [Google Scholar] [CrossRef]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-Grain Wheat Consumption Reduces Inflammation in a Randomized Controlled Trial on Overweight and Obese Subjects with Unhealthy Dietary and Lifestyle Behaviors: Role of Polyphenols Bound to Cereal Dietary Fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef]
- Rębas, E. Role of Flavonoids in Protecting Against Neurodegenerative Diseases—Possible Mechanisms of Action. Int. J. Mol. Sci. 2025, 26, 4763. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Ma, N.; Chen, Z.Y. Beneficial Effects of Dietary Polyphenols on High-Fat Diet-Induced Obesity Linking with Modulation of Gut Microbiota. J. Agric. Food Chem. 2020, 68, 33–47. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and Their Role in Oxidative Stress, Inflammation, and Human Diseases. Chem. Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef]
- Noberasco, G.; Odetti, P.; Boeri, D.; Maiello, M.; Adezati, L. Malondialdehyde (MDA) Level in Diabetic Subjects. Relationship with Blood Glucose and Glycosylated Hemoglobin. Biomed. Pharmacother. 1991, 45, 193–196. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Martin, K.R.; Appel, C.L. Polyphenols as Dietary Supplements: A Double-Edged Sword. Nutr. Diet Suppl. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Granato, D.; Mocan, A.; Câmara, J.S. Is a Higher Ingestion of Phenolic Compounds the Best Dietary Strategy? A Scientific Opinion on the Deleterious Effects of Polyphenols in Vivo. Trends Food Sci. Technol. 2020, 98, 162–166. [Google Scholar] [CrossRef]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-Oxidant Activity of Polyphenols and Its Implication on Cancer Chemoprevention and Chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, E.B.; Tang, W.Y.; Wong, K.P.; Mack, P. Inhibition of Glutathione Reductase by Plant Polyphenols. Biochem. Pharmacol. 1997, 54, 1047–1053. [Google Scholar] [CrossRef]
- Dzah, C.S.; Zhang, H.; Gobe, V.; Asante-Donyinah, D.; Duan, Y. Anti- and pro-Oxidant Properties of Polyphenols and Their Role in Modulating Glutathione Synthesis, Activity and Cellular Redox Potential: Potential Synergies for Disease Management. Adv. Redox Res. 2024, 11, 100099. [Google Scholar] [CrossRef]
- Scapagnini, G.; Sonya, V.; Nader, A.G.; Calogero, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE Pathway by Food Polyphenols: A Nutritional Neuroprotective Strategy for Cognitive and Neurodegenerative Disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef]
- Hou, Y.; Peng, S.; Song, Z.; Bai, F.; Li, X.; Fang, J. Oat Polyphenol Avenanthramide-2c Confers Protection from Oxidative Stress by Regulating the Nrf2-ARE Signaling Pathway in PC12 Cells. Arch. Biochem. Biophys. 2021, 706, 108857. [Google Scholar] [CrossRef]
- Lv, L.; Shu, H.; Mo, X.; Tian, Y.; Guo, H.; Sun, H.Y. Activation of the Nrf2 Antioxidant Pathway by Longjing Green Tea Polyphenols in Mice Livers. Nat. Prod. Commun. 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Murakami, A. Impact of Hormesis to Deepen Our Understanding of the Mechanisms Underlying the Bioactivities of Polyphenols. Curr. Opin. Biotechnol. 2024, 86, 103074. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic Effect of Dietary Polyphenols: A Systematic Review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Huang, J.; Ding, Y.; Pan, Z.; Zhao, Y.; Zhang, R.; Hu, B.; Zeng, X. In Vitro Extraction and Fermentation of Polyphenols from Grape Seeds (Vitis vinifera) by Human Intestinal Microbiota. Food Funct. 2016, 7, 1959–1967. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Suleria, H.; Zhang, P.; Gu, C.; Ng, K. Sugarcane Polyphenol and Fiber to Affect Production of Short-Chain Fatty Acids and Microbiota Composition Using in Vitro Digestion and Pig Faecal Fermentation Model. Food Chem. 2022, 385, 132665. [Google Scholar] [CrossRef]
- He, Z.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H.; Yuan, L.; Li, W. Changes in Polyphenol Fractions and Bacterial Composition after in Vitrofermentation of Apple Peel Polyphenol by Gut Microbiota. Int. J. Food Sci. Technol. 2022, 57, 4268–4276. [Google Scholar] [CrossRef]
- Van Dorsten, F.A.; Peters, S.; Gross, G.; Gomez-Roldan, V.; Klinkenberg, M.; De Vos, R.C.; Vaughan, E.E.; Van Duynhoven, J.P.; Possemiers, S.; Van De Wiele, T.; et al. Gut Microbial Metabolism of Polyphenols from Black Tea and Red Wine/Grape Juice Is Source-Specific and Colon-Region Dependent. J. Agric. Food Chem. 2012, 60, 11331–11342. [Google Scholar] [CrossRef]
- Havlik, J.; Marinello, V.; Gardyne, A.; Hou, M.; Mullen, W.; Morrison, D.J.; Preston, T.; Combet, E.; Edwards, C.A. Dietary Fibres Differentially Impact on the Production of Phenolic Acids from Rutin in an In Vitro Fermentation Model of the Human Gut Microbiota. Nutrients 2020, 12, 1577. [Google Scholar] [CrossRef]
- Sadeghi Ekbatan, S.; Sleno, L.; Sabally, K.; Khairallah, J.; Azadi, B.; Rodes, L.; Prakash, S.; Donnelly, D.J.; Kubow, S. Biotransformation of Polyphenols in a Dynamic Multistage Gastrointestinal Model. Food Chem. 2016, 204, 453–462. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Yan, H.; Wang, Q.; Wang, H.; et al. Sodium Acetate, Propionate, and Butyrate Reduce Fat Accumulation in Mice via Modulating Appetite and Relevant Genes. Nutrition 2021, 87–88, 111198. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Zhou, Y.; Tan, Y.; Feng, W.; Peng, C. Interactions between Gut Microbiota and Polyphenols: A Mechanistic and Metabolomic Review. Phytomedicine 2023, 119, 154979. [Google Scholar] [CrossRef]
- Nakatsu, C.H.; Armstrong, A.; Clavijo, A.P.; Martin, B.R.; Barnes, S.; Weaver, C.M. Fecal Bacterial Community Changes Associated with Isoflavone Metabolites in Postmenopausal Women after Soy Bar Consumption. PLoS ONE 2014, 9, e108924. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcumin on Serum Cytokine Concentrations in Subjects with Metabolic Syndrome: A Post-Hoc Analysis of a Randomized Controlled Trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-Alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-Controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Akbari, M.; Lankarani, K.B.; Tabrizi, R.; Ghayour-Mobarhan, M.; Peymani, P.; Ferns, G.; Ghaderi, A.; Asemi, Z. The Effects of Curcumin on Weight Loss among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2019, 10, 439144. [Google Scholar] [CrossRef]
- Alappat, L.; Awad, A.B. Curcumin and Obesity: Evidence and Mechanisms. Nutr. Rev. 2010, 68, 729–738. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green Tea Supplementation Affects Body Weight, Lipids, and Lipid Peroxidation in Obese Subjects with Metabolic Syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Thielecke, F.; Boschmann, M. The Potential Role of Green Tea Catechins in the Prevention of the Metabolic Syndrome—A Review. Phytochemistry 2009, 70, 11–24. [Google Scholar] [CrossRef]
- Phung, O.J.; Baker, W.L.; Matthews, L.J.; Lanosa, M.; Thorne, A.; Coleman, C.I. Effect of Green Tea Catechins with or without Caffeine on Anthropometric Measures: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2010, 91, 73–81. [Google Scholar] [CrossRef]
- Hursel, R.; Viechtbauer, W.; Westerterp-Plantenga, M.S. The Effects of Green Tea on Weight Loss and Weight Maintenance: A Meta-Analysis. Int. J. Obes. 2009, 33, 956–961. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Tang, R.; Lu, H. Resveratrol and Its Derivates Improve Inflammatory Bowel Disease by Targeting Gut Microbiota and Inflammatory Signaling Pathways. Food Sci. Hum. Wellness 2022, 11, 22–31. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, Á.; Halmai, R.; et al. Resveratrol Improves Insulin Sensitivity, Reduces Oxidative Stress and Activates the Akt Pathway in Type 2 Diabetic Patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- Dash, S.; Xiao, C.; Morgantini, C.; Szeto, L.; Lewis, G.F. High-Dose Resveratrol Treatment for 2 Weeks Inhibits Intestinal and Hepatic Lipoprotein Production in Overweight/Obese Men. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2895–2901. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Russo, G.L.; Daglia, M.; Nabavi, S.M. Role of Quercetin as an Alternative for Obesity Treatment: You Are What You Eat! Food Chem. 2015, 179, 305–310. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, X.; Zhang, L.; Bian, H.X.; Xu, N.; Bao, B.; Liu, J. Quercetin Reduces Obesity-Associated ATM Infiltration and Inflammation in Mice: A Mechanism Including AMPKα1/SIRT1. J. Lipid Res. 2014, 55, 363–374. [Google Scholar] [CrossRef]
- Tanaka, M.; Sugama, A.; Sumi, K.; Shimizu, K.; Kishimoto, Y.; Kondo, K.; Iida, K. Gallic Acid Regulates Adipocyte Hypertrophy and Suppresses Inflammatory Gene Expression Induced by the Paracrine Interaction between Adipocytes and Macrophages in Vitro and in Vivo. Nutr. Res. 2020, 73, 58–66. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, A.; Kishimoto, Y.; Mabashi-Asazuma, H.; Kondo, K.; Iida, K. Gallic Acid Inhibits Lipid Accumulation via AMPK Pathway and Suppresses Apoptosis and Macrophage-Mediated Inflammation in Hepatocytes. Nutrients 2020, 12, 1479. [Google Scholar] [CrossRef]
| Author (Year) | Country | Study Design | Duration | n | Age | Sex (M/F) | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|
| Fava et al. (2022) [56] | Italy | RCT, double-blind, placebo-controlled | 4 weeks | 67 | 47.2 | 31 M/36 F | 31.4 |
| González-Sarrías et al. (2017) [58] | Spain | RCT, double-blind, placebo-controlled, crossover | 6 months (two 3-week phases + washout) | 49 | 46,2 | 32 M/17 F | 30.4 |
| Guevara-Cruz et al. (2020) [57] | Mexico | RCT, double-blind, placebo-controlled, parallel | 8 weeks | 45 | 44 | NR | 34.1 |
| Hou et al. (2024) [59] | China | RCT, double-blind, placebo-controlled | 3 weeks | 67 | 47 | 35 M/32 F | 28 |
| Jamar et al. (2020) [60] | Brazil | RCT, double-blind, placebo-controlled | 6 weeks | 34 | 46.5 | 14 M/21 F | 34.4 |
| Jin et al. (2024) [61] | China | RCT, double-blind, placebo-controlled | 24 weeks | 83 | 42.9 | 49 M/34 F | 27.3 |
| Lee et al. (2022) [62] | South Korea | RCT (secondary analysis) | 12 weeks | 40 | 37.4 | 17 M/23 F | 27.7 |
| Lúcio et al. (2023) [63] | Brazil | RCT, single-blind, controlled | 8 weeks | 21 | 25.6 ± 4.6 years | 21M | 28.5 |
| Machado et al. (2020) [64] | Brazil | RCT, double-blind, placebo-controlled | 6 weeks | 26 | 31.3 | 11 M/15 F | 30.4 |
| Santamarina et al. (2025) [65] | Brazil | RCT, double-blind | 90 days | 77 | 54.5 | 21 M/56 F | 27.8 |
| Solch-Ottaiano et al. (2022) [66] | USA | RCT, double-blind, placebo-controlled, crossover | 2 weeks per intervention (12 weeks total with washout) | 36 | 35.4 | 10 M/26 F | 37.4 |
| van der Merwe et al. (2021) [67] | USA | RCT, double-blind, placebo-controlled | 16 weeks (+4 weeks in subgroup) | 57 | 36.2 | 57 F | 30.6 |
| Vitaglione et al. (2015) [68] | Italy | RCT, placebo-controlled, parallel | 8 weeks | 68 | 38.5 years | 11 M/25 F | 29.8 |
| Author (Year) | Intervention | Comparator | Study Focus | Methods of Analysis | Main Conclusions | |
|---|---|---|---|---|---|---|
| Polyphenol Type | Polyphenol Dose | |||||
| Fava et al. (2022) [56] | Aleurone (ferulic, cinnamic, benzoic acids) | 27 g/day aleurone | Placebo (cellulose) | CVD biomarkers, gut microbiota, metabolites in overweight/obese adults | qPCR, FCM-FISH, 16S rRNA, ELISA, untargeted metabolomics | ↑ Bifidobacterium, Lactobacillus; no change in inflammatory/oxidative markers |
| González-Sarrías et al. (2017) [58] | Pomegranate polyphenols (extract): Punicalin, valoneic acid dilactone, sanguisorbic acid, gallagic acid dilactone, ellagic acid, gallic acid | 160 (1 capsule) or 640 mg (4 capsules) phenolics/day | Placebo (maltodextrin) | Gut microbiota, urolithin metabotypes, inflammation, oxidative stress | qPCR, 16S rRNA, HPLC-DAD, ELISA | ↓ LDLc/oxLDL in UM-B individuals; Gordonibacter correlated with urolithin production |
| Guevara-Cruz et al. (2020) [57] | Genistein (isoflavone) | 50 mg/day | Placebo | Insulin sensitivity, gut microbiota, metabolic endotoxemia in obesity | 16S rRNA, blood glucose/lipids, HOMA-IR, muscle AMPK phosphorylation, serum metabolomics | ↑ Akkermansia, insulin sensitivity; ↓ LPS; AMPK activation in muscle |
| Hou et al. (2024) [59] | Pomegranate polyphenols (juice) | 200 mL/day juice | Placebo (flavored drink) | Gut microbiota/metabolites in overweight/obese individuals | 16S rRNA, HPLC (polyphenols), GC–MS (SCFAs) | ↑ Akkermansia, Bifidobacterium, SCFAs, urolithins; no anthropometric changes |
| Jamar et al. (2020) [60] | Anthocyanins (cyanidin-3-glucoside, rutinoside) | 5 g/day lyophilized juçara | Placebo (maltodextrin) | Prebiotic potential of juçara berry on gut microbiota and SCFAs in obese individuals | qPCR (Akkermansia, Bifidobacterium), GC-FID (SCFAs), serum LPS | ↑ Akkermansia, Bifidobacterium, acetate; no LPS changes |
| Jin et al. (2024) [61] | Silymarin (flavonolignans) | 103.2 mg/day (4 tablets/day) | Placebo (dextrin) | Effects of silymarin on liver stiffness and gut microbiota in MASLD patients | FibroScan for liver stiffness/steatosis, 16S rRNA sequencing for gut microbiota, blood biochemical tests | ↓ Liver stiffness, GGT; ↑ Oscillospiraceae; no hepatic steatosis improvement |
| Lee et al. (2022) [62] | Phlorotannins from Ecklonia cava | 360 mg/day | Placebo | EP effects on adiposity and gut microbiota in abdominal obesity | 16S rRNA, anthropometrics, oxidative stress markers, Tax4Fun | ↓ Adiposity, oxidative stress; ↑ Butyricimonas, Gordonibacter; improved Firmicutes/Bacteroidetes ratio |
| Lúcio et al. (2023) [63] | Proanthocyanidins, 3-deoxyanthocyanidins | 40 g/day SC319 sorghum | Whole wheat (38 g/day) + diet (−500 kcal/day) | Effects on gut microbiota, anthropometric markers, and inflammatory markers in overweight men | DXA, ELISA (IL-6/IL-10/TNF-α), 16S rRNA, qPCR, HPLC (SCFAs), fecal pH | ↓ Weight, body fat; modulated microbiota (↓ Clostridium); ↑ IL-6 in wheat group |
| Machado et al. (2020) [64] | Chlorogenic acid | 25 g/day yacon flour | Placebo (control drink) + diet (−500 kcal/day) | Effects on intestinal permeability, fecal SCFAs, oxidative stress, and inflammation in overweight/obese adults | HPLC (SCFAs, lactulose/mannitol), FRAP, carbonyls, catalase, GST, MDA, NO, CRP, leukocytes, NLR, PLR | ↑ Plasma antioxidants, Akkermansia; ↓ carbonyls; fecal SCFAs ↓ (weight loss effect) |
| Santamarina et al. (2025) [65] | Silymarin | 4 capsules/day | Nutraceutical blend (FOS + GOS + β-glucans + minerals) | Gut microbiota, inflammation, sleep in overweight adults | 16S rRNA (QIIME 2), CBA (cytokines), HPLC (silymarin), PSQI/ESS/MSQ-BR/BRUMS | ↑ Faecalibacterium, Lactobacillus; ↓ weight, TNF-α/IL-10; improved sleep, Silymarin enhanced anti-inflammatory effects |
| Solch-Ottaiano et al. (2022) [66] | Proanthocyanidins, anthocyanins, phenolics | 480 mL/day; 11 mg anthocyanins, 407 mg phenolics, 535 mg proanthocyanidins | Placebo (matched drink, no polyphenols) | Gut permeability, microbiota, and inflammation after aspirin challenge in obese adults | LC-MS/MS (sugar probes), 16S rRNA (QIIME 2), qPCR, ELISA (hs-CRP/IL-6/TNF-α/zonulin) | ↑ Faecalibacterium prausnitzii, Eggerthella lenta; no gut permeability/inflammation changes |
| van der Merwe et al. (2021) [67] | Quercetin, kaempferol, catechin, epicatechin, chlorogenic acid, rutin, hesperidin, narirutin | 6 capsules/day JuicePlus+ | Placebo (cellulose); habitual breakfast | Gut microbiota, SCFAs, glucose metabolism, inflammation, permeability | 16S rRNA (QIIME), ion chromatography (SCFAs), Luminex (cytokines), OGTT, DXA | ↓ Bacteroides; ↑ butyrate; no effect on α/β-diversity, lipids or inflammation; improved glucose clearance with FVC |
| Vitaglione et al. (2015) [68] | Ferulic acid, sinapic acid, caffeic acid, p-coumaric acid | 70 g/day whole grain wheat | Refined wheat products | Polyphenol bioavailability, gut microbiota, inflammation in overweight/obese | HPLC-MS/MS (phenolic acids), Luminex (cytokines), 16S rRNA (MiSeq), bioelectrical impedance | ↑ Ferulic acid metabolites; ↓ TNF-α, ↑ IL-10; ↑ Bacteroidetes/Firmicutes; no weight/lipid changes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Gómez, Á.; Cantone, M.; García-Muñoz, A.M.; Victoria-Montesinos, D.; Lucas-Abellán, C.; Serrano-Martínez, A.; Muñoz-Morillas, A.M.; Morillas-Ruiz, J.M. Effect of Polyphenol-Rich Interventions on Gut Microbiota and Inflammatory or Oxidative Stress Markers in Adults Who Are Overweight or Obese: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 2468. https://doi.org/10.3390/nu17152468
González-Gómez Á, Cantone M, García-Muñoz AM, Victoria-Montesinos D, Lucas-Abellán C, Serrano-Martínez A, Muñoz-Morillas AM, Morillas-Ruiz JM. Effect of Polyphenol-Rich Interventions on Gut Microbiota and Inflammatory or Oxidative Stress Markers in Adults Who Are Overweight or Obese: A Systematic Review and Meta-Analysis. Nutrients. 2025; 17(15):2468. https://doi.org/10.3390/nu17152468
Chicago/Turabian StyleGonzález-Gómez, Álvaro, Martina Cantone, Ana María García-Muñoz, Desirée Victoria-Montesinos, Carmen Lucas-Abellán, Ana Serrano-Martínez, Alejandro M. Muñoz-Morillas, and Juana M. Morillas-Ruiz. 2025. "Effect of Polyphenol-Rich Interventions on Gut Microbiota and Inflammatory or Oxidative Stress Markers in Adults Who Are Overweight or Obese: A Systematic Review and Meta-Analysis" Nutrients 17, no. 15: 2468. https://doi.org/10.3390/nu17152468
APA StyleGonzález-Gómez, Á., Cantone, M., García-Muñoz, A. M., Victoria-Montesinos, D., Lucas-Abellán, C., Serrano-Martínez, A., Muñoz-Morillas, A. M., & Morillas-Ruiz, J. M. (2025). Effect of Polyphenol-Rich Interventions on Gut Microbiota and Inflammatory or Oxidative Stress Markers in Adults Who Are Overweight or Obese: A Systematic Review and Meta-Analysis. Nutrients, 17(15), 2468. https://doi.org/10.3390/nu17152468