Advances in Nanotechnology Research in Food Production, Nutrition, and Health
Abstract
1. Introduction
2. Classification of Nanomaterials
2.1. Inorganic Nanomaterials
2.2. Organic Nanomaterials
2.2.1. Polymer Nanomaterials
2.2.2. Liposomes
2.2.3. Solid Lipid Nanoparticles
2.2.4. Nanoemulsions
2.2.5. Carbon-Based Nanomaterials
2.2.6. Protein/Peptide Nanomaterials
3. Nanotechnology in Food Production, Nutrition, and Health
3.1. Application of Nanotechnology in Food Production
3.1.1. Application of Nanotechnology in Food Processing
3.1.2. Application of Nanotechnology in Food Packaging
3.1.3. Application of Nanotechnology in Food Testing
3.2. Application of Nanotechnology in Functional Food Delivery
3.2.1. Application of Nanotechnology as Functional Ingredient Delivery System
3.2.2. Application of Nanotechnology as Probiotic Delivery System
3.3. Nanotechnology in Human Health
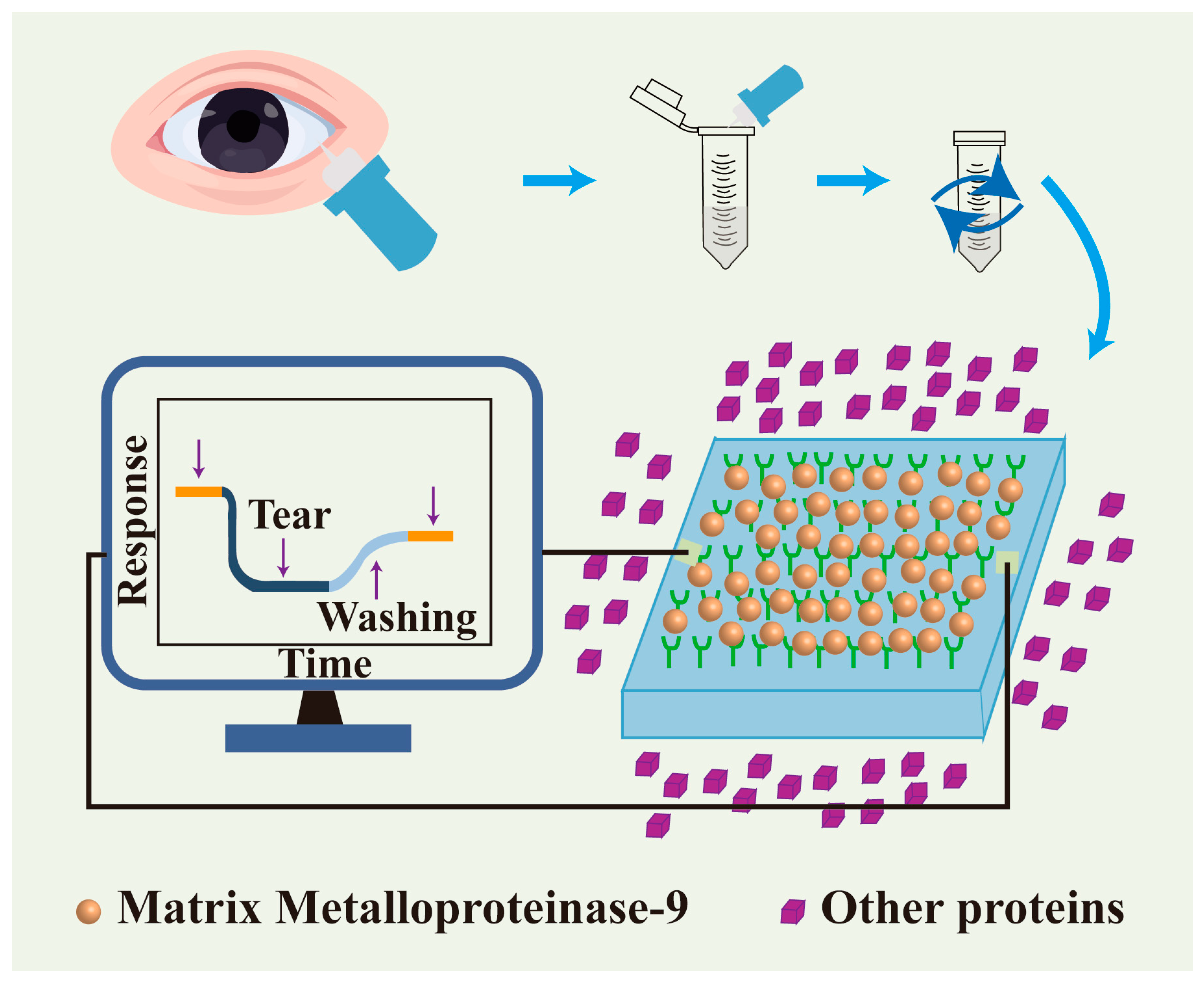
3.3.1. Nanotechnology in Disease Diagnosis
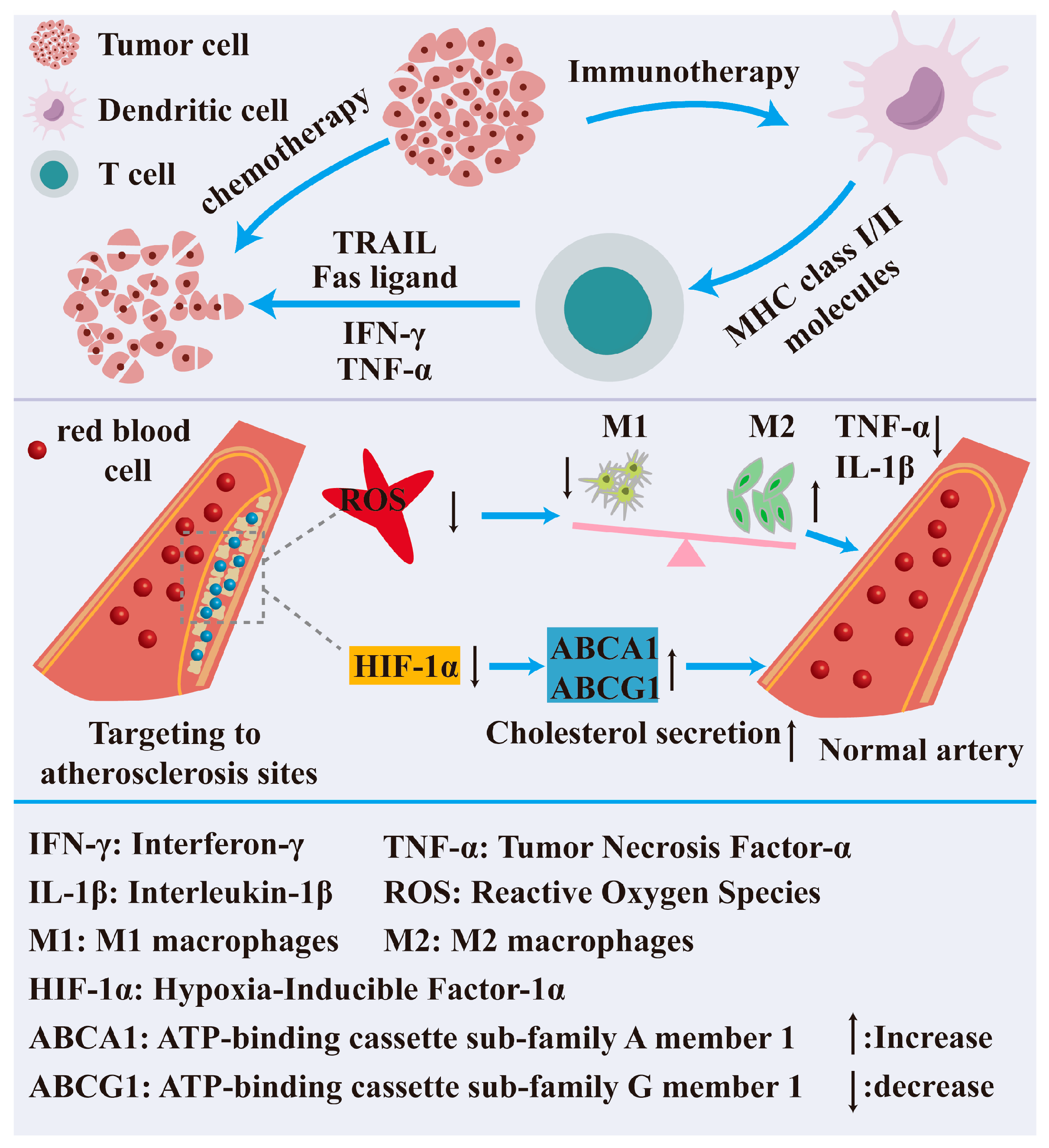
3.3.2. Nanotechnology in Disease Treatment
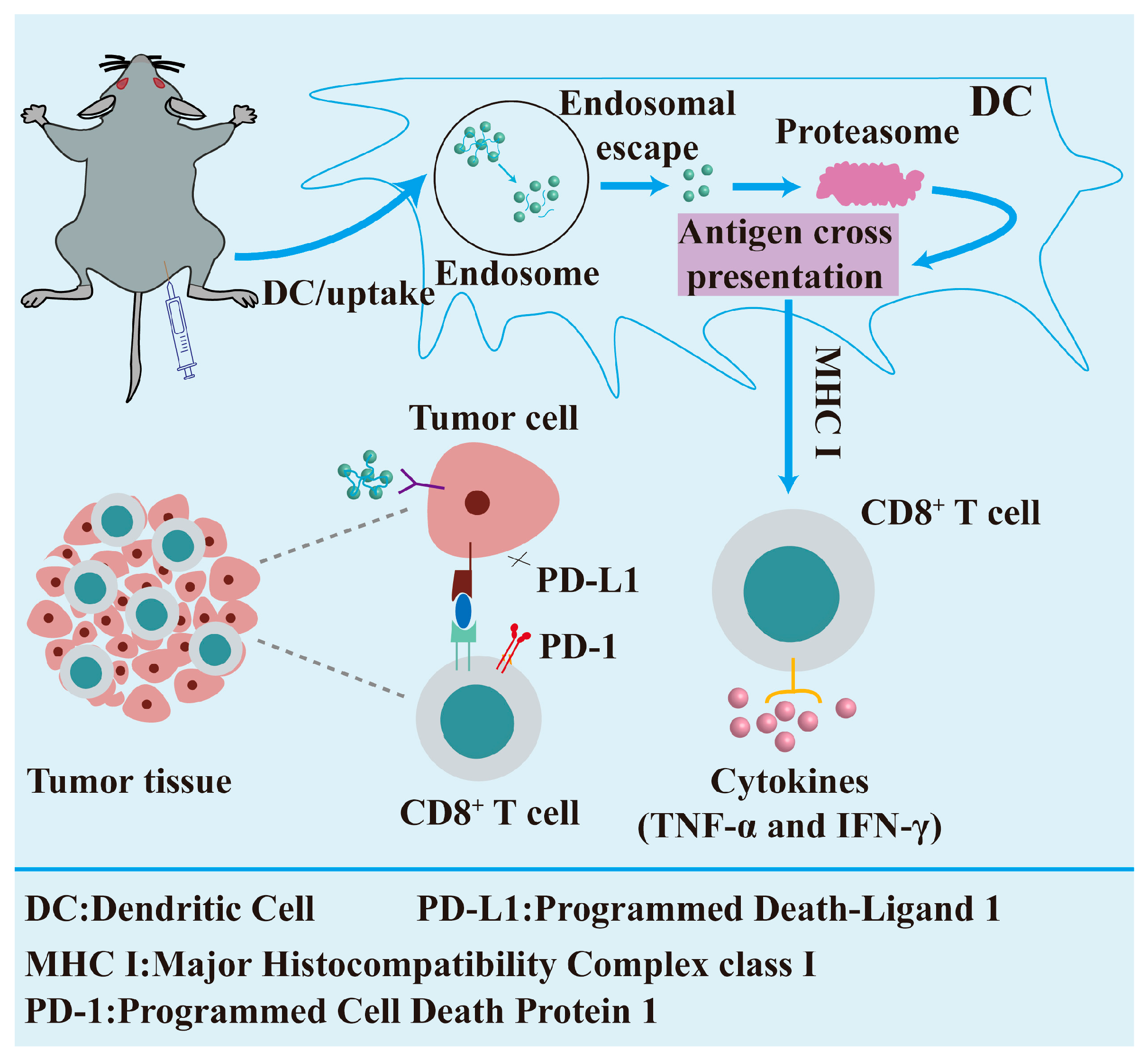
3.3.3. Nanotechnology in Regenerative Medicine
3.3.4. Nanotechnology in Preventive Medicine
4. Related Health Risks and Safety Issues
5. Summary and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Mulvaney, P. Nanoscience vs Nanotechnology Defining the Field. Paul Mulvaney 2015, 9, 2215–2217. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Pandit, K.H.; Patil, P.B.; Goswami, A.D.; Pinjari, D.V. Fabrications from renewable sources and agricultural wastes and characterization strategies of green nanomaterials. In Handbook of Green and Sustainable Nanotechnology: Fundamentals, Developments and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–15. [Google Scholar]
- Paradva, K.C.; Jangir, R.; Kalla, S. Green nanomaterials: Synthesis and applications in wastewater treatment. Inorg. Chem. Commun. 2023, 158, 111584. [Google Scholar] [CrossRef]
- Li, S.; Xu, X.; Xu, L.; Lin, H.; Kuang, H.; Xu, C. Emerging trends in chiral inorganic nanomaterials for enantioselective catalysis. Nat. Commun. 2024, 15, 3506. [Google Scholar] [CrossRef]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Fojtů, M.; Latiff, N.M.; Pumera, M. Two-dimensional materials in biomedical, biosensing and sensing applications. Chem. Soc. Rev. 2021, 50, 619–657. [Google Scholar] [CrossRef]
- Wan, M.; Wang, Q.; Wang, R.; Wu, R.; Li, T.; Fang, D.; Huang, Y.; Yu, Y.; Fang, L.; Wang, X. Platelet-derived porous nanomotor for thrombus therapy. Sci. Adv. 2020, 6, eaaz9014. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, S.; Zhang, Q.; Gao, W.; Mu, S.; Wang, B. Wound healing potential of silver nanoparticles from hybanthus enneaspermus on rats. Heliyon 2024, 10, e36118. [Google Scholar] [CrossRef]
- Yang, Z.; Wen, T.; Peng, F. Integrating green synthesized lichenan-silver nanoparticles into lichenan/chitosan matrix for photo-responsive multifunctional food packaging application. Chem. Eng. J. 2025, 514, 163197. [Google Scholar] [CrossRef]
- Jacob, J.; Robert, V.; Valapa, R.B.; Kuriakose, S.; Thomas, S.; Loganathan, S. Poly (lactic acid)/polyethylenimine functionalized mesoporous silica biocomposite films for food packaging. ACS Appl. Polym. Mater. 2022, 4, 4632–4642. [Google Scholar] [CrossRef]
- Mu, Y.; Li, M.; Zhao, X.; Gong, C.; Luo, Z.; Li, B.; Zhang, W.; Ge, X.; Chen, S.; Zhou, J.; et al. TiO2 nanotube implants modified with silk fibroin and mesoporous silica nanocomposite coatings enable efficient drug release to promote osteogenesis. ACS Appl. Mater. Interfaces 2025, 17, 30600–30612. [Google Scholar] [CrossRef]
- Gu, L.; Lin, J.; Wang, Q.; Meng, F.; Niu, G.; Lin, H.; Chi, M.; Feng, Z.; Zheng, H.; Li, D.; et al. Mesoporous zinc oxide-based drug delivery system offers an antifungal and immunoregulatory strategy for treating keratitis. J. Control. Release 2024, 368, 483–497. [Google Scholar] [CrossRef]
- Wu, R.; Wang, H.; Hai, L.; Wang, T.; Hou, M.; He, D.; He, X.; Wang, K. A photosensitizer-loaded zinc oxide-polydopamine core-shell nanotherapeutic agent for photodynamic and photothermal synergistic therapy of cancer cells. Chin. Chem. Lett. 2020, 31, 189–192. [Google Scholar] [CrossRef]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 2014, 5, 3132. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Luo, D.; Akakuru, O.U.; Mushtaq, A.; Hou, Y.; Ali, I.; Ijaz, G.; Khalid, B.; Kong, X.; Wu, A. Facile synthesis of biocompatible magnetic titania nanorods for t 1-magnetic resonance imaging and enhanced phototherapy of cancers. J. Mater. Chem. B 2021, 9, 6623–6633. [Google Scholar] [CrossRef]
- Vélez-Peña, E.; Jiménez, V.A.; Manzo-Merino, J.; Melin, V.; Contreras, D.; Alderete, J.B.; Campos, C.H. Technology. Visible light-activated mesoporous black titania nanorods for enhanced chemo-photodynamic cancer therapy. J. Drug Deliv. Sci. Technol. 2025, 106, 106713. [Google Scholar] [CrossRef]
- Kanelli, M.; Bardhan, N.M.; Sarmadi, M.; Eshaghi, B.; Alsaiari, S.K.; Rothwell, W.T.; Pardeshi, A.; Varshney, D.; De Fiesta, D.C.; Mak, H.; et al. A machine learning-optimized system for pulsatile, photo-and chemotherapeutic treatment using near-infrared responsive mos2-based microparticles in a breast cancer model. ACS Nano. 2024, 18, 30433–30447. [Google Scholar] [CrossRef]
- Xia, L.; Chen, J.; Xie, Y.; Zhang, S.; Xia, W.; Feng, W.; Chen, Y. Photo-/piezo-activated ultrathin molybdenum disulfide nanomedicine for synergistic tumor therapy. J. Mater. Chem. B 2023, 11, 2895–2903. [Google Scholar] [CrossRef]
- Bruna, J.E.; Muñoz-Shugulí, C.; Espinoza, L.; Herrera, A.; Rodríguez-Mercadoz, F.J.; Ganga, M.A.; Guarda, A.; Galotto, M.J. Poly (lactic acid) and copper-modified montmorillonite nanocomposite films for antimicrobial food packaging. J. Appl. Polym. Sci. 2025, 142, e56406. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Jayan, H.; Gao, S.; Zhou, R.; Yosri, N.; Zou, X.; Guo, Z. Recent and emerging trends of metal-organic frameworks (MOFs)-based sensors for detecting food contaminants: A critical and comprehensive review. Food Chem. 2024, 448, 139051. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Zheng, C.; Hu, C.; Yang, L.; Kong, Q.; Zhang, H.; Wang, Y. A versatile glycopeptide hydrogel promotes chronic refractory wound healing through bacterial elimination, sustained oxygenation, immunoregulation, and neovascularization. Adv. Funct. Mater. 2023, 33, 2305992. [Google Scholar] [CrossRef]
- Liang, T.; Feng, Z.; Zhang, X.; Li, T.; Yang, T.; Yu, L. Research progress of calcium carbonate nanomaterials in cancer therapy: Challenge and opportunity. Front. Bioeng. Biotechnol. 2023, 11, 1266888. [Google Scholar] [CrossRef]
- Singh, P.; Manori, S.; Raina, K.K.; Shukla, R.K. Inorganic, organic and polymer-based nanomaterials. In Nanomaterials for Drug Delivery and Neurological Diseases Management; Springer: Berlin/Heidelberg, Germany, 2024; pp. 21–46. [Google Scholar]
- Fang, H.; Zhang, L.; Wu, Y.; Chen, L.; Deng, Z.; Zheng, Z.; Wang, Y.; Yang, Y.; Chen, Q. Carrier-free multifunctional nanomedicine for enhanced hyperthermic intraperitoneal chemotherapy against abdominal pelvic tumors. Chem. Eng. J. 2024, 498, 155781. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Polymer nanomedicines. Adv. Drug Deliv. Rev. 2020, 156, 40–64. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Li, J.; Qi, Y.; Peng, C.; Wang, N.; Fan, H.; Li, Y. Zwitterionic polymers: Addressing the barriers for drug delivery. Chin. Chem. Lett. 2023, 34, 108177. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Y.; McClements, D.J.; Xu, D.; Chen, S. Production, characterization, delivery, and cholesterol-lowering mechanism of phytosterols: A review. J. Agric. Food Chem. 2022, 70, 2483–2494. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Biomater. Adv. 2016, 60, 560–568. [Google Scholar] [CrossRef]
- Geyik, G.; Güncüm, E.; Işıklan, N. Design and development of ph-responsive alginate-based nanogel carriers for etoposide delivery. Int. J. Biol. Macromol. 2023, 250, 126242. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Manne, G.K.; Srinivasan, K.; Obularajugari, G.N.; Kumar, R.; Pathi, P.; Hanumanthappa, N.; Pitchika, G.K.; Battana, S.; Mandala, R. PVA/CMC-Ag polymer blend nanocomposites prepared by doping bio-derived ag nanoparticles and their structural, thermal, optical, antimicrobial, and electrical characterization. J. Appl. Polym. Sci. 2025, 142, e56811. [Google Scholar] [CrossRef]
- Dardeer, H.M.; Toghan, A.; Zaki, M.E.; Elamary, R.B. Design, synthesis and evaluation of novel antimicrobial polymers based on the inclusion of polyethylene glycol/TiO2 nanocomposites in cyclodextrin as drug carriers for sulfaguanidine. Polymers 2022, 14, 227. [Google Scholar] [CrossRef]
- Correa, J.P.; Molina, V.; Sanchez, M.; Kainz, C.; Eisenberg, P.; Massani, M.B. Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Packag. Shelf Life 2017, 11, 31–39. [Google Scholar] [CrossRef]
- Cai, J.X.; Liu, J.H.; Wu, J.Y.; Li, Y.J.; Qiu, X.H.; Xu, W.J.; Xu, P.; Xiang, D.X. Hybrid cell membrane-functionalized biomimetic nanoparticles for targeted therapy of osteosarcoma. Int. J. Nanomed. 2022, 17, 837–854. [Google Scholar] [CrossRef]
- Alanazi, N.; Akhdar, H.; Alinad, T.; Pandiaraj, S.; Alodhayb, A.N. Technology. Optical interaction of CdTe/ZnS quantum dots with sodium alginate biopolymer paa-modified for glucose detection. ECS J. Solid State Sci. Technol. 2024, 13, 107006. [Google Scholar] [CrossRef]
- Lei, F.; Zeng, F.; Yu, X.; Deng, Y.; Zhang, Z.; Xu, M.; Ding, N.; Tian, J.; Li, C. Oral hydrogel nanoemulsion co-delivery system treats inflammatory bowel disease via anti-inflammatory and promoting intestinal mucosa repair. J. Nanobiotechnol. 2023, 21, 275. [Google Scholar] [CrossRef]
- Chen, Q.; Jia, C.; Xu, Y.; Jiang, Z.; Hu, T.; Li, C.; Cheng, X. Dual-ph responsive chitosan nanoparticles for improving in vivo drugs delivery and chemoresistance in breast cancer. Carbohydr. Polym. 2022, 290, 119518. [Google Scholar] [CrossRef]
- Fu, C.P.; Cai, X.Y.; Chen, S.L.; Yu, H.W.; Fang, Y.; Feng, X.C.; Zhang, L.M.; Li, C.Y. Hyaluronic acid-based nanocarriers for anticancer drug delivery. Polymers 2023, 15, 2317. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161–176. [Google Scholar] [CrossRef]
- Hu, J.; Jia, X.; Li, M.; Duan, G.; Man, K.; Dai, H.; Wen, L.; Geng, H. Enhanced delivery of photothermal gelatin nanoparticle for redox balanced nanocatalytic tumor chemotherapy. Small 2025, 21, 2411018. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Benech, R.-O.; Kheadr, E.; Laridi, R.; Lacroix, C.; Fliss, I. Inhibition of listeria innocua in cheddar cheese by addition of nisin z in liposomes or by in situ production in mixed culture. Appl. Environ. Microbiol. 2002, 68, 3683–3690. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, L.; Ren, Z.; Yuan, Y.; Yu, J.; Zhang, Y.; Gu, L.; Wang, X.; Wang, Y.; Xu, H.; et al. Stepwise-targeting and hypoxia-responsive liposome amvy@ nps carrying siyap and verteporfin for glioblastoma therapy. J. Nanobiotechnol. 2024, 22, 495. [Google Scholar] [CrossRef]
- Shehata, T.; Ogawara, K.I.; Higaki, K.; Kimura, T. Prolongation of residence time of liposome by surface-modification with mixture of hydrophilic polymers. Int. J. Pharm. 2008, 359, 272–279. [Google Scholar] [CrossRef]
- Guan, Y.; Ning, Y.; Xu, Z.; Zhou, C.; Zhao, Z. Chondroitin sulfate and chitosan-coated liposomes as a novel delivery system for betanin: Preparation, characterization and in vitro digestion behavior. Int. J. Biol. Macromol. 2024, 254, 128001. [Google Scholar] [CrossRef]
- Aditya, N.; Ko, S. Solid lipid nanoparticles (slns): Delivery vehicles for food bioactives. RSC Adv. 2015, 5, 30902–30911. [Google Scholar] [CrossRef]
- Daneshmand, S.; Golmohammadzadeh, S.; Jaafari, M.R.; Movaffagh, J.; Rezaee, M.; Sahebkar, A.; Malaekeh-Nikouei, B. Encapsulation challenges, the substantial issue in solid lipid nanoparticles characterization. J. Cell. Biochem. 2018, 119, 4251–4264. [Google Scholar] [CrossRef]
- Lin, L.; Chen, W.; Li, C.; Cui, H. Products. Enhancing stability of eucalyptus citriodora essential oil by solid nanoliposomes encapsulation. Ind. Crops Prod. 2019, 140, 111615. [Google Scholar] [CrossRef]
- Westesen, K.; Bunjes, H.; Koch, M. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J. Control. Release 1997, 48, 223–236. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Ghisleni, D.D.M.; Pinto, T.J.A.; Bou-Chacra, N.A. Nanoemulsion: Process selection and application in cosmetics–a review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef]
- Chatterjee, B.; Gorain, B.; Mohananaidu, K.; Sengupta, P.; Mandal, U.K.; Choudhury, H. Targeted drug delivery to the brain via intranasal nanoemulsion: Available proof of concept and existing challenges. Int. J. Pharm. 2019, 565, 258–268. [Google Scholar] [CrossRef]
- Gonçalves, A.; Nikmaram, N.; Roohinejad, S.; Estevinho, B.N.; Rocha, F.; Greiner, R.; McClements, D.J. Production, properties, and applications of solid self-emulsifying delivery systems (s-seds) in the food and pharmaceutical industries. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 108–126. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, C.; Liu, X.; Zhao, X.; Zhao, S.; Tong, S.; Ren, G.; Wei, Q. Formulation, characterization, antibacterial activity, antioxidant activity, and safety evaluation of camphora longepaniculata essential oil nanoemulsions through high-pressure homogenization. Antioxidants 2024, 14, 33. [Google Scholar] [CrossRef]
- Safaya, M.; Rotliwala, Y. Nanoemulsions: A review on low energy formulation methods, characterization, applications and optimization technique. Mater. Today Proc. 2020, 27, 454–459. [Google Scholar] [CrossRef]
- Tuček, J.; Błoński, P.; Ugolotti, J.; Swain, A.K.; Enoki, T.; Zbořil, R. Emerging chemical strategies for imprinting magnetism in graphene and related 2d materials for spintronic and biomedical applications. Chem. Soc. Rev. 2018, 47, 3899–3990. [Google Scholar] [CrossRef]
- Pereda, M.; Marcovich, N.E.; Ansorena, M.R. Nanotechnology in food packaging applications: Barrier materials, antimicrobial agents, sensors, and safety assessment. In Handbook of Ecomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–22. [Google Scholar]
- Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003, 21, 1171–1178. [Google Scholar] [CrossRef]
- Acharya, R.; Dutta, S.D.; Mallik, H.; Patil, T.V.; Ganguly, K.; Randhawa, A.; Kim, H.; Lee, J.; Park, H.; Mo, C.; et al. Physical stimuli-responsive DNA hydrogels: Design, fabrication strategies, and biomedical applications. J. Nanobiotechnol. 2025, 23, 233. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Manning, L.; Soon, J.M. Developing systems to control food adulteration. Food Policy 2014, 49, 23–32. [Google Scholar] [CrossRef]
- Biswas, R.; Alam, M.; Sarkar, A.; Haque, M.I.; Hasan, M.M.; Hoque, M.J.H. Application of nanotechnology in food: Processing, preservation, packaging and safety assessment. Heliyon 2022, 8, e11795. [Google Scholar] [CrossRef]
- Stanković, M.; Gabrovska, M.; Krstić, J.; Tzvetkov, P.; Shopska, M.; Tsacheva, T.; Banković, P.; Edreva-Kardjieva, R.; Jovanović, D. Effect of silver modification on structure and catalytic performance of ni-mg/diatomite catalysts for edible oil hydrogenation. J. Mol. Catal. A Chem. 2009, 297, 54–62. [Google Scholar] [CrossRef]
- Moraru, C.; Huang, Q.; Takhistov, P.; Dogan, H.; Kokini, J. Food nanotechnology: Current developments and future prospects. In Global Issues in Food Science and Technology; Academic Press: Cambridge, MA, USA, 2009; pp. 369–399. [Google Scholar] [CrossRef]
- Ansari, M.A. Nanotechnology in food and plant science: Challenges and future prospects. Plants 2023, 12, 2565. [Google Scholar] [CrossRef]
- del Rosario Herrera-Rivera, M.; Torres-Arellanes, S.P.; Cortés-Martínez, C.I.; Navarro-Ibarra, D.C.; Hernández-Sánchez, L.; Solis-Pomar, F.; Pérez-Tijerina, E.; Román-Doval, R. Nanotechnology in food packaging materials: Role and application of nanoparticles. RSC Adv. 2024, 14, 21832–21858. [Google Scholar] [CrossRef]
- Sun, M.Z.; Kim, D.-Y.; Baek, Y.; Lee, H.G. The effect of multilayer nanoemulsion on the in vitro digestion and antioxidant activity of β-carotene. Antioxidants 2024, 13, 1218. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef]
- Javed, M.; Matloob, A.; Ettoumi, F.E.; Sheikh, A.R.; Zhang, R.; Xu, Y. Novel nanobubble technology in food science: Application and mechanism. Food Innov. Adv. 2023, 2, 135–144. [Google Scholar] [CrossRef]
- Aliabbasi, N.; Emam-Djomeh, Z. Application of nanotechnology in dairy desserts and ice cream formulation with the emphasize on textural, rheological, antimicrobial, and sensory properties. Efood 2024, 5, e170. [Google Scholar] [CrossRef]
- Lu, N.; Chen, Z.; Song, J.; Weng, Y.; Yang, G.; Liu, Q.; Yang, K.; Lu, X.; Liu, Y. Size effect of TiO2 nanoparticles as food additive and potential toxicity. Food Biophys. 2022, 17, 75–83. [Google Scholar] [CrossRef]
- Voci, S.; Gagliardi, A.; Fresta, M.; Cosco, D. Ascorbic acid-loaded gliadin nanoparticles as a novel nutraceutical formulation. Food Res. Int. 2022, 161, 111869. [Google Scholar] [CrossRef]
- Subhashree, S.; Kumar, P.S. New analytical strategies amplified with carbon-based nanomaterial for sensing food pollutants. Chemosphere 2022, 295, 133847. [Google Scholar] [CrossRef]
- Dib, T.; Pan, H.; Chen, S. Recent advances in pectin-based nanoencapsulation for enhancing the bioavailability of bioactive compounds: Curcumin oral bioavailability. Food Rev. Int. 2023, 39, 3515–3533. [Google Scholar] [CrossRef]
- Yoon, S.H.; Rungraeng, N.; Song, W.; Jun, S. Superhydrophobic and superhydrophilic nanocomposite coatings for preventing escherichia coli k-12 adhesion on food contact surface. J. Food Eng. 2014, 131, 135–141. [Google Scholar] [CrossRef]
- Munir, Y.; Gul, S.; Khan, M.I.; Khan, S.B. Ecofriendly synthesis of copper oxide-chitosan nanocomposites and their efficacy as nano-pesticide and nano-fertilizer. Inorg. Chem. Commun. 2024, 168, 112769. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, W. Technology. Effective strategies to enhance ultraviolet barrier ability in biodegradable polymer-based films/coatings for fruit and vegetable packaging. Trends Food Sci. Technol. 2023, 139, 104139. [Google Scholar] [CrossRef]
- Nath, D.; Santhosh, R.; Pal, K.; Sarkar, P. Nanoclay-based active food packaging systems: A review. Food Packag. Shelf Life 2022, 31, 100803. [Google Scholar] [CrossRef]
- Liu, G.; Min, T.; Li, X.; Zhao, Y.; Li, Z.; Liu, J.; Wen, Y. Biomimetic functional food packaging materials. Chem. Eng. J. 2024, 499, 156146. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Li, H.; Chen, Y.; Lian, R.; Wang, Y. Innovative packaging materials and methods for flavor regulation of prepared aquatic products: Mechanism, classification and future prospective. Food Innov. Adv. 2023, 2, 145–155. [Google Scholar] [CrossRef]
- Sahoo, M.; Vishwakarma, S.; Panigrahi, C.; Kumar, J. Nanotechnology: Current applications and future scope in food. Food Front. 2021, 2, 3–22. [Google Scholar] [CrossRef]
- Shan, Y.; Li, T.; Qu, H.; Duan, X.; Farag, M.A.; Xiao, J.; Gao, H.; Jiang, Y. Nano-preservation: An emerging postharvest technology for quality maintenance and shelf life extension of fresh fruit and vegetable. Food Front. 2023, 4, 100–130. [Google Scholar] [CrossRef]
- Yang, X.; Niu, Y.; Fan, Y.; Zheng, T.; Fan, J. Green synthesis of poria cocos polysaccharides-silver nanoparticles and their applications in food packaging. Int. J. Biol. Macromol. 2024, 269, 131928. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Y.; Li, X.; Li, Q.; Xiu, W.; He, A.; Dai, Z.; Dong, H.; Shan, J.; Mou, Y. Simultaneous biofilm disruption, bacterial killing, and inflammation elimination for wound treatment using silver embellished polydopamine nanoplatform. Small 2024, 20, 2400927. [Google Scholar] [CrossRef]
- Dardari, O.; Sair, S.; El Idrissi, A.; Benjelloun, G.R.; Ait Ousaleh, H.; Maati, H.; Essamlali, Y.; Zahouily, M.; Amadine, O. Development of temperature-regulating cr/pva bionanocomposite films with phase change materials and antibacterial properties for ice cream packaging. Food Chem. 2025, 480, 143492. [Google Scholar] [CrossRef]
- Kumari, S.; Kumari, A.; Ahmed, J.; Jasrotia, R.; Sillanpää, M.; Lakshmaiya, N.; Kondal, N.; Kandwal, A.; Sharma, R. Enhancing uv protection and antimicrobial properties in food packaging through the use of copper nanoparticles and κ-carrageenan based nanocomposite film. J. Inorg. Organomet. Polym. Mater. 2024, 34, 5538–5550. [Google Scholar] [CrossRef]
- Sheng, W.; Yang, L.; Yang, Y.; Wang, C.; Jiang, G.; Tian, Y. Photo-responsive cu-tannic acid nanoparticle-mediated antibacterial film for efficient preservation of strawberries. Food Chem. 2025, 464, 141711. [Google Scholar] [CrossRef]
- Wang, L.; Huo, X.; Jiang, F.; Xi, X.; Li, Y.; Lin, J. Dual-functional manganese dioxide nanoclusters for power-free microfluidic biosensing of foodborne bacteria. Sens. Actuators B Chem. 2023, 393, 134242. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.E.; Morsi, S.M.; Hasanin, M.S.; Youssef, A.M. Applications. Synthesis and characterization of cellulose/polylactic acid/MnO2 bio-nanocomposite as a promising coating of paper sheet for food packaging application. Carbohydr. Polym. Technol. Appl. 2025, 10, 100789. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Kowalonek, J.; Stachowiak-Trojanowska, N.; Cieciurska, Z.; Richert, A. Zinc oxide nanoparticles and sage (salvia officinalis) essential oil as active components of alginate films for food packaging. Polym. Degrad. Stab. 2025, 237, 111328. [Google Scholar] [CrossRef]
- Perumal, M.K.K.; Rajasekaran, M.B.S.; Renuka, R.R.; Samrot, A.V.; Nagarajan, M. Zinc oxide nanoparticles and their nanocomposites as an imperative coating for smart food packaging. Appl. Food Res. 2025, 5, 100849. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of multifunctional active food packaging from chitosan-titanium dioxide nanocomposite as ethylene scavenging and antimicrobial film. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; Kim, J.T.; Rhim, J.W.; Parameswaranpillai, J.; Nandi, D.; Srisuk, R.; Siengchin, S. Titanium dioxide nanoparticles and elderberry extract incorporated starch based polyvinyl alcohol films as active and intelligent food packaging wraps. Food Packag. Shelf Life 2022, 34, 100967. [Google Scholar] [CrossRef]
- Chen, C.; Ding, R.; Yang, S.; Wang, J.; Chen, W.; Zong, L.; Xie, J. Development of thermal insulation packaging film based on poly (vinyl alcohol) incorporated with silica aerogel for food packaging application. LWT 2020, 129, 109568. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun antimicrobial films of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) containing eugenol essential oil encapsulated in mesoporous silica nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef]
- Lawal, U.; Kumar, N.; Samyuktha, R.; Gopi, A.; Robert, V.; Pugazhenthi, G.; Loganathan, S.; Valapa, R.B. Poly (lactic acid)/amine grafted mesoporous silica-based composite for food packaging application. Int. J. Biol. Macromol. 2024, 277, 134567. [Google Scholar] [CrossRef]
- Sani, M.A.; Khezerlou, A.; Rezvani-Ghalhari, M.; McClements, D.J.; Varma, R.S. Advanced carbon-based nanomaterials: Application in the development of multifunctional next-generation food packaging materials. Adv. Coll. Interface Sci. 2025, 339, 103422. [Google Scholar] [CrossRef]
- Sangeetha, U.; Sudhakaran, N.; Parvathy, P.; Abraham, M.; Das, S.; De, S.; Sahoo, S.K. Coconut husk-lignin derived carbon dots incorporated carrageenan based functional film for intelligent food packaging. Int. J. Biol. Macromol. 2024, 266, 131005. [Google Scholar] [CrossRef]
- Lyn, F.H.; Tan, C.P.; Zawawi, R.; Hanani, Z.N. Physicochemical properties of chitosan/graphene oxide composite films and their effects on storage stability of palm-oil based margarine. Food Hydrocoll. 2021, 117, 106707. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cheigh, C.I.; Chung, M.S. Preparation and characteristic evaluation of ldpe/clay nanocomposites for food packaging. Food Sci. Biotechnol. 2025, 34, 2799–2805. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Ren, H.; Jin, J.; He, H.; Jin, P.; Wu, Z.; Zheng, Y. Research progress of nanocellulose-based food packaging. Food Sci. Technol. 2024, 143, 104289. [Google Scholar] [CrossRef]
- Swaroop, C.; Shukla, M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. Int. J. Biol. Macromol. 2018, 113, 729–736. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Ling, Z.; Yang, L.; Zhang, W.; Yao, T.; Xu, H. Detection of food contaminants: A review of established rapid analytical techniques and their applications and limitations. Food Saf. Health 2024, 2, 72–95. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Chepngeno, J.; Imathiu, S.; Owino, W.O.; Morlock, G.E. Baobab pulp authenticity and quality control by multi-imaging high-performance thin-layer chromatography. Food Chem. 2022, 390, 133108. [Google Scholar] [CrossRef]
- Estevez, P.; Prieto, J.O.; Burlingame, A.; Martinez, A.G. Characterization of the ciguatoxin profile in fish samples from the eastern atlantic ocean using capillary liquid chromatography-high resolution mass spectrometry. Food Chem. 2023, 418, 135960. [Google Scholar] [CrossRef]
- Spanu, D.; Butti, L.; Boldrocchi, G.; Bettinetti, R.; Recchia, S.; Monticelli, D. Selective organomercury determination by icp-ms made easy. Anal. Chim. Acta 2022, 1206, 339553. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, S.P.; Kumar, P.; Khan, M.A.; Singh, S. Emerging electrochemical portable methodologies on carbon-based electrocatalyst for the determination of pharmaceutical and pest control pollutants: State of the art. J. Environ. Chem. Eng. 2023, 11, 109023. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Auger, S.; Vidic, J. Metal oxide nanoparticles for safe active and intelligent food packaging. Trends Food Sci. Technol. 2021, 116, 655–668. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y.; Zhang, Z.; Shen, Y.; Li, Y.; Ma, T.; Zhang, Q.; Ying, Y.; Fu, Y. Portable and durable sensor based on porous mofs hybrid sponge for fluorescent-visual detection of organophosphorus pesticide. Biosens. Bioelectron. 2022, 216, 114659. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S. Revolutionizing food safety with quantum dot–polymer nanocomposites: From monitoring to sensing applications. Foods 2023, 12, 2195. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, S.; Li, H.; Yang, T.; Zheng, K.; Guo, Z.M.; Shi, J.; Huang, X.; Zou, X.; Picchetti, P.; et al. Design principles of nanosensors for multiplex detection of contaminants in food. Small 2025, 21, e2412271. [Google Scholar] [CrossRef]
- Lu, D.; Tian, X.; Yang, Y.; Fu, Z.; Zhao, Q.; Li, B.; Wang, W. Rapid and sensitive aptamer-linked immunosorbent assay with aptamer-templated silver nanoparticles for detection of aflatoxin b1 in medicinal and edible food. Food Biosci. 2024, 60, 104387. [Google Scholar] [CrossRef]
- Tao, C.; Wang, J.; Zhu, Y.; Ding, C.; Shen, Z.; Sun, D.; Cao, S.; Jiang, X.; Li, Y.; Liu, C.; et al. A highly sensitive fluorescence biosensor for aflatoxins b1 detection based on polydiacetylene liposomes combined with exonuclease iii-assisted recycling amplification. Microchim. Acta 2024, 191, 397. [Google Scholar] [CrossRef]
- Tai, W.; Hu, Q.; Dong, X.; Zhao, F.; Wu, W.; Wang, Y.; Yu, L. Hydrogel-integrated multimodal biosensor for the detection of glucose and carcinoembryonic antigen. Sens. Actuators B Chem. 2024, 412, 135800. [Google Scholar] [CrossRef]
- Lu, D.; Jiang, H.; Zhang, G.; Luo, Q.; Zhao, Q.; Shi, X. An in situ generated prussian blue nanoparticle-mediated multimode nanozyme-linked immunosorbent assay for the detection of aflatoxin B1. ACS Appl. Mater. Interfaces 2021, 13, 25738–25747. [Google Scholar] [CrossRef]
- Rahaiee, S.; Assadpour, E.; Esfanjani, A.F.; Silva, A.S.; Jafari, S.M. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Coll. Interface Sci. 2020, 279, 102153. [Google Scholar] [CrossRef]
- Zare, M.; Norouzi Roshan, Z.; Assadpour, E.; Jafari, S.M. Improving the cancer prevention/treatment role of carotenoids through various nano-delivery systems. Crit. Rev. Food Sci. Nutr. 2021, 61, 522–534. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Navarro, A.; Yamul, D.; Libonatti, C.; Tabera, A.; Basualdo, M. The use of propolis as a functional food ingredient: A review. Trends Food Sci. Technol. 2021, 115, 297–306. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, M.; You, X.; Sela, D.A.; Xiao, H.; McClements, D.J. Encapsulation of bifidobacterium in alginate microgels improves viability and targeted gut release. Food Hydrocoll. 2021, 116, 106634. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-grade pickering emulsions for encapsulation and delivery of bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.J. Microencapsulated saffron floral waste extracts as functional ingredients for antioxidant fortification of yogurt: Stability during the storage. LWT 2023, 184, 114976. [Google Scholar] [CrossRef]
- Li, B.-L.; Zhang, J.; Jin, W.; Chen, X.-Y.; Yang, J.-M.; Chi, S.-M.; Ruan, Q.; Zhao, Y. Oral administration of ph-responsive polyamine modified cyclodextrin nanoparticles for controlled release of anti-tumor drugs. React. Funct. Polym. 2022, 172, 105175. [Google Scholar] [CrossRef]
- Sevindik, M.; Krupodorova, T.; Sevindik, E.; Koçer, O.; Uysal, I.; Ünal, O. A comprehensive review of its biological activities, phenolic and chemical constituents, and applications. Appl. Fruit Sci. 2025, 67, 70. [Google Scholar] [CrossRef]
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and delivery of bioactive citrus pomace polyphenols: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8028–8044. [Google Scholar] [CrossRef]
- Zhang, D.; Tian, W.; Chen, L.-H.; Chen, T.; Wu, D.; Du, Y.; Hu, J. Synergistic effects of oleanolic acid and curcumin nanoparticles in gastric ulcer prevention. Int. J. Pharm. 2025, 674, 125465. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Yang, K.; Li, G.; Li, Q.; Wang, W.; Zhao, X.; Shao, N.; Qiu, H.; Liu, J.; Xu, L.; Zhao, J.J.C.; et al. Distribution of gut microbiota across intestinal segments and their impact on human physiological and pathological processes. Cell Biosci. 2025, 15, 47. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ansari, B.; Gharsallaoui, A. Polyelectrolytes-stabilized liposomes for efficient encapsulation of lactobacillus rhamnosus and improvement of its survivability under adverse conditions. Food Chem. 2022, 372, 131358. [Google Scholar] [CrossRef]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of vital signs with flexible and wearable medical devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Y.; Wang, P. Recent advances in hydrogels for the diagnosis and treatment of dry eye disease. Gels 2022, 8, 816. [Google Scholar] [CrossRef]
- Li, X.; Ai, S.; Lu, X.; Liu, S.; Guan, W. Nanotechnology-based strategies for gastric cancer imaging and treatment. RSC Adv. 2021, 11, 35392–35407. [Google Scholar] [CrossRef]
- Han, C.Y.; Choi, S.H.; Chi, S.-H.; Hong, J.H.; Cho, Y.E.; Kim, J. Nano-fluorescence imaging: Advancing lymphatic disease diagnosis and monitoring. Nano Converg. 2024, 11, 1–23. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, M.; Zheng, W.; Xu, T.; Deng, H.; Liu, J. Se/ru-decorated porous metal–organic framework nanoparticles for the delivery of pooled sirnas to reversing multidrug resistance in taxol-resistant breast cancer cells. ACS Appl. Mater. Interfaces 2017, 9, 6712–6724. [Google Scholar] [CrossRef]
- Raveendran, R.; Prabakaran, L.; Senthil, R.; Yesudhason, B.V.; Dharmalingam, S.; Sathyaraj, W.V.; Atchudan, R. Current innovations in intraocular pressure monitoring biosensors for diagnosis and treatment of glaucoma—Novel strategies and future perspectives. Biosensors 2023, 13, 663. [Google Scholar] [CrossRef]
- Mondal, H.; Kim, H.J.; Mohanto, N.; Jee, J.P. A review on dry eye disease treatment: Recent progress, diagnostics, and future perspectives. Pharmaceutics 2023, 15, 990. [Google Scholar] [CrossRef]
- Minaříková, M.; Fík, Z.; Štorm, J.; Helisová, K.; Ferrová, K.; Mahelková, G.J. Tear matrix metalloproteinase-9 levels may help to follow a ocular surface injury in lagophthalmic eyes. PLoS ONE 2022, 17, e0274173. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, T.; Zhou, X.; Yang, Y.; Liu, Y.; Zhou, H.; Wei, S.; Zhai, Z.; Wu, Y.; Sun, F.; et al. Rapid and quantitative detection of tear mmp-9 for dry eye patients using a novel silicon nanowire-based biosensor. Biosens. Bioelectron. 2022, 214, 114498. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Chang, X.; Wu, Q.; Wu, Y.; Xi, X.; Cao, J.; Chu, H.; Liu, Q.; Li, Y.; Wu, W.; Fang, X.; et al. Multifunctional au modified Ti3C2-MXene for photothermal/enzyme dynamic/immune synergistic therapy. Nano Lett. 2022, 22, 8321–8330. [Google Scholar] [CrossRef]
- Lan, Z.; Liu, W.J.; Yin, W.W.; Yang, S.R.; Cui, H.; Zou, K.L.; Cheng, G.W.; Chen, H.; Han, Y.H.; Rao, L.; et al. Biomimetic mdscs membrane coated black phosphorus nanosheets system for photothermal therapy/photodynamic therapy synergized chemotherapy of cancer. J. Nanobiotechnol. 2024, 22, 174. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, Z.; Wang, Z.; Zhao, Q.; Chen, W.; Chen, G.; Jiang, Z.; Cai, Q.; Gong, L.; Lai, Y.; et al. Synergizing catalysis with post-catalysis pseudo-iron release by building dynamic catalytic active sites in diatomic nanozymes for boosting cancer therapy. J. Am. Chem. Soc. 2025, 147, 15814–15826. [Google Scholar] [CrossRef]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S. Global epidemiology of ischemic heart disease: Results from the global burden of disease study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Tolppanen, A.-M.; Solomon, A.; Soininen, H.; Kivipelto, M.; de la Torre, J. Midlife vascular risk factors and alzheimer’s disease: Evidence from epidemiological studies. J. Alzheimers Dis. 2012, 32, 531–540. [Google Scholar] [CrossRef]
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; Kumar, N.V.A.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Peluso, I.; et al. Plant-derived bioactives and oxidative stress-related disorders: A key trend towards healthy aging and longevity promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Docea, A.O.; Calina, D.; Tsarouhas, K.; Zamfira, L.M.; Mitrut, R.; Sharifi-Rad, J.; Kovatsi, L.; Siokas, V.; Dardiotis, E.; et al. A mechanistic and pathophysiological approach for stroke associated with drugs of abuse. J. Clin. Med. 2019, 8, 1295. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Yin, Y.; Chen, S.; Guo, C.; Wu, S.; Yuan, Y. New-onset age of metabolic-associated fatty liver disease and incident cardiovascular diseases: Findings from prospective cohort. Innov. Med. 2024, 2, 100064. [Google Scholar] [CrossRef]
- Stefanadis, C.; Antoniou, C.K.; Tsiachris, D.; Pietri, P. Coronary atherosclerotic vulnerable plaque: Current perspectives. J. Am. Heart Assoc. 2017, 6, e005543. [Google Scholar] [CrossRef]
- An, H.; Qiu, X.; Wang, X.; Du, C.; Guo, X.; Hou, S.; Xu, M.; Wang, J.; Cheng, C.; Ran, H. Lifu-unlocked endogenous H2S generation for enhancing atherosclerosis-specific gas-enzymatic therapy. Biomaterials 2025, 315, 122972. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Tang, C.; Rong, X.; Liu, Y.; Luo, Y.; Xu, L.; Xu, Z.; Wang, J.; Wang, Y.; et al. Macrophage membrane-functionalized manganese dioxide nanomedicine for synergistic treatment of atherosclerosis by mitigating inflammatory storms and promoting cholesterol efflux. J. Nanobiotechnol. 2024, 22, 664. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The treatment of impaired wound healing in diabetes: Looking among old drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef]
- Hoversten, K.P.; Kiemele, L.J.; Stolp, A.M.; Takahashi, P.Y.; Verdoorn, B.P. In Prevention, diagnosis, and management of chronic wounds in older adults. Mayo Clin. Proc. 2020, 95, 2021–2034. [Google Scholar] [CrossRef]
- Yu, H.; Sun, J.; She, K.; Lv, M.; Zhang, Y.; Xiao, Y.; Liu, Y.; Han, C.; Xu, X.; Yang, S. Sprayed PAA-CaO2 nanoparticles combined with calcium ions and reactive oxygen species for antibacterial and wound healing. Regen. Biomater. 2023, 10, rbad071. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, B.; Xiang, F.; Tan, J.; Chen, Z.; Zhang, X.; Wu, C.; Mao, Z.; Luo, G.; Chen, X. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020, 11, 2788. [Google Scholar] [CrossRef]
- Cao, F.; Ju, E.; Zhang, Y.; Wang, Z.; Liu, C.; Li, W.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. An efficient and benign antimicrobial depot based on silver-infused MoS2. ACS Nano 2017, 11, 4651–4659. [Google Scholar] [CrossRef]
- Xu, H.; Lv, F.; Zhang, Y.; Yi, Z.; Ke, Q.; Wu, C.; Liu, M.; Chang, J. Hierarchically micro-patterned nanofibrous scaffolds with a nanosized bio-glass surface for accelerating wound healing. Nanoscale 2015, 7, 18446–18452. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, M.; Bao, F.; Xing, M.; Wang, E.; Xu, Q.; Huan, Z.; Guo, F.; Chang, J. Multifunctional Zn doped hollow mesoporous silica/polycaprolactone electrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale 2019, 11, 6315–6333. [Google Scholar] [CrossRef]
- Shi, R.; Li, H.; Jin, X.; Huang, X.; Ou, Z.; Zhang, X.; Luo, G.; Deng, J. Promoting re-epithelialization in an oxidative diabetic wound microenvironment using self-assembly of a ros-responsive polymer and p311 peptide micelles. Acta Biomater. 2022, 152, 425–439. [Google Scholar] [CrossRef]
- Xia, G.; Liu, Y.; Tian, M.; Gao, P.; Bao, Z.; Bai, X.; Yu, X.; Lang, X.; Hu, S.; Chen, X. Nanoparticles/thermosensitive hydrogel reinforced with chitin whiskers as a wound dressing for treating chronic wounds. J. Mater. Chem. B 2017, 5, 3172–3185. [Google Scholar] [CrossRef]
- Chen, S.A.; Chen, H.M.; Yao, Y.D.; Hung, C.F.; Tu, C.S.; Liang, Y.J. Topical treatment with anti-oxidants and au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur. J. Pharm. Sci. 2012, 47, 875–883. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Wang, Z.; Feng, J.; Zou, J.; Gao, B. Consequences of aging on bone. Aging Dis. 2023, 15, 2417. [Google Scholar] [CrossRef]
- Aghali, A. Craniofacial bone tissue engineering: Current approaches and potential therapy. Cells 2021, 10, 2993. [Google Scholar] [CrossRef]
- Li, X.; Shu, X.; Shi, Y.; Li, H.; Pei, X. MOFs and bone: Application of mofs in bone tissue engineering and bone diseases. Chin. Chem. Lett. 2023, 34, 107986. [Google Scholar] [CrossRef]
- Tan, B.; Zheng, Y.; Hao, J.; Yang, Q.; Luo, X.; Li, Q.; Zhang, X.; Ouyang, J.; Wang, J.; Hu, Z.; et al. Intra-articular injection of mof-based nanomaterials for the treatment of osteoarthritis by modulating the bone microenvironment. Adv. Compos. Hybrid Mater. 2025, 8, 126. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface. 2016, 13, 20160462. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Zhang, B.; Shi, A.; Pei, X.; Zhang, X.; Zhu, Z.; Chen, J.; Wang, J. Optimized fabrication of L-Asp-Cu(II) Bio-MOF for enhanced vascularized bone regeneration. Chem. Eng. J. 2025, 505, 159617. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Z.; Zhang, X.; Xu, Z.; Zhang, Y.; He, B.; Yang, R.; Zhang, Q.; Yang, Q.; Liu, W.; et al. 3D-printed, bi-layer, biomimetic artificial periosteum for boosting bone regeneration. Bio Design Manuf. 2022, 5, 540–555. [Google Scholar] [CrossRef]
- Qin, D.; Zhao, Y.; Cheng, R.; Liu, Y.; Guo, S.; Sun, L.; Guo, Y.; Hao, F.; Zhao, B. Mussel-inspired immunomodulatory and osteoinductive dual-functional hydroxyapatite nanoplatform for promoting bone regeneration. J. Nanobiotechnol. 2024, 22, 320. [Google Scholar] [CrossRef]
- Pitrolino, K.A.; Felfel, R.M.; Pellizzeri, L.M.; McLaren, J.; Popov, A.A.; Sottile, V.; Scotchford, C.A.; Scammell, B.E.; Roberts, G.A.; Grant, D.M. Development and in vitro assessment of a bi-layered chitosan-nano-hydroxyapatite osteochondral scaffold. Carbohydr. Polym. 2022, 282, 119126. [Google Scholar] [CrossRef]
- Tatara, A.M.; Mikos, A.G.; Kontoyiannis, D.P. Immunoengineering: An emerging field in infectious diseases. J. Infect. Dis. 2025, jiaf209. [Google Scholar] [CrossRef]
- Delany, I.; Rappuoli, R.; De Gregorio, E. Vaccines for the 21st century. Vaccines 2014, 6, 708–720. [Google Scholar] [CrossRef]
- Kennedy, R.B.; Ovsyannikova, I.G.; Palese, P.; Poland, G.A. Current challenges in vaccinology. Front. Immunol. 2020, 11, 1181. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Poudel, K.; Vithiananthan, T.; Kim, J.O.; Tsao, H. Recent progress in cancer vaccines and nanovaccines. Biomaterials 2024, 314, 122856. [Google Scholar] [CrossRef]
- Simnani, F.Z.; Singh, D.; Patel, P.; Choudhury, A.; Sinha, A.; Nandi, A.; Samal, S.K.; Verma, S.K.; Panda, P.K. Nanocarrier vaccine therapeutics for global infectious and chronic diseases. Mater. Today 2023, 66, 371–408. [Google Scholar] [CrossRef]
- McMichael, A.J.; Phillips, R.E. Escape of human immunodeficiency virus from immune control. Annu. Rev. Immunol. 1997, 15, 271–296. [Google Scholar] [CrossRef]
- Hoagland, D.T.; Liu, J.; Lee, R.B.; Lee, R.E. New agents for the treatment of drug-resistant mycobacterium tuberculosis. Adv. Drug Deliv. Rev. 2016, 102, 55–72. [Google Scholar] [CrossRef]
- Amin, R.; Melody Devi, C.; Sarkar, D.; Sharifi-Rad, J.; Sönmez Gürer, E.; Oana Docea, A.; Calina, D. Curcumin-loaded nanomedicines as therapeutic strategy in malaria management. Efood 2023, 4, e113. [Google Scholar] [CrossRef]
- Ko, H.J.; Kim, Y.J. Antigen delivery systems: Past, present, and future. Biomol. Ther. 2023, 31, 370. [Google Scholar] [CrossRef]
- Melo, M.; Porter, E.; Zhang, Y.; Silva, M.; Li, N.; Dobosh, B.; Liguori, A.; Skog, P.; Landais, E.; Menis, S. Immunogenicity of rna replicons encoding hiv env immunogens designed for self-assembly into nanoparticles. Mol. Ther. 2019, 27, 2080–2090. [Google Scholar] [CrossRef]
- Venkatesan, P. Preliminary phase 1 results from an hiv vaccine candidate trial. Lancet Microb. 2021, 2, e95. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Feng, C.; Li, Y.; Ferdows, B.E.; Patel, D.N.; Ouyang, J.; Tang, Z.; Kong, N.; Chen, E.; Tao, W. Emerging vaccine nanotechnology: From defense against infection to sniping cancer. Acta Pharm. Sin. B 2022, 12, 2206–2223. [Google Scholar] [CrossRef]
- Taylor, P.R.; Gordon, S.; Martinez-Pomares, L. The mannose receptor: Linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005, 26, 104–110. [Google Scholar] [CrossRef]
- van der Zande, H.J.; Nitsche, D.; Schlautmann, L.; Guigas, B.; Burgdorf, S. The mannose receptor: From endocytic receptor and biomarker to regulator of (meta) inflammation. Front. Immunol. 2021, 12, 765034. [Google Scholar] [CrossRef]
- Moku, G.; Vangala, S.; Gulla, S.K.; Yakati, V. In vivo targeting of DNA vaccines to dendritic cells via the mannose receptor induces long-lasting immunity against melanoma. ChemBioChem 2021, 22, 523–531. [Google Scholar] [CrossRef]
- Chen, J.; Fang, H.; Hu, Y.; Wu, J.; Zhang, S.; Feng, Y.; Lin, L.; Tian, H.; Chen, X. Combining mannose receptor mediated nanovaccines and gene regulated PD-L1 blockade for boosting cancer immunotherapy. Bioact. Mater. 2022, 7, 167–180. [Google Scholar] [CrossRef]
- van Den Brule, S.; Ambroise, J.; Lecloux, H.; Levard, C.; Soulas, R.; De Temmerman, P.J.; Palmai-Pallag, M.; Marbaix, E.; Lison, D. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part. Fibre Toxicol. 2015, 13, 38. [Google Scholar] [CrossRef]
- Pedata, P.; Ricci, G.; Malorni, L.; Venezia, A.; Cammarota, M.; Volpe, M.G.; Iannaccone, N.; Guida, V.; Schiraldi, C.; Romano, M.; et al. In vitro intestinal epithelium responses to titanium dioxide nanoparticles. Food Res. Int. 2019, 119, 634–642. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Dekkers, S.; Noordam, M.Y.; Hagens, W.I.; Bulder, A.S.; De Heer, C.; Ten Voorde, S.E.; Wijnhoven, S.W.; Marvin, H.J.; Sips, A.; et al. Review of health safety aspects of nanotechnologies in food production. Regul. Toxicol. Pharmacol. 2009, 53, 52–62. [Google Scholar] [CrossRef]
- Morais, L.d.O.; Macedo, E.V.; Granjeiro, J.M.; Delgado, I.F. Critical evaluation of migration studies of silver nanoparticles present in food packaging: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3083–3102. [Google Scholar] [CrossRef]
- Truzzi, E.; Bertelli, D.; Bilia, A.R.; Vanti, G.; Maretti, E.; Leo, E. Combination of nanodelivery systems and constituents derived from novel foods: A comprehensive review. Pharmaceutics 2023, 15, 2614. [Google Scholar] [CrossRef]
- Asfour, M.H.; Abd El-Alim, S.H.; Kassem, A.A.; Salama, A.; Gouda, A.S.; Nazim, W.S.; Nashaat, N.H.; Hemimi, M.; Abdel Meguid, N.A. Vitamin D3-loaded nanoemulsions as a potential drug delivery system for autistic children: Formulation development, safety, and pharmacokinetic studies. AAPS PharmSciTech 2023, 24, 58. [Google Scholar] [CrossRef]
- Crosera, M.; Prodi, A.; Mauro, M.; Pelin, M.; Florio, C.; Bellomo, F.; Adami, G.; Apostoli, P.; De Palma, G.; Bovenzi, M.; et al. Titanium dioxide nanoparticle penetration into the skin and effects on hacat cells. Int. J. Environ. Res. Public Health 2015, 12, 9282–9297. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Nishanth, R.P.; Jyotsna, R.G.; Schlager, J.J.; Hussain, S.M.; Reddanna, P. Inflammatory responses of raw 264.7 macrophages upon exposure to nanoparticles: Role of ros-nfκb signaling pathway. Nanotoxicology 2011, 5, 502–516. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-activated protein kinases and reactive oxygen species: How can ros activate mapk pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Walter, P.L.; Kampkötter, A.; Eckers, A.; Barthel, A.; Schmoll, D.; Sies, H.; Klotz, L.O. Modulation of FoxO signaling in human hepatoma cells by exposure to copper or zinc ions. Arch. Biochem. Biophys. 2006, 454, 107–113. [Google Scholar] [CrossRef]
- Frame, M.C. Src in cancer: Deregulation and consequences for cell behaviour. Biochim. Biophys. Acta Rev. Cancer 2002, 1602, 114–130. [Google Scholar] [CrossRef]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- Kuempel, E.D.; Geraci, C.L.; Schulte, P.A. Risk assessment and risk management of nanomaterials in the workplace: Translating research to practice. Ann. Occup. Hyg. 2012, 56, 491–505. [Google Scholar] [CrossRef]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef]
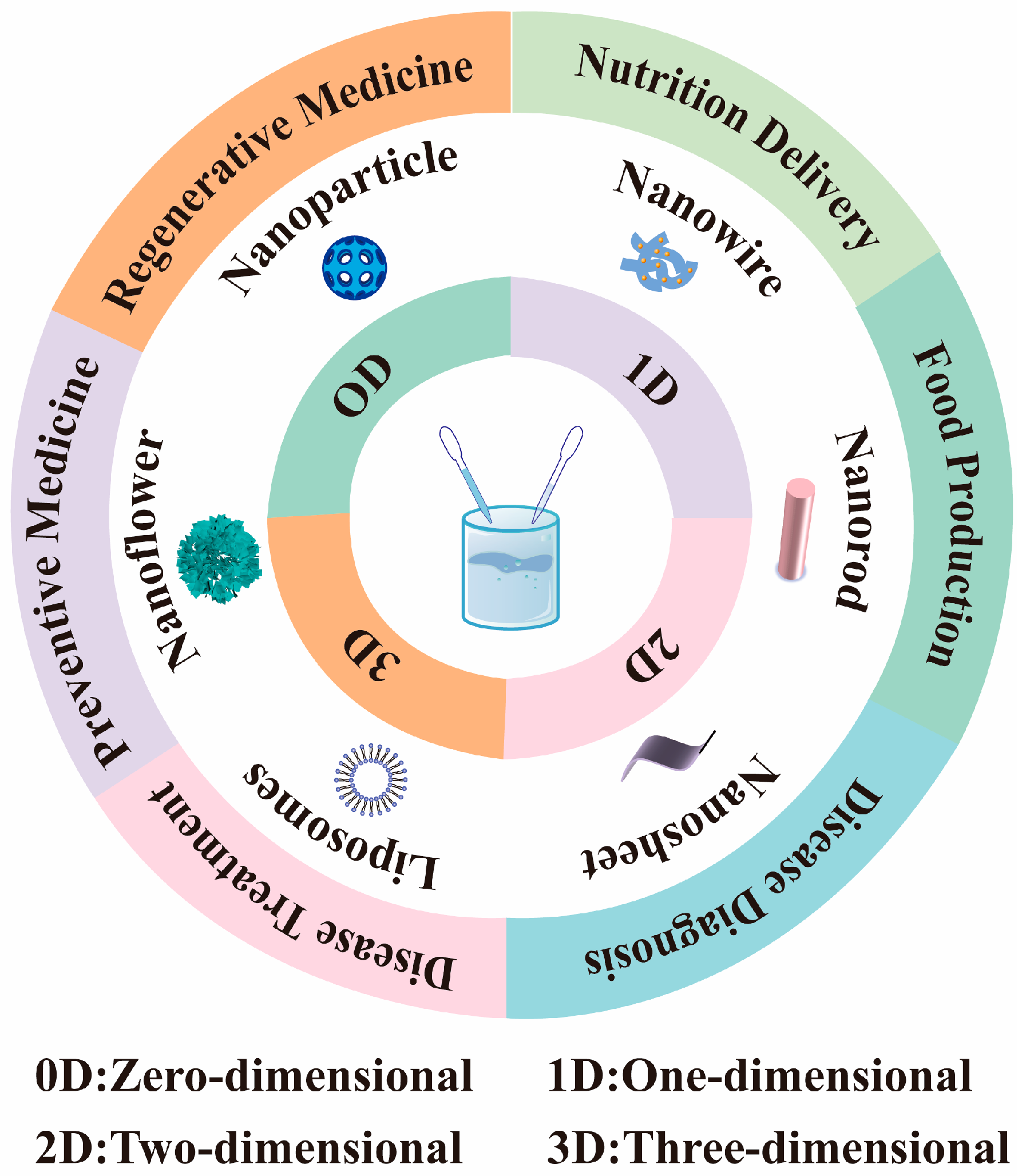


| Typical Shapes | Nanostructured Material | Application | References |
|---|---|---|---|
| Nanoparticle | Ag | Active packaging for food preservation and wound antimicrobials. | [8,9] |
| SiO2 | For the controlled release of pharmaceuticals, the development of very sensitive biosensors, food packaging, and seed treatments. | [10,11] | |
| Nanowire | ZnO | Utilizing photothermal treatment for precise drug delivery and sustained release. | [12,13] |
| Au | For the production of wearable high-sensitivity pressure sensors, photothermal therapy, and cardiac tissue restoration. | [14] | |
| Nanorod | TiO2 | For the sustained release of pharmaceuticals, cellular imaging and biomolecule tracking, and photothermal therapy. | [15,16] |
| Nanoplates | MoS2 | Utilization of drug carriers; advancement of intelligent packaging materials; identification of illness markers, pathogens, and cells; and implementation of photothermal therapy. | [17,18] |
| Nanofilm | Cu | Advancement of intelligent packaging materials, identification of glucose, cholesterol, and other substances. | [19] |
| Nanoporous material | MOFs | Extended release of pharmaceuticals, including antimicrobials, oxygen scavengers, ethylene scavengers, and the detection of viral nucleic acids and antibodies. | [20] |
| Nanoflower | MnO2 | Facilitating accurate drug loading and release, for food packaging, as fluorescent indicators, for wound healing. | [21] |
| CaCO3 | Utilizing drug carriers to enhance the strength, toughness, and barrier qualities of packing materials, as well as to identify biomolecules such as dopamine. | [22] |
| Classification | Name of Polymer | Structure | Advantages | References |
|---|---|---|---|---|
| Synthetic polymer | PVA | 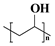 | High mechanical strength and flexibility, excellent water solubility and film-forming properties, chemical modification flexibility | [31] |
| PEG | 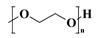 | Highly hydrophilic, can be modified into amphiphilic block copolymers | [32] | |
| PCL |  | Slow degradation rate, good flexibility, low melting point | [33] | |
| PLGA | 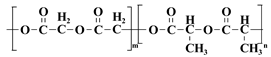 | Biodegradable, high mechanical strength, but relatively brittle | [34] | |
| PAA | 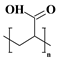 | pH-responsive, highly absorbent | [35] | |
| Natural polymer | Sodium alginate | 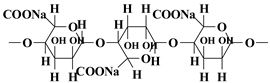 | pH responsiveness, easy surface functionalization, high water absorption and water retention | [36] |
| Chitosan |  | Natural antibacterial properties, good biocompatibility, and chemically modifiable | [37] | |
| Hyaluronic acid |  | Superior water retention, targets CD44 receptor | [38] | |
| Sericin |  | Ultra-high mechanical strength, controlled degradability | [39] | |
| Gelatin | 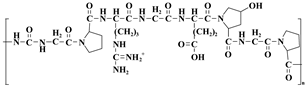 | Temperature sensitivity, promotion of cell adhesion | [40] |
| Nanostructured Materials/Particles | Activity | Applications in Food Technology | Reference |
|---|---|---|---|
| Nanoemulsification technology | Employed to stabilize immiscible components (e.g., oil and water) and enhance the mouthfeel of sauces, dressings, and beverages. | Nanoscale emulsion droplets to improve the bioavailability of beta-carotene. | [67] |
| Nanopackaging technology | Encapsulates sensitive ingredients (vitamins, probiotics, antioxidants) and protects them from damage by the processing or digestive environment. | Nanoliposomes encapsulate Omega-3 fatty acids to extend shelf life and mask fish oil odor. | [68] |
| Nanobubble technology | Improvement of food texture and taste. | Texture optimization of low-fat ice cream. | [69,70] |
| Food additive | Improvement of color, taste, and stability of food, extension of shelf life. | Titanium dioxide (TiO2) as food additive coloring. | [71] |
| Nutrient enhancer | Improve the absorption efficiency of vitamins, minerals, and other nutrients. | Ascorbic acid-embedded maltolysin nanoparticles as food fortification agents. | [72] |
| Nanofilm | Improvement of food texture. | Nanoencapsulated nanoclusters for improved milkshake aroma. | [73] |
| Nano lipid rolls | Improving the quality of processed foods. | [74] | |
| Nanocoatings | Improved abrasion resistance and antimicrobial properties of food processing equipment. | Both superhydrophobic and superhydrophilic coatings may minimize microbial adhesion to solid substrates. | [75] |
| Nano fertilizers/pesticides | Slow-release nanoparticles improve nutrient utilization and reduce chemical use. | Nano-copper hydroxide-controlled release pesticides. | [76] |
| Nanostructured Material | Activity | Applications in Food Technology | Reference |
|---|---|---|---|
| silver-based | Demonstrates broad-spectrum efficacy against foodborne pathogens, including bacteria, fungi, yeasts, and viruses | Active packaging for food preservation | [83,84] |
| copper-based | UV blocking, gas barrier, mechanical, moisture sensitivity, and antimicrobial properties | Active packaging for food preservation | [85,86,87] |
| manganese-based | Antioxidant, enhanced barrier properties of packaging materials, environmental friendliness, intelligent sensing | For packaging of fruits and seafood, for RFID smart labels | [88,89] |
| zinc oxide | Antimicrobial effect, enhanced UV protection, and better barrier properties | Maintaining food quality and extending shelf life | [90,91,92] |
| titanium dioxide | Self-cleaning packaging surface, photocatalytic properties, stability, antimicrobial and UV-blocking capabilities, intelligent sensing | Active packaging for food preservation, inhibition of microbial growth | [93,94] |
| silicon dioxide | High specific surface area, chemical inertness, tunable pore structure, and good biocompatibility | Enhanced mechanical properties of packaging materials, food preservation | [95,96,97] |
| carbon nanomaterials | Unique mechanical, electrical, thermal, and antimicrobial properties, sustainable packaging | Antimicrobial freshness and ultra-high barrier packaging film for foodstuffs | [98,99,100] |
| nanoclay | Unique layered structure, high specific surface area, and modifiability | Used for fresh meat packaging, enhance the mechanical properties of packaging materials | [101] |
| nanocellulose | Renewable, degradable, high strength and surface area, inhibits antimicrobial effect on both Gram-negative and -positive microorganisms | Replacement of plastic laminates and super-barrier functional coatings for high-end gift packages | [102] |
| polymer-based | Excellent mechanical properties, renewability, crystallinity, biodegradability, and processability | Maintaining food quality and extending shelf life | [103,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, K.; Yang, H.; Fan, D.; Deng, J. Advances in Nanotechnology Research in Food Production, Nutrition, and Health. Nutrients 2025, 17, 2443. https://doi.org/10.3390/nu17152443
Han K, Yang H, Fan D, Deng J. Advances in Nanotechnology Research in Food Production, Nutrition, and Health. Nutrients. 2025; 17(15):2443. https://doi.org/10.3390/nu17152443
Chicago/Turabian StyleHan, Kangran, Haixia Yang, Daidi Fan, and Jianjun Deng. 2025. "Advances in Nanotechnology Research in Food Production, Nutrition, and Health" Nutrients 17, no. 15: 2443. https://doi.org/10.3390/nu17152443
APA StyleHan, K., Yang, H., Fan, D., & Deng, J. (2025). Advances in Nanotechnology Research in Food Production, Nutrition, and Health. Nutrients, 17(15), 2443. https://doi.org/10.3390/nu17152443








