A Refined Carbohydrate-Rich Diet Reduces Vascular Reactivity Through Endothelial Oxidative Stress and Increased Nitric Oxide: The Involvement of Inducible Nitric Oxide Synthase
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Groups
2.3. Glucose and Insulin Tolerance Tests
2.4. Body Parameters, Tissue Collection, and Glycemic Profile
2.5. Lipid Profile Assessment
2.6. Vascular Reactivity
2.7. RNA Extraction and Real-Time PCR
2.8. Superoxide Dismutase (SOD) Activity
2.9. Drugs and Reagents
2.10. Statistical Analysis
3. Results
3.1. Effects of Refined Carbohydrate-Rich Diet Consumption on Body Parameters
3.2. Effects of Refined Carbohydrate-Rich Diet Consumption on Glycemic and Lipid Profiles
3.3. Effects of Refined Carbohydrate-Rich Diet Consumption on Glucose Metabolism
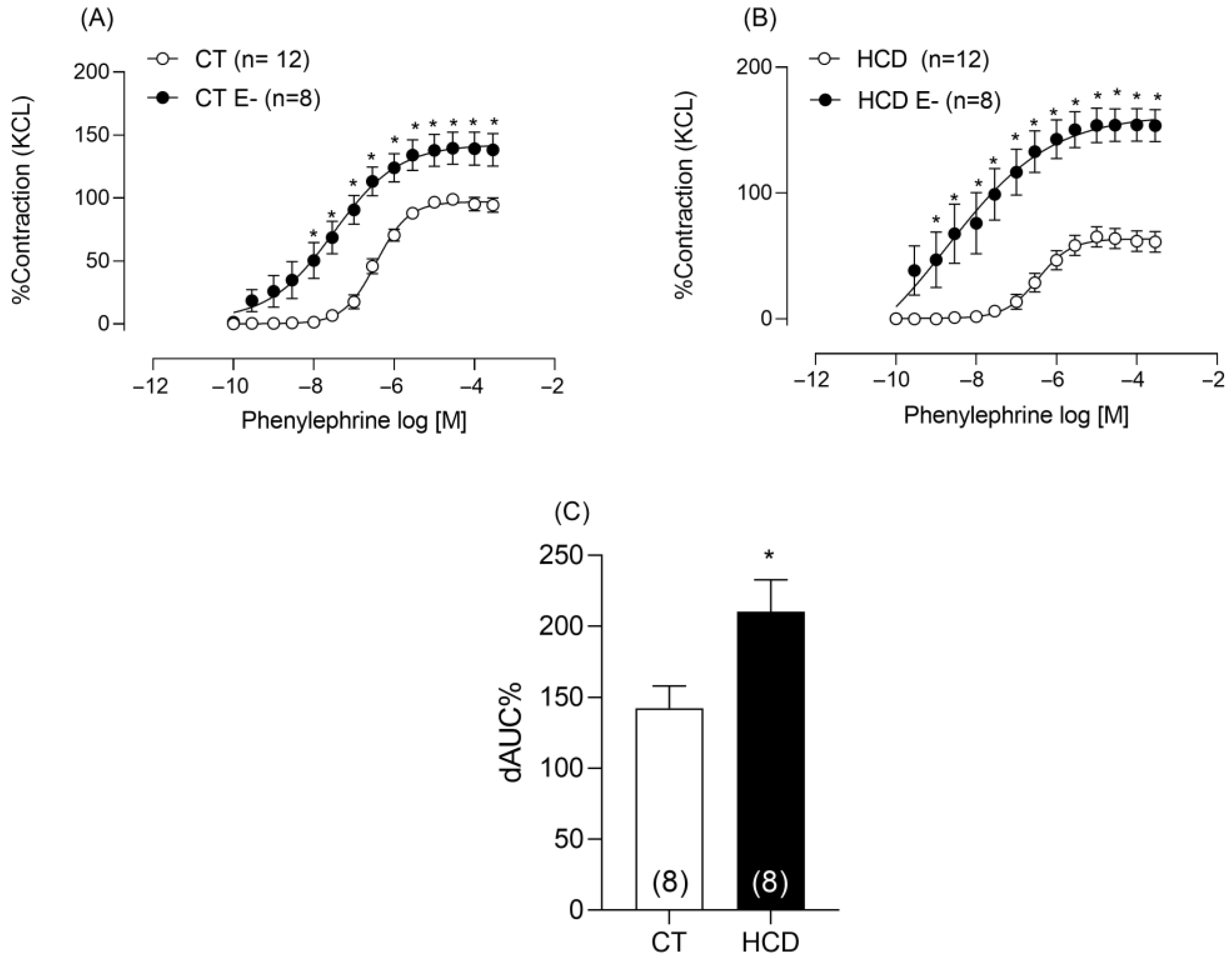
3.4. Effects of Refined Carbohydrate-Rich Diet Consumption on Aortic Vascular Reactivity
3.5. Effects of Refined Carbohydrate-Rich Diet Consumption on the Regulation of Genes Associated with Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD86 | Cluster of differentiation 86. |
| CT | Control. |
| CVDs | Cardiovascular diseases. |
| DETCA | Diethyldithiocarbamic acid. |
| eNOS | Endothelial nitric oxide synthase. |
| GTT | Glucose tolerance test. |
| HCD | High-carbohydrate diet. |
| iNOS | Inducible nitric oxide synthase. |
| ITT | Insulin tolerance test. |
| mRNA | Messenger ribonucleic acid. |
| NO | Nitric oxide. |
| NOS | Nitric oxide synthase. |
| PCR | Polymerase chain reaction. |
| PVAT | Perivascular adipose tissue. |
| qPCR | Quantitative polymerase chain reaction. |
| RNA | Ribonucleic acid. |
| ROS | Reactive oxygen species. |
| SOD | Superoxide dismutase. |
| TyG | Triglyceride–glucose index. |
References
- Arnone, D.; Chabot, C.; Heba, A.-C.; Kökten, T.; Caron, B.; Hansmannel, F.; Dreumont, N.; Ananthakrishnan, A.N.; Quilliot, D.; Peyrin-Biroulet, L. Sugars and Gastrointestinal Health. Clin. Gastroenterol. Hepatol. 2022, 20, 1912–1924.e7. [Google Scholar] [CrossRef] [PubMed]
- Azais-Braesco, V.; Sluik, D.; Maillot, M.; Kok, F.; Moreno, L.A. A review of total & added sugar intakes and dietary sources in Europe. Nutr. J. 2017, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Witek, K.; Wydra, K.; Filip, M. A high-sugar diet consumption, metabolism and health impacts with a focus on the development of substance use disorder: A narrative review. Nutrients 2022, 14, 2940. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 February 2024).
- Zhang, Y.; Giovannucci, E.L. Ultra-processed foods and health: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 63, 10836–10848. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wellens, J.; Yang, N.; Li, D.; Wang, J.; Wang, L.; Yuan, S.; He, Y.; Song, P.; Munger, R.; et al. Ultra-processed foods and human health: An umbrella review and updated meta-analyses of observational evidence. Clin. Nutr. 2024, 43, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.-L.; Wei, Y.-F.; Sun, J.-N.; Shi, Y.-C.; Liu, F.-H.; Sun, M.-H.; Chang, Q.; Wu, Q.-J.; Zhao, Y.-H. Ultra-processed food consumption and metabolic disease risk: An umbrella review of systematic reviews with meta-analyses of observational studies. Front. Nutr. 2024, 11, 1306310. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med. 2014, 174, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Mansourian, M.; Firouzi, S.; Taheri, M.; Haghighatdoost, F. Longitudinal association of dietary carbohydrate and the risk cardiovascular disease: A dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 62, 6277–6292. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhu, W.; Lei, Z.; Yan, X.; Zhang, X.; Wei, S.; Wang, Q. Intake of added sugar from different sources and risk of all-cause mortality and cardiovascular diseases: The role of body mass index. J. Nutr. 2024, 154, 3457–3464. [Google Scholar] [CrossRef] [PubMed]
- Jo, U.; Park, K. Carbohydrate intake and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Nutrients 2023, 15, 1740. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liang, Z.; Ma, J.; Hu, D.; Yao, F.; Qin, P. Total sugar, added sugar, fructose, and sucrose intake and all-cause, cardiovascular, and cancer mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Nutrition 2023, 111, 112032. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Sugars Intake for Adults and Children [Internet]; World Health Organization: Geneva, Switzerland, 2015; p. 59. ISBN 978-92-4-154902-8. Available online: https://www.who.int/publications/i/item/9789241549028/ (accessed on 16 July 2025).
- Ferreira, A.V.M.; Mario, É.G.; Porto, L.C.J.; Andrade, S.P.; Botion, L.M. High-carbohydrate diet selectively induces tumor necrosis factor-α production in mice liver. Inflammation 2010, 34, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Niño, O.M.; da Costa, C.S.; Torres, K.M.; Zanol, J.F.; Freitas-Lima, L.C.; Miranda-Alves, L.; Graceli, J.B. High-refined carbohydrate diet leads to polycystic ovary syndrome-like features and reduced ovarian reserve in female rats. Toxicol. Lett. 2020, 332, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Zanol, J.F.; Niño, O.M.; da Costa, C.S.; Zimerman, J.; Silva, N.P.; Oliveira, T.M.; Maas, E.M.; dos Santos, F.C.; Miranda-Alves, L.; Graceli, J.B. High-refined carbohydrate diet alters different metabolic functions in female rats. Mol. Cell. Endocrinol. 2022, 558, 111774. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Menezes-Garcia, Z.; Henriques, M.C.C.; Soriani, F.M.; Pinho, V.; Faria, A.M.C.; Santiago, A.F.; Cara, D.C.; Souza, D.G.; Teixeira, M.M.; et al. Acute and sustained inflammation and metabolic dysfunction induced by high refined carbohydrate-containing diet in mice. Obesity 2012, 21, E396–E406. [Google Scholar] [CrossRef] [PubMed]
- dos Reis Costa, D.E.F.; Silveira, A.L.M.; Campos, G.P.; Nóbrega, N.R.C.; De Araújo, N.F.; de Figueiredo Borges, L.; dos Santos Aggum Capettini, L.; Ferreira, A.V.M.; Bonaventura, D. High-carbohydrate diet enhanced the anticontractile effect of perivascular adipose tissue through activation of renin-angiotensin system. Front. Physiol. 2021, 11, 628101. [Google Scholar] [CrossRef]
- Bruno, A.S.; Castor, R.G.M.; Berg, B.; Costa, D.E.F.d.R.; Monteiro, A.L.L.; Scalzo, S.; Oliveira, K.C.M.; Bello, F.L.M.; Aguiar, G.C.; Melo, M.B.; et al. Cardiac disturbances and changes in tissue cytokine levels in mice fed with a high-refined carbohydrate diet. Cytokine 2023, 166, 156192. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Roles of oxidative stress and inflammation in vascular endothelial dysfunction-related disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Masi, L.N.; Martins, A.R.; Crisma, A.R.; do Amaral, C.L.; Davanso, M.R.; Serdan, T.D.A.; da Cunha de Sá, R.D.C.; Cruz, M.M.; Alonso-Vale, M.I.C.; Torres, R.P.; et al. Combination of a high-fat diet with sweetened condensed milk exacerbates inflammation and insulin resistance induced by each separately in mice. Sci. Rep. 2017, 7, 3937. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Guerrero-Romero, F. The correct formula for the triglycerides and glucose index. Eur. J. Pediatr. 2020, 179, 1171. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.Z.; Fioresi, M.; Marques, V.B.; Vassallo, D.V. Acute copper overload induces vascular dysfunction in aortic rings due to endothelial oxidative stress and increased nitric oxide production. J. Toxicol. Environ. Health Part A 2018, 81, 218–228. [Google Scholar] [CrossRef]
- Bolsoni-Lopes, A.; Festuccia, W.T.; Farias, T.S.M.; Chimin, P.; Torres-Leal, F.L.; Derogis, P.B.M.; de Andrade, P.B.; Miyamoto, S.; Lima, F.B.; Curi, R.; et al. Palmitoleic acid (n-7) increases white adipocyte lipolysis and lipase content in a PPARα-dependent manner. Am. J. Physiol. Metab. 2013, 305, E1093–E1102. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M. Calcium-activated potassium channels and endothelial dysfunction: Therapeutic options? Br. J. Pharmacol. 2009, 156, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.T.; Quayle, J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995, 268, C799–C822. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 2021, 73, 924. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of endothelial dysfunction in cardiovascular diseases: The link between inflammation and hydrogen sulfide. Front. Pharmacol. 2020, 10, 1568. [Google Scholar] [CrossRef]
- Allbritton-King, J.D.; García-Cardeña, G. Endothelial cell dysfunction in cardiac disease: Driver or consequence? Front. Cell Dev. Biol. 2023, 11, 1278166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ray, A.; Maharana, K.C.; Meenakshi, S.; Singh, S. Endothelial dysfunction and its relation in different disorders: Recent update. Heal. Sci. Rev. 2023, 7, 100084. [Google Scholar] [CrossRef]
- Förstermann, U.; Nakane, M.; Tracey, W.R.; Pollock, J.S. Isoforms of nitric oxide synthase: Functions in the cardiovascular system. Eur. Heart J. 1993, 14 (Suppl I), 10–15. [Google Scholar] [PubMed]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’gallagher, K. Dysfunctional and dysregulated nitric oxide synthases in cardiovascular disease: Mechanisms and therapeutic potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2019, 40, 158–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible nitric oxide synthase: Good or bad? Biomed. Pharmacother. 2017, 93, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, J. Deciphering Oxidative stress in cardiovascular disease progression: A blueprint for mechanistic understanding and therapeutic innovation. Antioxidants 2024, 14, 38. [Google Scholar] [CrossRef]
- Wattanapitayakul, S.K.; Bauer, J.A. Oxidative pathways in cardiovascular disease: Roles, mechanisms, and therapeutic implications. Pharmacol. Ther. 2001, 89, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Sahranavard, T.; Carbone, F.; Montecucco, F.; Xu, S.; Al-Rasadi, K.; Jamialahmadi, T.; Sahebkar, A. The role of potassium in atherosclerosis. Eur. J. Clin. Investig. 2020, 51, e13454. [Google Scholar] [CrossRef]
- Lemke, J.; Gollasch, M.; Tsvetkov, D.; Schulig, L. Advances in the design and development of chemical modulators of the voltage-gated potassium channels K V 7.4 and K V 7.5. Expert Opin. Drug Discov. 2025, 20, 47–62. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Lucan, S.C.; O’kEefe, J.H. The evidence for saturated fat and for sugar related to coronary heart disease. Prog. Cardiovasc. Dis. 2016, 58, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, P.A.; Naug, H.L.; DU Toit, E.F.; Donner, D.; Colson, N.J. A refined high carbohydrate diet is associated with changes in the serotonin pathway and visceral obesity. Genet. Res. 2015, 97, e23. [Google Scholar] [CrossRef]
- Te Morenga, L.A.; Howatson, A.J.; Jones, R.M.; Mann, J. Dietary sugars and cardiometabolic risk: Systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am. J. Clin. Nutr. 2014, 100, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P. Refined carbohydrates, phenotypic plasticity and the obesity epidemic. Med. Hypotheses 2019, 131, 109317. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive intake of sugar: An accomplice of inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Wang, L.; Wang, Z.; Chai, S.; Zhu, X.; Ren, W.; Chang, X. High-glucose administration induces glucose intolerance in mice: A critical role of toll-like receptor 4. J. Clin. Biochem. Nutr. 2019, 64, 194–200. [Google Scholar] [CrossRef]
- Gounden, V.; Devaraj, S.; Jialal, I. The role of the triglyceride-glucose index as a biomarker of cardio-metabolic syndromes. Lipids Heal. Dis. 2024, 23, 416. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Rawish, E.; Nording, H.M.; Langer, H.F. Inflammation in metabolic and cardiovascular disorders—Role of oxidative stress. Life 2021, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A. Estrogens in adipose tissue physiology and obesity-related dysfunction. Biomedicines 2023, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Deutz, N.E.P.; Biolo, G.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cuerda, C.; Delzenne, N.; Leon Sanz, M.; Ljungqvist, O.; et al. Carbohydrates and insulin resistance in clinical nutrition: Recommendations from the ESPEN expert group. Clin. Nutr. 2017, 36, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin resistance and cardiovascular disease. J. Int. Med. Res. 2023, 51, 3000605231164548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant Protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Ramji, D.P.; Davies, T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor. Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Y.; Yu, J. Role of inflammation and immune response in atherosclerosis: Mechanisms, modulations, and therapeutic targets. Hum. Immunol. 2023, 84, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Mantani, P.T.; Ljungcrantz, I.; Andersson, L.; Alm, R.; Hedblad, B.; Björkbacka, H.; Nilsson, J.; Fredrikson, G.N. Circulating CD40+ and CD86+ B cell subsets demonstrate opposing associations with risk of stroke. Arter. Thromb. Vasc. Biol. 2014, 34, 211–218. [Google Scholar] [CrossRef] [PubMed]










| CT | HCD | |
|---|---|---|
| Initial body weight (g) | 367 ± 39 | 354 ± 27 |
| Final body weight (g) | 418 ± 43 | 405 ± 30 |
| Weigh gain (g) | 50 ± 3.1 | 50 ± 2.7 |
| Food intake (g/day) | 27 ± 1.2 | 20 ± 0.5 * |
| Water intake (mL/day) | 39 ± 6.3 | 23 ± 3.4 * |
| Energy efficiency (Kcal) | 0.45 ± 0.07 | 0.54 ± 0.07 * |
| Adipose Tissue | CT | HCD |
|---|---|---|
| Epididymal (g) | 12 ± 1.01 | 11.72 ± 1.16 |
| Mesenteric (g) | 4.41 ± 0.38 | 4.35 ± 0.32 |
| Subcutaneous (g) | 12.22 ± 0.75 | 11.8 ± 0.54 |
| Perirenal (g) | 1.98 ± 0.17 | 1.68 ± 0.13 |
| Retroperitoneal (g) | 8.93 ± 0.56 | 9.38 ± 0.67 |
| Thoracic PVAT (g) | 0.26 ± 0.012 | 0.22 ± 0.013 |
| Adipose Tissue | CT | HCD |
|---|---|---|
| Glucose (mg/dL) | 117.5 ± 5.3 | 130.3 ± 11.1 * |
| Triglyceride (mg/dL) | 105 ± 23.8 | 197.04 ± 29.1 * |
| Total Cholesterol (mg/dL) | 110.4 ± 17.4 | 126.8 ± 9.0 |
| HDL (mg/dL) | 141.65 ± 10.5 | 144.12 ± 15.5 |
| TyG index | 12.4 ± 2.9 | 25.4 ± 4.5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, K.; Mawandji, N.B.d.S.; Schereider, I.R.G.; de Oliveira, E.C.; Vieira, J.M.; Bolsoni-Lopes, A.; Graceli, J.B.; Dantas, J.A.; Cardoso, L.S.; Vassallo, D.V.; et al. A Refined Carbohydrate-Rich Diet Reduces Vascular Reactivity Through Endothelial Oxidative Stress and Increased Nitric Oxide: The Involvement of Inducible Nitric Oxide Synthase. Nutrients 2025, 17, 2395. https://doi.org/10.3390/nu17152395
Neumann K, Mawandji NBdS, Schereider IRG, de Oliveira EC, Vieira JM, Bolsoni-Lopes A, Graceli JB, Dantas JA, Cardoso LS, Vassallo DV, et al. A Refined Carbohydrate-Rich Diet Reduces Vascular Reactivity Through Endothelial Oxidative Stress and Increased Nitric Oxide: The Involvement of Inducible Nitric Oxide Synthase. Nutrients. 2025; 17(15):2395. https://doi.org/10.3390/nu17152395
Chicago/Turabian StyleNeumann, Karoline, Nina Bruna de Souza Mawandji, Ingridy Reinholz Grafites Schereider, Emanuelle Coutinho de Oliveira, Julia Martins Vieira, Andressa Bolsoni-Lopes, Jones Bernardes Graceli, Julia Antonietta Dantas, Lorena Silveira Cardoso, Dalton Valentim Vassallo, and et al. 2025. "A Refined Carbohydrate-Rich Diet Reduces Vascular Reactivity Through Endothelial Oxidative Stress and Increased Nitric Oxide: The Involvement of Inducible Nitric Oxide Synthase" Nutrients 17, no. 15: 2395. https://doi.org/10.3390/nu17152395
APA StyleNeumann, K., Mawandji, N. B. d. S., Schereider, I. R. G., de Oliveira, E. C., Vieira, J. M., Bolsoni-Lopes, A., Graceli, J. B., Dantas, J. A., Cardoso, L. S., Vassallo, D. V., & Nunes, K. Z. (2025). A Refined Carbohydrate-Rich Diet Reduces Vascular Reactivity Through Endothelial Oxidative Stress and Increased Nitric Oxide: The Involvement of Inducible Nitric Oxide Synthase. Nutrients, 17(15), 2395. https://doi.org/10.3390/nu17152395







