The Effects of Intermittent Fasting on Inflammatory Markers in Adults: A Systematic Review and Pairwise and Network Meta-Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
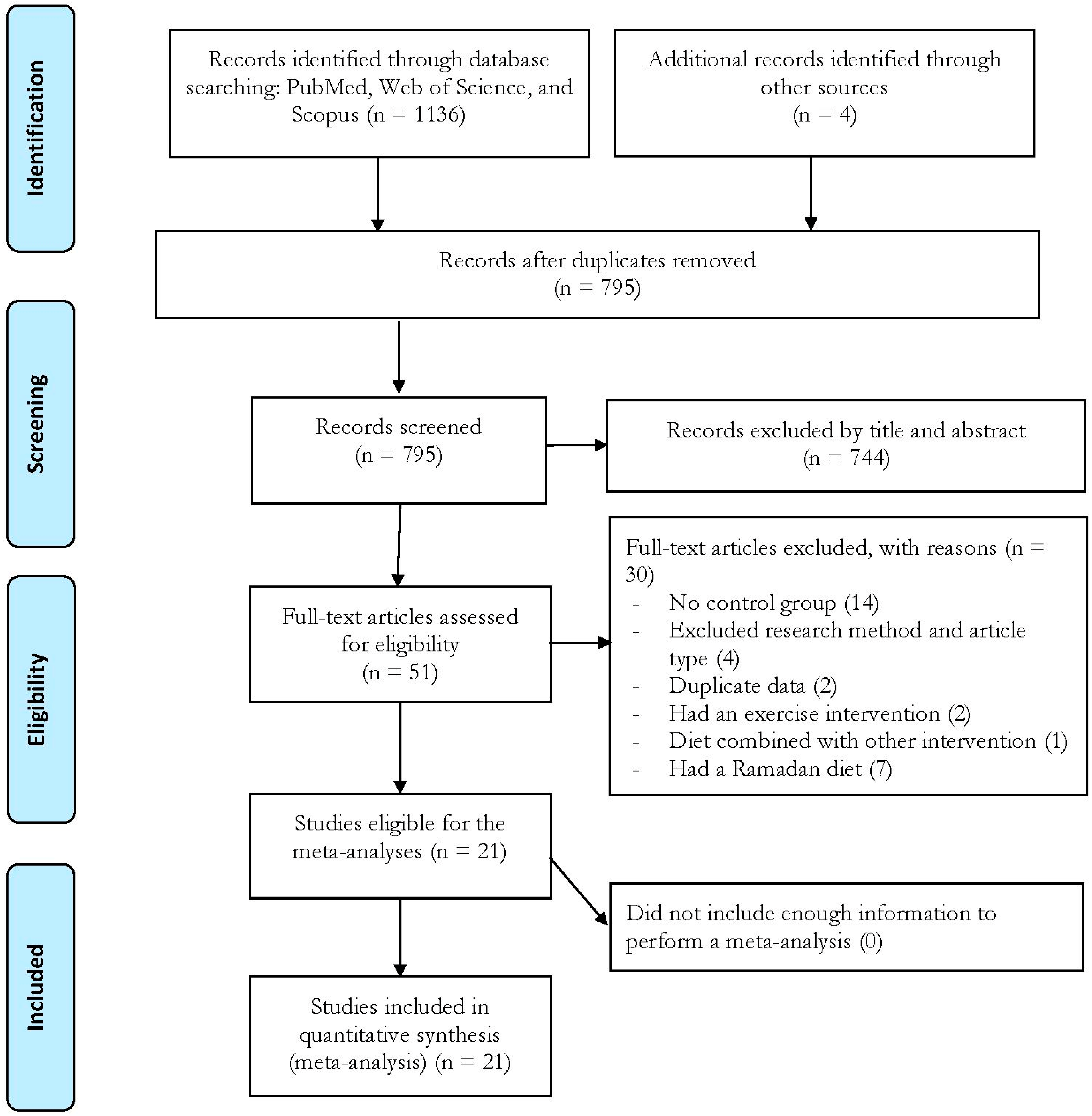
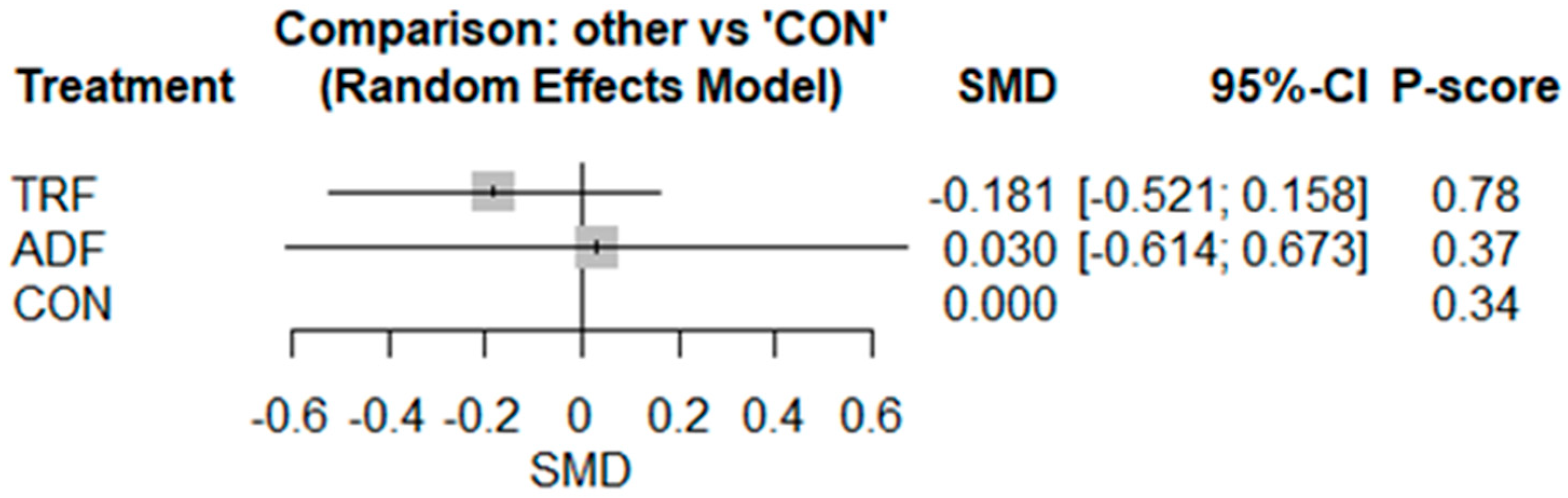
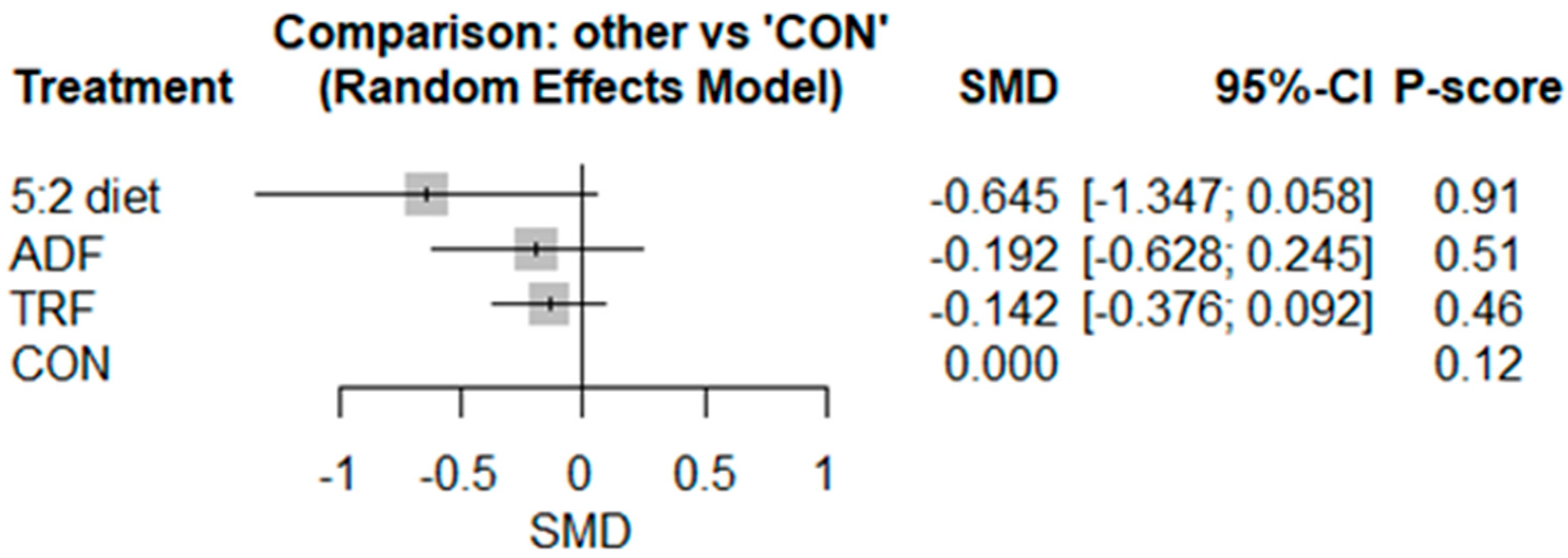
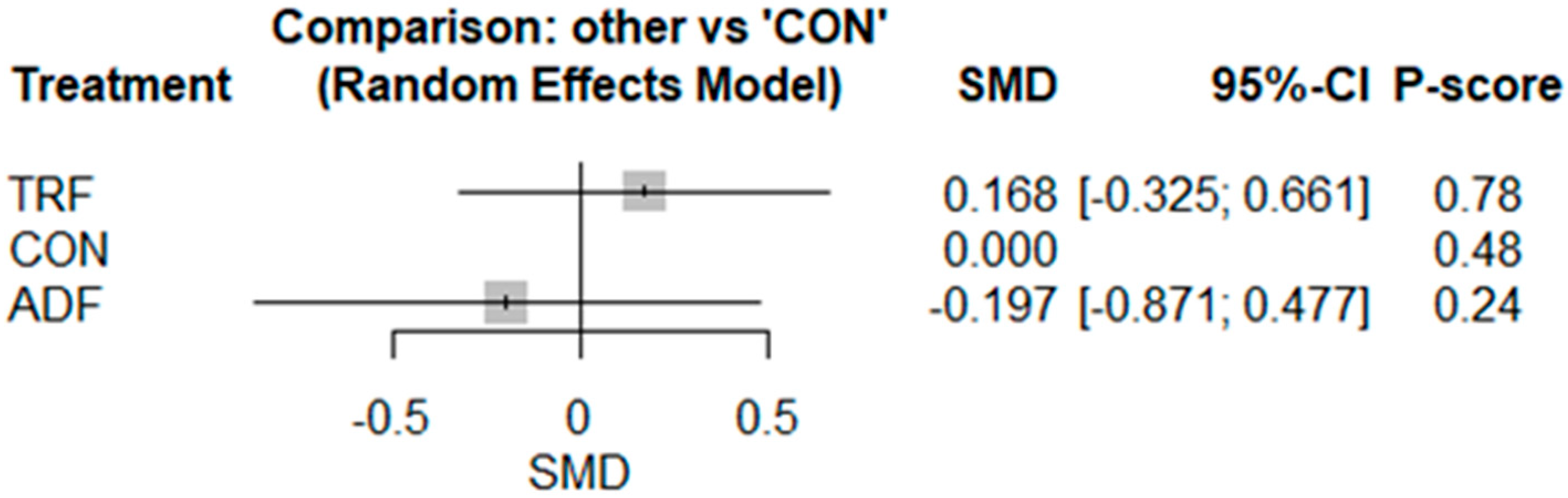
3. Results
3.1. Study Characteristics
3.2. Meta-Analyses
3.2.1. IL-6
3.2.2. TNF-α
3.2.3. CRP
3.2.4. Leptin
3.2.5. Adiponectin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| IF | Intermittent Fasting |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor-Alpha |
| CRP | C-Reactive Protein |
| hsCRP | High-Sensitivity C-Reactive Protein |
| SMD | Standardized Mean Difference |
| TRF | Time-Restricted Feeding |
| ADF | Alternate-Day Fasting |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| BMI | Body Mass Index |
References
- Frühbeck, G.; Busetto, L.; Dicker, D.; Yumuk, V.; Goossens, G.H.; Hebebrand, J.; Halford, J.G.; Farpour-Lambert, N.J.; Blaak, E.E.; Woodward, E. The ABCD of obesity: An EASO position statement on a diagnostic term with clinical and scientific implications. Obes. Facts 2019, 12, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Alsalhe, T.A.; Chalghaf, N.; Riccò, M.; Bragazzi, N.L.; Wu, J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17, e1003198. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Charrouf, R.; Martínez-Vizcaíno, V.; Hutchison, A.; Heilbronn, L.K.; Fernández-Rodríguez, R. The effects of time-restricted eating versus habitual diet on inflammatory cytokines and adipokines in the general adult population: A systematic review with meta-analysis. Am. J. Clin. Nutr. 2024, 119, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H. The metabolic phenotype in obesity: Fat mass, body fat distribution, and adipose tissue function. Obes. Facts 2017, 10, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Aamir, A.B.; Kumari, R.; Latif, R.; Ahmad, S.; Rafique, N.; Salem, A.M.; Alasoom, L.I.; Alsunni, A.; Alabdulhadi, A.S.; Chander, S. Effects of intermittent fasting and caloric restriction on inflammatory biomarkers in individuals with obesity/overweight: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2025, 26, e13838. [Google Scholar] [CrossRef] [PubMed]
- Haase, C.L.; Lopes, S.; Olsen, A.H.; Satylganova, A.; Schnecke, V.; McEwan, P. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: Evidence from a UK primary care database. Int. J. Obes. 2021, 45, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E. Weight loss is a critical factor to reduce inflammation. Clin. Nutr. ESPEN 2018, 28, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, L.K.; Wallace, J.M.; Livingstone, M.B.E. Obesity and inflammation: The effects of weight loss. Nutr. Res. Rev. 2008, 21, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Patikorn, C.; Roubal, K.; Veettil, S.K.; Chandran, V.; Pham, T.; Lee, Y.Y.; Giovannucci, E.L.; Varady, K.A.; Chaiyakunapruk, N. Intermittent fasting and obesity-related health outcomes: An umbrella review of meta-analyses of randomized clinical trials. JAMA Netw. Open 2021, 4, e2139558. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Maleki, A.H.; Ehsanifar, M.; Symonds, M.E.; Rosenkranz, S.K. Longer-term effects of intermittent fasting on body composition and cardiometabolic health in adults with overweight and obesity: A systematic review and meta-analysis. Obes. Rev. 2025, 26, e13855. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Metabolic effects of intermittent fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Habibi Maleki, A.; Symonds, M.E.; Rosenkranz, S.K.; Rohani, H.; Ehsanifar, M. The effects of intermittent fasting on body composition and cardiometabolic health in adults with prediabetes or type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2024, 26, 3830–3841. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Rosenkranz, S.K.; Ghasemi, F.; Kheradmand, S.; Habibi Maleki, A.; Korivi, M.; Tsao, J.-P. Efficacy of intermittent fasting on improving liver function in individuals with metabolic disorders: A systematic review and meta-analysis. Nutr. Metab. 2025, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent fasting and metabolic health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O. Intermittent fasting in the management of diabetes: A review of glycemic control and safety. Nutr. Rev. 2024, 82, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Mulas, A.; Cienfuegos, S.; Ezpeleta, M.; Lin, S.; Pavlou, V.; Varady, K.A. Effect of intermittent fasting on circulating inflammatory markers in obesity: A review of human trials. Front. Nutr. 2023, 10, 1146924. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Q.; Liao, Q.; Li, M.; Zhang, P.; Santos, H.O.; Kord-Varkaneh, H.; Abshirini, M. Effects of intermittent fasting diets on plasma concentrations of inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2020, 79, 110974. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Clinical application of intermittent fasting for weight loss: Progress and future directions. Nat. Rev. Endocrinol. 2022, 18, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-E.; Tsai, H.-L.; Tu, Y.-K.; Chen, L.-W. Effects of different types of intermittent fasting on metabolic outcomes: An umbrella review and network meta-analysis. BMC Med. 2024, 22, 529. [Google Scholar] [CrossRef] [PubMed]
- Xiaoyu, W.; Yuxin, X.; Li, L. The effects of different intermittent fasting regimens in people with type 2 diabetes: A network meta-analysis. Front. Nutr. 2024, 11, 1325894. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, L.; Mu, Z. Network meta-analysis of three different forms of intermittent energy restrictions for overweight or obese adults. Int. J. Obes. 2024, 48, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Collaboration and John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Tufanaru, C.; Munn, Z.; Stephenson, M.; Aromataris, E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. JBI Evid. Implement. 2015, 13, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.; Phillips, S.A.; Norkeviciute, E.; Varady, K.A. Alternate day fasting with or without exercise: Effects on endothelial function and adipokines in obese humans. e-SPEN J. 2013, 8, e205–e209. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.-R.; Moon, J.-Y.; Kim, S.; An, K.-Y.; Oh, M.; Jeon, J.Y.; Jung, D.-H.; Choi, M.H.; Lee, J.-W. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: A pilot randomized controlled trial. Metabolism 2019, 93, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4-and 6-h time-restricted feeding on weight and cardiometabolic health: A randomized controlled trial in adults with obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Kroeger, C.M.; Trepanowski, J.F.; Hoddy, K.K.; Cienfuegos, S.; Kalam, F.; Varady, K.A. Differential effects of alternate-day fasting versus daily calorie restriction on insulin resistance. Obesity 2019, 27, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Haganes, K.L.; Silva, C.P.; Eyjolfsdottir, S.K.; Steen, S.; Grindberg, M.; Lydersen, S.; Hawley, J.A.; Moholdt, T. Time-restricted eating and exercise training improve HbA1c and body composition in women with overweight/obesity: A randomized controlled trial. Cell Metab. 2022, 34, 1457–1471.e4. [Google Scholar] [CrossRef] [PubMed]
- Kord Varkaneh, H.; Salehi Sahlabadi, A.; Găman, M.-A.; Rajabnia, M.; Sedanur Macit-Çelebi, M.; Santos, H.O.; Hekmatdoost, A. Effects of the 5: 2 intermittent fasting diet on non-alcoholic fatty liver disease: A randomized controlled trial. Front. Nutr. 2022, 9, 948655. [Google Scholar] [CrossRef] [PubMed]
- Kord Varkaneh, H.; Salehi-Sahlabadi, A.; Tinsley, G.M.; Santos, H.O.; Hekmatdoost, A. Effects of time-restricted feeding (16/8) combined with a low-sugar diet on the management of non-alcoholic fatty liver disease: A randomized controlled trial. Nutrition 2023, 105, 111847. [Google Scholar] [CrossRef] [PubMed]
- Kotarsky, C.J.; Johnson, N.R.; Mahoney, S.J.; Mitchell, S.L.; Schimek, R.L.; Stastny, S.N.; Hackney, K.J. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol. Rep. 2021, 9, e14868. [Google Scholar] [CrossRef] [PubMed]
- Lao, B.-N.; Luo, J.-H.; Xu, X.-Y.; Fu, L.-Z.; Tang, F.; Ouyang, W.-W.; Xu, X.-Z.; Wei, M.-T.; Xiao, B.-J.; Chen, L.-Y. Time-restricted feeding’s effect on overweight and obese patients with chronic kidney disease stages 3–4: A prospective non-randomized control pilot study. Front. Endocrinol. 2023, 14, 1096093. [Google Scholar] [CrossRef] [PubMed]
- Manoogian, E.N.; Zadourian, A.; Lo, H.C.; Gutierrez, N.R.; Shoghi, A.; Rosander, A.; Pazargadi, A.; Ormiston, C.K.; Wang, X.; Sui, J. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: The Healthy Heroes randomized control trial. Cell Metab. 2022, 34, 1442–1456.e7. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. GeroScience 2020, 42, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Miranda, E.R.; Fuller, K.N.; Perkins, R.K.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A.; Haus, J.M. Endogenous secretory RAGE increases with improvements in body composition and is associated with markers of adipocyte health. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Longo, G.; Grigoletto, D.; Bianco, A.; Ferraris, C.; Guglielmetti, M.; Veneto, A.; Tagliabue, A.; Marcolin, G. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Pacelli, F.Q.; Marcolin, G.; Bianco, A.; Paoli, A. Twelve months of time-restricted eating and resistance training improves inflammatory markers and cardiometabolic risk factors. Med. Sci. Sports Exerc. 2021, 53, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.D.; Falqueto, H.; Mânica, A.; Zanini, D.; de Oliveira, T.; de Sá, C.A.; Cardoso, A.M.; Manfredi, L.H. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J. Transl. Med. 2021, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.T.; Tinsley, G.M.; Alesi, M.G.; Hester, G.M.; Olmos, A.A.; Serafini, P.R.; Modjeski, A.S.; Mangine, G.T.; King, K.; Savage, S.N. Four weeks of time-restricted feeding combined with resistance training does not differentially influence measures of body composition, muscle performance, resting energy expenditure, and blood biomarkers. Nutrients 2020, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-M.; Liu, Z.; Wang, J.-Q.; Li, R.-Q.; Ren, J.-Y.; Gao, X.; Lv, S.-S.; Liang, L.-Y.; Zhang, F.; Yin, B.-W. Randomized controlled trial for time-restricted eating in overweight and obese young adults. iScience 2022, 25, 104870. [Google Scholar] [CrossRef] [PubMed]
- Schroor, M.M.; Joris, P.J.; Plat, J.; Mensink, R.P. Effects of intermittent energy restriction compared with those of continuous energy restriction on body composition and cardiometabolic risk markers–A systematic review and meta-analysis of randomized controlled trials in adults. Adv. Nutr. 2024, 15, 100130. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol.-Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.K.; Bunkin, D.A.; Greenberg, A.S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 1998, 83, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Tylutka, A.; Walas, Ł.; Zembron-Lacny, A. Level of IL-6, TNF, and IL-1β and age-related diseases: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1330386. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.-I.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Spiegelman, B.M. Tumor necrosis factor α: A key component of the obesity-diabetes link. Diabetes 1994, 43, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor necrosis factor-alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Lodish, H.F. Insulin resistance in adipose tissue: Direct and indirect effects of tumor necrosis factor-α. Cytokine Growth Factor Rev. 2003, 14, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Molnar, C.; Geiger, S.; Graziadei, I.; Ebenbichler, C.F.; Weiss, H.; Kaser, S.; Kaser, A.; Tilg, H. Anti-inflammatory effects of excessive weight loss: Potent suppression of adipose interleukin 6 and tumour necrosis factor α expression. Gut 2010, 59, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liao, Y.; Deng, Y.; Shuang, R. Unraveling the Health Benefits and Mechanisms of Time-Restricted Feeding: Beyond Caloric Restriction. Nutr. Rev. 2025, 83, e1209–e1224. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Bartha, P.; Zinder, O.; Kerner, A.; Markiewicz, W.; Avizohar, O.; Brook, G.; Levy, Y. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int. J. Obes. 2004, 28, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L. Effect of exercise training on C reactive protein: A systematic review and meta-analysis of randomised and non-randomised controlled trials. Br. J. Sports Med. 2017, 51, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Hayashino, Y.; Jackson, J.L.; Hirata, T.; Fukumori, N.; Nakamura, F.; Fukuhara, S.; Tsujii, S.; Ishii, H. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Metabolism 2014, 63, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Symonds, M.E.; Akbari, A. The impact of exercise training versus caloric restriction on inflammation markers: A systemic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4226–4241. [Google Scholar] [CrossRef] [PubMed]
- Kemalasari, I.; Fitri, N.A.; Sinto, R.; Tahapary, D.L.; Harbuwono, D.S. Effect of calorie restriction diet on levels of C reactive protein (CRP) in obesity: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102388. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, obesity, and leptin resistance: Where are we 25 years later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G.; Faggioni, R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukoc. Biol. 2000, 68, 437–446. [Google Scholar] [CrossRef] [PubMed]
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kord, H.V.; Tinsley, G.M.; Santos, H.O.; Zand, H.; Nazary, A.; Fatahi, S.; Mokhtari, Z.; Salehi-Sahlabadi, A.; Tan, S.C.; Rahmani, J. The influence of fasting and energy-restricted diets on leptin and adiponectin levels in humans: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Jequier, E. Leptin signaling, adiposity, and energy balance. Ann. N. Y. Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.K.; Ciaraldi, T.; Henry, R.R. Adiponectin in health and disease. Diabetes Obes. Metab. 2007, 9, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Gaeini, Z.; Mirmiran, P.; Bahadoran, Z. Effects of Ramadan intermittent fasting on leptin and adiponectin: A systematic review and meta-analysis. Hormones 2021, 20, 237–246. [Google Scholar] [CrossRef] [PubMed]


| Source, Year | Participant Characteristics | Intervention Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size (Sex) | Health Status | Age (Years) | BMI (kg/m2) | Design | Duration (Weeks or Months) | IF Mode | IF Protocol | CON or CR Protocol | Outcomes | |
| Bhutani et al. 2013 [31,32] | 32 (F and M) | Obese | IF: 42.0 ± 2.0 CON: 49.0 ± 2.0 | IF: 35.0 ± 1.0 CON: 35.0 ± 1.0 | RCT | 12 weeks | ADF | Consumed 25% of baseline energy on fasting days (24 h) and ate ad libitum on feeding days (24 h) | CON: Maintained normal diet | Adiponectin, Leptin, CRP |
| Cho et al. 2019 [33] | 13 (F and M) | Overweight/ Obese | IF: 33.5 ± 5.0 CON: 42.6 ± 10.6 | IF: 27.8 ± 3.4 CON: 25.8 ± 3.4 | RCT | 8 weeks | ADF | Consumed 25% of their daily recommended energy intake (approximately 500 kcal) | CON: Normal daily habits | CRP |
| Cienfuegos et al. 2020 [34] | 49 (F and M) | Obese | IF1: 49.0 ± 2.0 IF2: 46.0 ± 3.0 CON: 45.0 ± 2.0 | IF1: 36.0 ± 1.0 IF2: 37.0 ± 1.0 CON: 36.0 ± 1.0 | RCT | 8 weeks | TRF | IF1: Ate ad libitum from 3 to 7 p.m. daily (20 h fast) IF2: Ate ad libitum from 1 to 7 p.m. daily (18 h fast) | CON: No meal timing restrictions | TNF-α, IL-6 |
| Gabel et al. 2019 [35] | 43 (F and M) | Overweight/ Obese | IF: 43.1 ± 9.9 CR: 42.0 ± 12.4 CON: 41.0 ± 11.6 | IF: 34.0 ± 3.3 CR: 36.0 ± 4.1 CON: 35.0 ± 3.9 | RCT | 12 months | ADF | Consumed 25% of their baseline energy needs at lunch (between 12 and 2 p.m.) | CR: Consumed 75% baseline energy CON: Did not change their usual eating and activity habits | hsCRP, TNF-α, IL-6 |
| Haganes et al. 2022 [36] | 66 (F) | Overweight/ Obese | IF: 36.2 ± 5.9 CON: 36.4 ± 6.2 | IF: 31.8 ± 3.3 CON: 33.1 ± 4.2 | RCT | 7 weeks | TRF | A ≤10 h daily eating window with ad libitum energy intake | CON: Not to change their diets | Adiponectin, Leptin |
| Kord Varkaneh et al. 2022 [37] | 44 (F and M) | NAFLD | IF: 46.4 ± 13.4 CON: 44.2 ± 4.9 | IF: 30.4 ± 2.3 CON: 30.6 ± 3.1 | RCT | 12 weeks | 5:2 diet | On fasting days, 25% of recommended calorie intake from 12 to 2 p.m. | CON: Maintenance of usual diet | hsCRP |
| Kord Varkaneh et al. 2023 [38] | 45 (F and M) | NAFLD | IF: 41.4 ± 10.5 CON: 44.2 ± 4.9 | IF: 29.1 ± 2.6 CON: 30.6 ± 3.1 | RCT | 12 weeks | TRF | Maintained 16 h fasting/8 h feeding daily plus a low-sugar diet | CON: Diet based on traditional meals | hsCRP |
| Kotarsky et al. 2021 [39] | 21 (F and M) | Overweight/ Obese | IF: 45.0 ± 9.9 CON: 44.0 ± 6.3 | IF: 29.8 ± 2.6 CON: 29.4 ± 2.5 | RCT | 8 weeks | TRF | Consumed all their calories between 12:00 p.m. and 8:00 p.m. each day, inducing a fasting window of 16 h | CON: Maintained their regular eating schedule | CRP |
| Lao et al. 2023 [40] | 27 (F and M) | CKD | IF: 51.8 ± 7.7 CON: 52.5 ± 11.3 | IF: 29.3 ± 2.3 CON: 28.0 ± 2.4 | NRCT | 12 weeks | TRF | Followed a low-protein diet, eating three meals within an 8 h window starting between 7:00 a.m. and noon. During fasting periods, only water and non-caloric beverages were allowed | CON: Received a high-quality low-protein diet with no restrictions on what time they could eat each day, following their daily routines | CRP, TNF-α, IL-6 |
| Manoogian et al. 2022 [41] | 137 (F and M) | Healthy | IF: 41.1 ± 8.7 CON: 39.6 ± 9.4 | IF: 27.8 ± 3.6 CON: 27.7 ± 3.9 | RCT | 12 weeks | TRF | Followed a 14 h fast, 10 h eating window; self-selected: 09:00–19:00 (60% CHO, 25% fat, 15% protein); average 11.3 h eating window | CON: Standard care (Mediterranean diet) | CRP |
| Martens et al. 2020 [42] | 22 (F and M) | Healthy | 67.0 ± 1.0 | 24.7 ± 0.6 | RXT | 6 weeks | TRF | Maintained 16 h of daily fasting and eating during the other 8 h | CON: Standard care | CRP, IL-6 |
| Miranda et al. 2018 [43] | 42 (F and M) | Obese | IF: 44.0 ± 21.4 CON: 43.0 ± 24.8 | IF: 33.0 ± 6.3 CON: 34.5 ± 7.2 | RCT | 12 weeks | ADF | Consumed 25% of baseline energy needs as a lunch (12 p.m.–2 p.m.) on “fast days” and 125% of their energy needs across three meals on subsequent “feast days” | CON: Not to change their diet | Adiponectin, Leptin, TNF-α, IL-6 |
| Moro et al. 2016 [44] | 34 (M) | Healthy | IF: 29.9 ± 4.1 CON: 28.5 ± 3.5 | ND | RCT | 8 weeks | TRF | Consumed 100% of daily energy needs within an 8 h time window (at 1 p.m., 4 p.m., and 8 p.m.) | CON: Caloric intake as three meals consumed at 8 a.m., 1 p.m., and 8 p.m. | Adiponectin, Leptin, TNF-α, IL-6 |
| Moro et al. 2020 [45] | 16 (M) | Healthy | IF: 19.4 ± 2.4 CON: 19.4 ± 1.6 | IF: 21.9 ± 1.7 CON: 22.5 ± 1.8 | RCT | 4 weeks | TRF | Consumed 100% of estimated daily energy needs in an 8 h time window (from 10:00 a.m. to 6:00 p.m.) | CON: Consumed a complete diet divided into three meals between 7 a.m. and 9 p.m. | Adiponectin, TNF-α, IL-6 |
| Moro et al. 2021 [46] | 20 (M) | Healthy | ND | ND | RCT | 12 months | TRF | Consumed 100% of daily energy needs within an 8 h time window (at 1 p.m., 4 p.m., and 8 p.m.) | CON: Caloric intake as three meals consumed at 8 a.m., 1 p.m., and 8 p.m. | Adiponectin, Leptin, TNF-α, IL-6 |
| Schroder et al. 2021 [47] | 22 (F) | Obese | IF: 36.6 ± 1.6 CON: 42.3 ± 3.5 | IF: 32.5 ± 1.1 CON: 43.5 ± 1.2 | NRCT | 3 months | TRF | Fasting period (no energy intake whatsoever) of 16 h (8 p.m. to 12 p.m.) and ad libitum feeding for 8 h (12 p.m. to 8 p.m.) | CON: Maintained their habitual nutrition throughout the whole period | CRP |
| Stratton et al. 2020 [48] | 26 (M) | Healthy | IF: 22.9 ± 3.6 CON: 22.5 ± 2.2 | ND | RCT | 4 weeks | TRF | Maintained 25% caloric deficit and only ate within an 8 h window each day | CON: 25% caloric deficit with participants’ usual daily feeding schedules | Adiponectin, Leptin |
| Sutton et al. 2018 [49] | 8 (M) | Prediabetes | 56.0 ± 9.0 | 32.2 ± 4.4 | RXT | 5 weeks | TRF | Maintained 6 h feeding period, with dinner before 3 p.m. | CON: 12 h feeding period | CRP, IL-6 |
| Varady et al. 2013 [50] | 30 (F and M) | Healthy | IF: 47.0 ± 11.6 CON: 48.0 ± 7.7 | IF: 26.0 ± 3.9 CON: 26.0 ± 3.9 | RCT | 12 weeks | ADF | Consumed 25% of their baseline energy needs on the fast day and then ate ad libitum on each alternating feed day | CON: Ate ad libitum | Adiponectin, Leptin, CRP |
| Xie et al. 2022 [51] | 82 (F and M) | Healthy | IF1: 28.7 ± 9.7 IF2: 31.1 ± 8.4 CON: 33.6 ± 11.6 | IF1: 22.7 ± 3.1 IF2: 21.4 ± 2.2 CON: 21.5 ± 2.9 | RCT | 5 weeks | TRF | IF1: (16 h fasting:8 h feeding) self-selected feeding window between 06:00 and 15:00 IF2: (16 h fasting:8 h feeding) self-selected feeding window between 11:00 and 20:00 | CON: Ate ad libitum | Leptin, TNF-α, CRP |
| Zhang et al. 2022 [52] | 60 (F and M) | Overweight/ Obese | IF1: 23.8 ± 2.7 IF2: 23.2 ± 2.2 CON: 21.1 ± 1.7 | IF1: 27.1 ± 3.2 IF2: 28.5 ± 3.6 CON: 27.8 ± 3.5 | RCT | 8 weeks | TRF | IF1: 6 h eating window from 7:00 to 13:00 IF2: 6 h eating window from 12:00 to 18:00 | CON: Ate ad libitum | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalafi, M.; Habibi Maleki, A.; Mojtahedi, S.; Ehsanifar, M.; Rosenkranz, S.K.; Symonds, M.E.; Tarashi, M.S.; Fatolahi, S.; Fernandez, M.L. The Effects of Intermittent Fasting on Inflammatory Markers in Adults: A Systematic Review and Pairwise and Network Meta-Analyses. Nutrients 2025, 17, 2388. https://doi.org/10.3390/nu17152388
Khalafi M, Habibi Maleki A, Mojtahedi S, Ehsanifar M, Rosenkranz SK, Symonds ME, Tarashi MS, Fatolahi S, Fernandez ML. The Effects of Intermittent Fasting on Inflammatory Markers in Adults: A Systematic Review and Pairwise and Network Meta-Analyses. Nutrients. 2025; 17(15):2388. https://doi.org/10.3390/nu17152388
Chicago/Turabian StyleKhalafi, Mousa, Aref Habibi Maleki, Shima Mojtahedi, Mahsa Ehsanifar, Sara K. Rosenkranz, Michael E. Symonds, Mohammad Sadegh Tarashi, Saeid Fatolahi, and Maria Luz Fernandez. 2025. "The Effects of Intermittent Fasting on Inflammatory Markers in Adults: A Systematic Review and Pairwise and Network Meta-Analyses" Nutrients 17, no. 15: 2388. https://doi.org/10.3390/nu17152388
APA StyleKhalafi, M., Habibi Maleki, A., Mojtahedi, S., Ehsanifar, M., Rosenkranz, S. K., Symonds, M. E., Tarashi, M. S., Fatolahi, S., & Fernandez, M. L. (2025). The Effects of Intermittent Fasting on Inflammatory Markers in Adults: A Systematic Review and Pairwise and Network Meta-Analyses. Nutrients, 17(15), 2388. https://doi.org/10.3390/nu17152388










