Abstract
Adequate antioxidant supply is essential for maintaining metabolic homeostasis and reducing oxidative stress during detoxification. The emerging evidence suggests that certain classes of phytonutrients can help support the detoxification process by stimulating the liver to produce detoxification enzymes or acting as antioxidants that neutralize the harmful effects of free radicals. This study was designed to examine the effects of a guided 28-day metabolic detoxification program in healthy adults. The participants were randomly assigned to consume a whole food, multi-ingredient supplement (n = 14, education and intervention) or control (n = 18, education and healthy meal) daily for the duration of the trial. The whole food supplement contained 37 g/serving of a proprietary, multicomponent nutritional blend in the form of a rehydratable shake. Program readiness was ensured at baseline using a validated self-perceived wellness score and a blood metabolic panel, indicating stable emotional and physical well-being in both groups. No significant changes or adverse effects were found on physical or emotional health, cellular glutathione (GSH) and the GSH:GSSG ratio, porphyrin, and hepatic detoxification biomarkers in urine. The intervention was positively associated with a 23% increase in superoxide dismutase (p = 0.06) and a 13% increase in glutathione S-transferase (p = 0.003) activities in the blood. This resulted in a 40% increase in the total cellular antioxidant capacity (p = 0.001) and a 13% decrease in reactive oxygen species (p = 0.002) in isolated PBMCs from participants in the detoxification group. Our findings indicate that consuming a whole food nutritional intervention as a part of the guided detoxification program supported phase II detoxification, in part, by promoting enhanced free radical scavenging and maintaining redox homeostasis under the body’s natural glutathione recycling capacity.
Keywords:
whole food; botanical; detoxification program; liver; antioxidants; free radicals; hepatic markers; GST; SOD; GSH; GSH:GSSG ratio 1. Introduction
The environment contains close to 80,000 novel chemicals, which are registered with the United States Environmental Protection Agency (EPA), but many have not had thorough reviews for their risk to human health [1]. Generally regarded as environmental toxins or pollutants, these chemicals belong to several identifiable groups, such as toxic elements (heavy metals), naturally occurring toxins (molds and their volatile metabolites, food allergens), pesticides, persistent organic pollutants, volatile organic compounds (solvents and fuels), and plastics [2]. As these compounds enter food chains and agricultural production systems [3], the assessment of the health risks associated with these chemicals is limited due to the lacking information on their long-term toxicity and exposure levels. Developmental and early years remain particularly susceptible to their effects [4].
Direct exposure, as well as the bioaccumulation of toxic chemicals in human tissues, make them a major continuous threat to human health. More so, the mechanisms by which environmental pollutants cause disruptive health effects may include tissue damage, endocrine signaling, genotoxicity, enzyme inhibition, oxidative stress, and changes in the activity of the gene expression networks that modulate them [5]. Severe pathological dysfunction may develop as a result once failure to maintain healthy levels of metabolic homeostasis [6], endocannabinoid signaling [7], immune responses [8], and tissue repair [9] are not timely resolved. Chronic exposure and improper clearance of environmental toxins, therefore, directly contribute to the development of obesity [10], cardiovascular disease [11], neurocognitive impairment [12], and reproductive concerns [13].
The human body largely relies on the liver to metabolize and direct exogenous chemicals to tissue storage or excretion [14]. This is achieved by the phase I enzyme-mediated oxidation or reduction of the toxic substrates, followed by the phase II enzymatic conjugation of the newly added or exposed functional groups to glucuronide, sulfate, or glutathione moieties. These conjugation reactions increase the water solubility of xenobiotics and tag them for carrier-mediated transport and excretion [15]. While the phase I reactions are mediated predominantly by the cytochrome P450 (CYP) superfamily of enzymes [16], the phase II system is more diverse and includes UDP-glucuronosyltransferases (UGTs), sulfotransferases (SULTs), methyltransferases (MTs), glutathione S-transferases (GSTs), and N-acetyltransferases (NATs) [17].
Additionally, the liver detoxification system is supported by endogenous antioxidants (GSH, reduced glutathione), dietary antioxidants (ascorbate), and antioxidant enzymes, such as superoxide dismutase (SOD), catalase, glutathione reductases, glutathione peroxidases (GPXs), and glutathione S-transferases (GSTs), that enable scavenging of reactive oxygen species (ROS) and free radicals arising from healthy cellular metabolism, as well as the detoxification of xenobiotics [18]. GSH is the most abundant endogenous antioxidant, which must be reduced and recycled from its inactive form (GSSG) in order to carry out additional antioxidant reactions [19]. The ratio of reduced to oxidized glutathione (GSH:GSSG) is an indicator of cellular health or cellular toxicity [20], as well as the redox status of the cell [21]. While there may be a need to repair low levels of glutathione, proper balance, rather than excess, is generally required [22].
Nutritional status and dietary intake patterns can have a profound effect on the body’s ability to absorb, conjugate, and excrete potentially dangerous toxins [1]. Certain botanically defined vegetable diets have been shown to enhance the hepatic conversion of xenobiotics [23] and support the body’s endogenous levels of glutathione [24]. A 12-week program combined with a 30-day dietary detoxification intervention (n = 12) resulted in significant changes to body composition as well as improved levels of zonulin and leptin [10]. Other clinical trials investigating strategies to enhance detoxification pathways exhibited high variability due to genetic and gender differences [25,26,27]. In this study, we focused on a cohort of healthy adults enrolled in a guided detoxification program that included a healthy diet education session with or without 28-day nutritional supplementation with a whole food, proprietary multicomponent blend. The primary objective was to quantify the functional markers of metabolic detoxification in blood and urine compared to the study baseline. The secondary outcomes were to determine the safety and tolerability of the intervention by assessing adverse event rates, comparisons of laboratory tests, and self-reported wellness data.
2. Materials and Methods
2.1. Participants
Flow of the participants through the study is shown in Figure 1. Using data generated previously [28], a sample size calculation with a p < 0.05 significance and 80% power revealed that 15–20 study participants would be needed to detect a 15% difference in the activity of detoxification enzymes in a randomized clinical study [29]. The study was a 4-week blinded, randomized controlled trial (RCT) with parallel assignment initiated in March of 2022 at Northwestern Health Sciences University’s De Rusha Clinic in Bloomington, Minnesota. The participants were heathy adults affiliated with the university who responded to an announcement regarding an upcoming lifestyle change program. The study staff completed an informational session that outlined the program and obtained health-related information from interested participants to determine study eligibility.
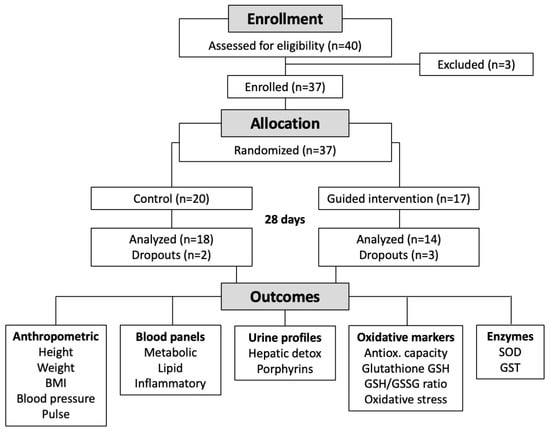
Figure 1.
Flowchart of the study.
Forty potential participants responded to a posting. Thirty-seven were subsequently offered participation in the program and were randomized to serve as controls (a healthy diet education session alone) or to receive a healthy diet education session and a guided 28-day metabolic detoxification program, including the allocated intervention. The healthy diet education session included a PowerPoint presentation on healthy dietary guidelines and sample recipes of healthy meals. The guided component of the detoxification program included an additional PowerPoint presentation with the information about the investigational product, directions, and dosing information for its consumption.
All healthy participants (29 female, 8 male, age range 20–60 years) met the inclusion criteria and provided informed consent. None had any exclusion criteria (Table 1). All participants completed the study procedures. Two control participants and three intervention participants were excluded from analyses due to study dropout. Females on birth control were excluded from the study. There were no protocol deviations. The investigators and outcome assessors were blinded to group allocation. Participant information and generated data were fully anonymized for data analysis and interpretation of results.

Table 1.
Inclusion and exclusion criteria of the study.
All research involving human participants was approved by the Advarra Institutional Review Board (IRB), protocol No. Pro00055058, and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. The study was registered on www.clinicaltrials.gov (accessed on 3 May 2023) (Identifier: NCT00000000).
2.2. Study Investigational Product
The proprietary, whole food blend was supplied as 27.4 oz (777 g) SP Detox Balance bottles by the manufacturer (Standard Process Inc., Palmyra, WI, USA). The serving size was defined as 2 scoops (37 g) and included a blend of organic pea protein, flax meal, organic oat flour, organic pumpkin seed protein, organic buckwheat flour, organic beet (leaf) juice powder, organic buckwheat (aerial parts), apple pectin, juniper (berry) powder, organic Spanish black radish (root), burdock (root) powder, organic beet (root), calcium citrate, organic barley (grass), dandelion (leaf), broccoli (aerial parts), inositol, organic alfalfa (aerial parts) juice powder, Oregon grape (root) powder, globe artichoke (leaf), sunflower lecithin powder, milk thistle extract (80% silymarins), organic cordyceps mushroom powder, organic carrot, and organic sweet potato. Other ingredients included creatine, L-leucine, xanthan gum, L-isoleucine, L-valine, DL-methionine, monk fruit extract, and choline bitartrate. The nutritional intervention was self-administered by participants orally in the form of a rehydratable shake with 1–2 servings daily on days 1–7, 3 servings daily on days 8–21, and 1–2 servings on days 22–28.
2.3. Anthropometrics
Height, weight, BMI, blood pressure, and pulse were measured by the study staff on day 1 and day 28 of the study. Procedures were followed as per the Centers for Disease Control and Prevention guidelines. The standard Food Frequency Questionnaire (FFQ), the 24 h Dietary Recall Questionnaire (24 h), and the Medical Symptoms Questionnaire (MSQ) were administered to all participants at baseline and the end of the study to monitor study readiness, dietary patterns, and health-related events during the study.
2.4. Laboratory Testing
Fasting blood samples were obtained at baseline (day 1) and on the last day of the study (day 28). The samples were collected in three BD Vacutainer SST tubes, allowed to clot at room temperature for 30–45 min. Then, serum was separated using centrifugation at 3400 rpm for 10 min and stored at −80 °C. Serum was analyzed for three panels of metabolic (ALT, ALK, AST, free T3, free T4, GGT, total T3, total T4, TSH, T-uptake, and Vitamin D), lipid (cholesterol, ultra HDL), and inflammatory (CRP, IgA, IgE, IgG, IgM) markers using the Abbott (Chicago, IL, USA) Architect ci4100 following the manufacturer’s instructions (Supplementary Table S1).
Morning first void urine samples on day 1 and day 28 were collected for assessment of the hepatic detox profile (phase I D-glucaric acid, phase II mercapturic acids, and creatinine) and the urine porphyrin profile (uroporphyrins, heptacarboxylporphyrins, hexacarboxylporphyrins, pentacarboxylporphyrins, coproporphyrins 1-3, total porphyrins, including precoproporphyrins 1-3, total precoproporphyrins, and creatinine) using commercial testing services (Doctor’s Data, St. Charles, IL, USA).
2.5. Antioxidant and Redox Status
Total antioxidant capacity was measured as the combined antioxidant activities of all serum constituents, including vitamins, proteins, lipids, glutathione, and uric acid. The assay relied on the ability of antioxidants in the sample to inhibit the oxidation of ABTS (2,2′-Azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS•+ by metmyoglobin and was quantified as Trolox, a water-soluble tocopherol analogue, equivalents. The decrease in ABTS oxidation was quantified colorimetrically at 734 nm using a Biotek Synergy H1 spectrophotometer (Agilent, Santa Clara, CA, USA).
Reduced glutathione (GSH), oxidized glutathione (GSSG), and total glutathione in serum samples were quantified in duplicate using fluorometric GSH/GSSG ratio detection assay kit (Abcam, Cambridge, MA, USA) at the excitation/emission of 490/520 nm (Biotek Synergy H1).
2.6. Oxidative Stress
Blood samples were collected in BD Vacutainer CPT mononuclear cell preparation tubes that contained blood separation media composed of a thixotropic polyester gel and a Ficoll Hypaque solution. The tubes were centrifuged to isolate live peripheral blood mononuclear cells (PBMCs), and oxidative stress was measured using a fluorogenic cell-permeant probe CellROX Orange (Thermo Fisher, Waltham, MA, USA). Fluorescence upon oxidation by reactive oxygen species was quantified on BD Accuri C6 flow cytometer with absorption/emission maxima of 545/565 nm and presented as relative fluorescent units (RFUs).
2.7. Detoxification Enzyme Activity
SOD activity was assessed using an assay that utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine using a superoxide dismutase assay kit colorimetrically at 450 nm (Cayman, Ann Arbor, MI, USA). One unit of SOD was defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
Total GST activity was measured by quantifying the conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) with reduced glutathione using a glutathione S-transferase assay kit colorimetrically at 340 nm (Cayman).
2.8. Self-Reported Wellness
Program readiness was ensured at baseline and the end of the study using self-reported wellness status as determined by PROMIS Global-10 questionnaire and a normal comprehensive blood metabolic panel result. The PROMIS Global short form was scored into a Global Physical Health component and Global Mental Health component. The summed raw scores from PROMIS Global were converted into standardized T-score distributions such that a 50 represents the average (mean) for the US general population [30].
2.9. Statistics
Statistical analyses were performed using JMP 15.2.1 (SAS Institute, Cary, NC, USA). Datasets were tested for normality using the goodness-of-fit Shapiro–Wilks test. Descriptive statistics and two-tailed paired and unpaired Student’s t-test were used to evaluate changes in clinical outcomes at baseline and after intervention. All values were reported as mean ± standard error of mean (SEM), and statistical significance was set at p ≤ 0.05. Asterisks *, **, and *** indicate significance levels of p < 0.05, p < 0.01, and p < 0.001, respectively.
3. Results
3.1. Subjects’ Characteristics
A total of 37 participants were selected for the study and randomized into the control (n = 20, a healthy diet education session) or detox intervention groups (n = 17, a healthy diet education session followed by a guided 28-day metabolic detoxification program using the study investigational product). Three additional participants were screened but excluded from participating in the study based on medical conditions that did not qualify them further.
Eighteen control and fourteen detox group participants completed the study as per the protocol (Figure 1). Two control and three detox group subjects were excluded from the study due to failing to comply with the study after the first visit (1); the remaining dropouts were excluded from the study based on voluntary withdrawal on the grounds of being unable to commit to the study protocol (4). The demographics and anthropometric data of the participants are shown in Table 2. While the detoxification group was able to maintain the parameters, including weight and BMI, throughout the study, control participants showed positive improvement in weight (p = 0.002) and BMI (p = 0.001) at the end of the study (Table 2).

Table 2.
Demographic and anthropometric data of the study participants (±SEM).
3.2. Safety and Tolerance Assessment
No adverse effects attributable to the intervention were reported during the study. The laboratory safety endpoints included data from metabolic, lipid, and inflammatory panels and showed no significant changes (Supplementary Table S1). There were no significant observations in the Food Frequency Questionnaire (FFQ), the 24 h Dietary Recall Questionnaire (24 h), and the Medical Symptoms Questionnaire (MSQ) completed at baseline and the end of the study, suggesting that all participants followed the dietary education instructions provided to them at baseline and experienced no undesirable events for 4 consecutive weeks of the study.
3.3. Wellness Status and Quality of Life
Baseline physical and emotional health scores captured with the PROMIS Global-10 questionnaire were similar for both the control and detoxification groups and remained stable after the trial (Figure 2). The average unadjusted PROMIS Global Physical Health scores were 49.9 and 50.6 at baseline and 50.1 and 51.9 at the end of the study for participants in the control and intervention groups, respectively. Similarly, the average unadjusted PROMIS Global Mental Health scores were 47.1 and 49.3 at baseline and slightly increased to 50.1 and 51.9 at the end of the study.

Figure 2.
PROMIS Global Health-10 scores at baseline and at the end of the study, separated by (a) physical health and (b) emotional health components. Results are expressed as means ± SEM. Data were analyzed using the paired sample t-test.
3.4. Biomarkers of Oxidative Stress
At the baseline, the total plasma antioxidant capacity was measured as 457.9 μM and 365.2 μM for participants in the control and intervention groups, respectively. A 4-week detoxification program was associated with a 40% increase in total antioxidant capacity only in subjects receiving the intervention (514.7 μM, p < 0.001) (Figure 3a). This change correlated with an observed 13% decrease in ROS-associated oxidative stress in the peripheral blood mononuclear cells isolated from subjects receiving the intervention (724.4 RFUs at baseline versus 635.1 RFUs at the end of the study, p < 0.001) (Figure 3b).
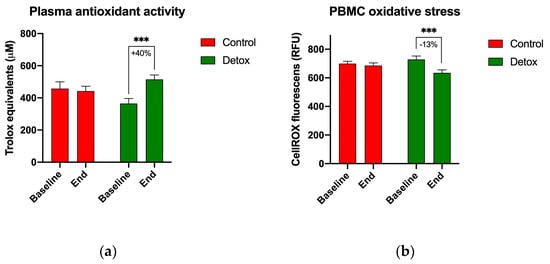
Figure 3.
Changes in biomarkers of oxidative stress during the 4-week detoxification study. (a) Plasma antioxidant capacity measured using TEAC assay; (b) oxidative stress in the peripheral blood mononuclear cells measured using CellROX orange reagent. Results are expressed as means ± SEM. Data were analyzed using the paired sample t-test (*** p < 0.001).
3.5. Redox Homeostasis
Reduced glutathione (GSH) and the GSH:GSSG ratio remained stable in both groups (Figure 4), suggesting balanced redox homeostasis under the body’s natural glutathione recycling capacity. However, apparent changes in the activity of the phase II antioxidant enzymes superoxide dismutase (SOD) and glutathione S-transferases (GSTs) suggested an increased glutathione turnover in the participants receiving the detoxification intervention. The baseline SOD activity increased by 23% from 2.7 to 3.9 units/mL (p = 0.067) by the end of the study (Figure 5a), and the baseline GST activity increased by 13% from 101.6 to 113.7 nmol/min/mL (p < 0.001) for the detox participants only (Figure 5b).
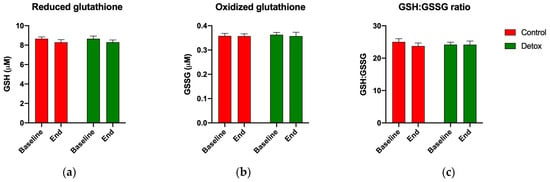
Figure 4.
Changes in redox homeostasis during the 4-week detoxification study. (a) Reduced glutathione (GSH); (b) oxidized glutathione (GSSG); and (c) GSH/GSSG ratio as quantified using the fluorometric GSH/GSSG ratio detection assay. Results are expressed as means ± SEM. Data were analyzed using the paired sample t-test.
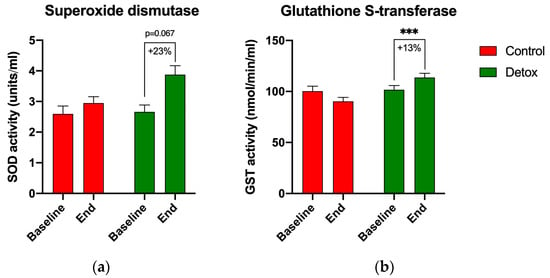
Figure 5.
Changes in the activity of the phase II antioxidant enzymes during the 4-week detoxification study. (a) Superoxide dismutase (SOD); (b) glutathione S-transferases (GSTs) as quantified using the respective enzymatic assays. Results are expressed as means ± SEM. Data were analyzed using the paired sample t-test (*** p < 0.001).
3.6. Detoxification Markers in Urine
Consistent with previous reports on high interindividual variation in the detoxification panels, we did not observe any significant reductions in the urine porphyrin biomarkers from both the control and detox cohorts (all p-values > 0.05)). A trend for increased urine D-glucaric acid (from 86.14 to 106.07 nM, p = 0.09) and decreased creatinine (from 103.50 to 91.50 mg/dL, p = 0.13) was observed only in the participants undergoing the detoxification treatment (Table 3).

Table 3.
Hepatic detoxification markers in urine (±SEM).
4. Discussion
The human body is exposed to a lifetime variety of xenobiotics that need to be properly recognized, metabolized, and excreted to preserve and maintain health. Ubiquitous sources of xenobiotics include natural foods and drink products that are routinely digested and detoxified, as well as byproducts of their cellular metabolism that can build up over time and become harmful [31]. The development of synthetic chemistry, agrochemical, and pharmaceutical industries exponentially increased the amount of foreign chemical substances that must be recognized and neutralized [1]. Chronic exposure and improper clearance of these chemicals increase the body’s susceptibility to tissue injury and insufficient repair over time [5].
The bulk of tissue detoxification reactions occur in the liver and the gastrointestinal tract, with the kidneys, lungs, lymphatics, and skin tissues contributing to the different aspects of this process. Central to this process is adequate nutritional support for the activity of phase I (activation) [14] and phase II (conjugation) [15] enzymes, as these mechanisms exhibit significant individual variability affected by the environment, lifestyle, and genetic factors [32]. The use of plants and the consumption of plant-based foods have played a fundamental role in regulating the metabolism and elimination of xenobiotics, with probably the most famous example being grapefruit juice and its effects on the activity of hepatic detoxification enzymes [33]. Food structure, as it applies to whole foods, including texture- and matrix-related effects, also has direct effects on biomolecular availability and absorption in the gastrointestinal tract [34]. Additionally, the whole food matrix changes the availability of nutrients, such as amino acids or vitamins, that are required for many phase I and phase II detoxification reactions [35,36]. The increased intake of vegetables is protective of nonalcoholic fatty liver disease [37], hepatocellular carcinoma [38], and the overall risk of mortality from all causes [39]. These data justify and stimulate continuous research on a selection of whole food ingredients or formulations which are nutritionally complete, shelf–table, and hold promise to support tissue detoxification mechanisms.
This randomized controlled study showed that a whole food, multi-ingredient nutritional intervention was well tolerated for 4 weeks with good palatability. The intervention showed no effect on general physiological outcomes, such as weight, BMI, blood pressure, and heart rate, in healthy volunteers, and did not affect the baseline values of the metabolic, lipid, and inflammatory panels. The main advantage of the tested intervention was the series of whole food ingredients used in its formulation. A mixture of pea protein, pumpkin seed protein, and oat flour was designed to deliver good levels of amino acids relevant to the detoxification reactions, including arginine (1300 mg), glycine (600 mg), isoleucine (850 mg), leucine (1600 mg), methionine (300 mg), and valine (900 mg) per serving. The total sugars (1 g) and total fats (5 g) were kept low using a mixture of flax meal, oat flour, and buckwheat flour. A series of functional ingredients was also selected to support the activity of the detoxification enzymes, including beets reported to activate glutathione S-transferase [40], Spanish black radish root for the activation of phase I/II enzymes [28], burdock root for reducing hepatotoxicity [41], apple pectin for its ability to detoxify heavy metals [42], juniper berries for their high antioxidant activity and ability to activate superoxide dismutase and glutathione peroxidase [43], dandelion leaf for protective effects against hepatic injuries [44], broccoli leaf for its capacity to modulate antioxidant and phase II enzymes, including SOD, via glucosinolate and isothiocyanate metabolism [45], globe artichoke leaf for the modulation of antioxidant and hepatic metabolism [46], milk thistle extract standardized to 80% silymarins to support healthy liver function [47], and cordyceps mushroom powder for its antioxidant and liver protection qualities [48].
The study also demonstrated that measurable biological effects on the total plasma antioxidant activity and oxidative stress in PBMCs can be achieved in healthy participants at intervention doses that are well tolerated. These findings correlated with increases in SOD and GST activities in the absence of the overall levels of reduced glutathione (GSH) or the GSH:GSSG ratio in the participating healthy volunteers. While multiple studies demonstrate the challenge associated with the maintenance of an endogenous glutathione pool, owing to a multitude of factors, including age and physiological and pathological conditions [49], proper balance, rather than excess, is generally required [22]. Many of the enzymes that participate in the antioxidant and glutathione recycling reactions require nutrient cofactors [50] that the current intervention was designed to supply. This may be responsible, in part, for the increased activities of SOD and GSTs, as many of the individual ingredients directly target or support these enzymes as discussed above. The ability of the intervention to increase phase II detoxification and antioxidant enzyme activity is in line with studies demonstrating the applicability of whole foods and certain phytonutrients to protect cells against electrophiles, DNA damage, and inflammation [51].
The current study also demonstrated high variability in hepatic detoxification (phase I D-glucaric acid, phase II mercapturic acids) and urine porphyrin profiles. The explanation for this intersubject variability is not obvious, however; individual responses to plant foods often depend on individual genetic polymorphisms and gender, as shown previously for GST enzymes [26] and cytochrome CYP enzyme families [52]. As the current intervention, similar to a regular healthy human diet, contains a wide variety of food ingredients, the precise individual effects of its supplementation on detoxification enzymes may be difficult to predict.
While this study offers novel insights into a whole food, nutritional intervention and its potential application to detoxification and antioxidant support, it does have notable limitations. We enrolled healthy volunteers, and it remains to be determined whether these findings apply generally to diverse patient populations with pre-existing disease states and metabolic pathologies. The majority of subjects were females, and this may limit our understanding of the effects of this supplementation in a male population. Furthermore, small but significant changes in BMI observed in the control population may be attributed to the placebo-like effects of the educational session and may be further explored in the future. This will be critical in determining the beneficial potential of the whole food, multi-ingredient supplementation in subsequent clinical studies in specific patient populations.
In summary, this study both reports the successful development of a whole food, multi-ingredient nutritional intervention and demonstrates that its oral administration for 4 weeks improves the total plasma antioxidant activity and oxidative stress in PBMCs, in part, by increasing phase II detoxification and antioxidant enzyme activity, such as SOD and GSTs, in healthy participants. These effects were observed in the absence of apparent clinical side effects and changes in the laboratory biomarkers. Our work highlights the feasibility of translating the whole food, multi-ingredient supplementation and positions it as a novel formulation capable of supporting the body’s endogenous detoxification pathways. These results also present a strong case for incorporating functional biomarkers and guided education into personalized metabolic detoxification programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15092209/s1, Supplementary Table S1: Metabolic, lipid, and inflammatory panel data (±SEM).
Author Contributions
M.N.F. and C.M. conceptualized and performed the study, coordinated participants and all visits, as well as collected study data and samples; S.K. performed oxidative and redox assays; C.P. outlined the study and analyzed data; C.P. and K.B. wrote the manuscript; S.K., S.L.B.-B., and B.M. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Standard Process Inc., grant number 032022.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Advarra (protocol No. Pro00055058, approved on 25 January 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
We greatly appreciate Northwestern Health Sciences University staff for the study coordination, participant enrolment, and sample and data collection, as well as Northwestern Health Sciences University staff and students for volunteering in the study. We are also extremely grateful to Patrick Shanley for coordinating the study, Ashley Dominique and Lluvia Gonzalez for blood biochemistry and panel testing, and Brea Nance for data entry (all Standard Process Inc.).
Conflicts of Interest
Chinmayee Panda, Keri Barron, Sara Le Brun-Blashka, and Brandon Metzger are employees of Standard Process. Financial support from Standard Process had no role in the design of the study, interpretation of the data, and the decision to publish the results. The remaining authors declare no conflict of interest.
References
- Sears, M.E.; Genuis, S.J. Environmental Determinants of Chronic Disease and Medical Approaches: Recognition, Avoidance, Supportive Therapy, and Detoxification. J. Environ. Public Health 2012, 2012, 356798. [Google Scholar] [CrossRef]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef]
- Komarnytsky, S.; Retchin, S.; Vong, C.I.; Lila, M.A. Gains and Losses of Agricultural Food Production: Implications for the Twenty-First Century. Annu. Rev. Food. Sci. Technol. 2022, 13, 239–261. [Google Scholar] [CrossRef]
- Kelishadi, R.; Mirghaffari, N.; Poursafa, P.; Gidding, S.S. Lifestyle and Environmental Factors Associated with Inflammation, Oxidative Stress and Insulin Resistance in Children. Atherosclerosis 2009, 203, 311–319. [Google Scholar] [CrossRef]
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic Transgenerational Actions of Environmental Factors in Disease Etiology. Trends Endocrinol. Metab. 2010, 21, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Marroqui, L.; Tudurí, E.; Alonso-Magdalena, P.; Quesada, I.; Nadal, Á.; Santos, R.S. dos Mitochondria as Target of Endocrine-Disrupting Chemicals: Implications for Type 2 Diabetes. J. Endocrinol. 2018, 239, R27–R45. [Google Scholar] [CrossRef] [PubMed]
- Komarnytsky, S.; Rathinasabapathy, T.; Wagner, C.; Metzger, B.; Carlisle, C.; Panda, C.; Le Brun-Blashka, S.; Troup, J.P.; Varadharaj, S. Endocannabinoid System and Its Regulation by Polyunsaturated Fatty Acids and Full Spectrum Hemp Oils. Int. J. Mol. Sci. 2021, 22, 5479. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; He, H.; Qiu, W.; Zheng, Y.; Chen, Y.; Hu, S.; Zhao, X. Oxidative Stress, Endocrine Disturbance, and Immune Interference in Humans Showed Relationships to Serum Bisphenol Concentrations in a Dense Industrial Area. Environ. Sci. Technol. 2021, 55, 1953–1963. [Google Scholar] [CrossRef]
- Jia, P.-P.; Junaid, M.; Xin, G.-Y.; Wang, Y.; Ma, Y.-B.; Pei, D.-S. Disruption of Intestinal Homeostasis Through Altered Responses of the Microbial Community, Energy Metabolites, and Immune System in Zebrafish After Chronic Exposure to DEHP. Front. Microbiol. 2021, 12, 729530. [Google Scholar] [CrossRef]
- Bonakdar, R.A.; Sweeney, M.; Dalhoumi, S.; Adair, V.; Garvey, C.; Hodge, T.; Herrala, L.; Barbee, A.; Case, C.; Kearney, J.; et al. Detoxification Enhanced Lifestyle Intervention Targeting Endotoxemia (DELITE) in the Setting of Obesity and Pain: Results of a Pilot Group Intervention. Integr. Med. 2020, 19, 16–28. [Google Scholar]
- Mostafalou, S.; Abdollahi, M. Pesticides and Human Chronic Diseases: Evidences, Mechanisms, and Perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef]
- Liu, J.; Lewis, G. Environmental Toxicity and Poor Cognitive Outcomes in Children and Adults. J. Environ. Health 2014, 76, 130–138. [Google Scholar]
- Pizzorno, J. Environmental Toxins and Infertility. Integr. Med. 2018, 17, 8–11. [Google Scholar]
- Grant, D.M. Detoxification Pathways in the Liver. J. Inherit. Metab. Dis. 1991, 14, 421–430. [Google Scholar] [CrossRef]
- Zamek-Gliszczynski, M.J.; Hoffmaster, K.A.; Nezasa, K.; Tallman, M.N.; Brouwer, K.L.R. Integration of Hepatic Drug Transporters and Phase II Metabolizing Enzymes: Mechanisms of Hepatic Excretion of Sulfate, Glucuronide, and Glutathione Metabolites. Eur. J. Pharm. Sci. 2006, 27, 447–486. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T.; Bhatnagar, V.; Poloyac, S.M.; Momper, J.D. The Systems Biology of Drug Metabolizing Enzymes and Transporters: Relevance to Quantitative Systems Pharmacology. Clin. Pharmacol. Ther. 2020, 108, 40–53. [Google Scholar] [CrossRef]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.-R.; Harrison, D.G.; Bhatnagar, A. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Kassab, G.S. Glutathione: A Samsonian Life-Sustaining Small Molecule That Protects against Oxidative Stress, Ageing and Damaging Inflammation. Front. Nutr. 2022, 9, 1007816. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox Status Expressed as GSH: GSSG Ratio as a Marker for Oxidative Stress in Paediatric Tumour Patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Chernov, N.N.; Novichkova, M.D. Role of Glutathione, Glutathione Transferase, and Glutaredoxin in Regulation of Redox-Dependent Processes. Biochemistry 2014, 79, 1562–1583. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef]
- Lampe, J.W.; Chen, C.; Li, S.; Prunty, J.; Grate, M.T.; Meehan, D.E.; Barale, K.V.; Dightman, D.A.; Feng, Z.; Potter, J.D. Modulation of Human Glutathione S-Transferases by Botanically Defined Vegetable Diets. Cancer Epidemiol. Biomark. Prev. 2000, 9, 787–793. [Google Scholar]
- Bettermann, E.L.; Hartman, T.J.; Easley, K.A.; Ferranti, E.P.; Jones, D.P.; Quyyumi, A.A.; Vaccarino, V.; Ziegler, T.R.; Alvarez, J.A. Higher Mediterranean Diet Quality Scores and Lower Body Mass Index Are Associated with a Less-Oxidized Plasma Glutathione and Cysteine Redox Status in Adults. J. Nutr. 2018, 148, 245–253. [Google Scholar] [CrossRef]
- Tinsley, G.; Urbina, S.; Santos, E.; Villa, K.; Foster, C.; Wilborn, C.; Taylor, L. A Purported Detoxification Supplement Does Not Improve Body Composition, Waist Circumference, Blood Markers, or Gastrointestinal Symptoms in Healthy Adult Females. J. Diet. Suppl. 2019, 16, 649–658. [Google Scholar] [CrossRef]
- Navarro, S.L.; Chang, J.-L.; Peterson, S.; Chen, C.; King, I.B.; Schwarz, Y.; Li, S.S.; Li, L.; Potter, J.D.; Lampe, J.W. Modulation of Human Serum Glutathione S-Transferase-A1/2 Concentration by Cruciferous Vegetables in a Controlled Feeding Study Is Influenced by GSTM1 and GSTT1 Genotypes. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2974–2978. [Google Scholar] [CrossRef]
- Gasper, A.V.; Al-Janobi, A.; Smith, J.A.; Bacon, J.R.; Fortun, P.; Atherton, C.; Taylor, M.A.; Hawkey, C.J.; Barrett, D.A.; Mithen, R.F. Glutathione S-Transferase M1 Polymorphism and Metabolism of Sulforaphane from Standard and High-Glucosinolate Broccoli. Am. J. Clin. Nutr. 2005, 82, 1283–1291. [Google Scholar] [CrossRef]
- Evans, M.; Paterson, E.; Barnes, D.M. An Open Label Pilot Study to Evaluate the Efficacy of Spanish Black Radish on the Induction of Phase I and Phase II Enzymes in Healthy Male Subjects. BMC Complement. Altern. Med. 2014, 14, 475. [Google Scholar] [CrossRef]
- Kadam, P.; Bhalerao, S. Sample size calculation. Int. J. Ayurveda Res. 2010, 1, 55–57. [Google Scholar] [CrossRef]
- Hays, R.D.; Bjorner, J.B.; Revicki, D.A.; Spritzer, K.L.; Cella, D. Development of Physical and Mental Health Summary Scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) Global Items. Qual. Life Res. 2009, 18, 873–880. [Google Scholar] [CrossRef]
- Mega, A.; Marzi, L.; Kob, M.; Piccin, A.; Floreani, A. Food and Nutrition in the Pathogenesis of Liver Damage. Nutrients 2021, 13, 1326. [Google Scholar] [CrossRef]
- Liska, D.J. The Detoxification Enzyme Systems. Altern. Med. Rev. 1998, 3, 187–198. [Google Scholar]
- Bailey, D.G.; Dresser, G.K. Natural Products and Adverse Drug Interactions. Can. Med. Assoc. J. 2004, 170, 1531–1532. [Google Scholar] [CrossRef]
- Forde, C.G.; Bolhuis, D. Interrelations Between Food Form, Texture, and Matrix Influence Energy Intake and Metabolic Responses. Curr. Nutr. Rep. 2022, 11, 124–132. [Google Scholar] [CrossRef]
- Capuano, E.; Oliviero, T.; Fogliano, V.; Pellegrini, N. Role of the Food Matrix and Digestion on Calculation of the Actual Energy Content of Food. Nutr. Rev. 2018, 76, 274–289. [Google Scholar] [CrossRef]
- Aguilera, J.M. The Food Matrix: Implications in Processing, Nutrition and Health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Anania, C.; Perla, F.M.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean Diet and Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2018, 24, 2083–2094. [Google Scholar] [CrossRef]
- Guo, X.; Shao, X.; Li, J.; Li, S.; Li, K.; Li, D. Fruit and Vegetable Intake and Liver Cancer Risk: A Meta-Analysis of Prospective Cohort Studies. Food Funct. 2019, 10, 4478–4485. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food Groups and Risk of All-Cause Mortality: A Systematic Review and Meta-Analysis of Prospective Studies 1, 2. Am. J. Clin. Nutr. 2017, 105, 1462–1473. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The Potential Benefits of Red Beetroot Supplementation in Health and Disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- El-Kott, A.F.; Bin-Meferij, M.M. Use of Arctium Lappa Extract Against Acetaminophen-Induced Hepatotoxicity in Rats. Curr. Ther. Res. Clin. Exp. 2015, 77, 73–78. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef]
- Pfingstgraf, I.O.; Taulescu, M.; Pop, R.M.; Orăsan, R.; Vlase, L.; Uifalean, A.; Todea, D.; Alexescu, T.; Toma, C.; Pârvu, A.E. Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure. Antioxidants 2021, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Modulation of Phase II Enzymes by Sulforaphane: Implications for Its Cardioprotective Potential. J. Agric. Food Chem. 2009, 57, 5615–5622. [Google Scholar] [CrossRef]
- Rangboo, V.; Noroozi, M.; Zavoshy, R.; Rezadoost, S.A.; Mohammadpoorasl, A. The Effect of Artichoke Leaf Extract on Alanine Aminotransferase and Aspartate Aminotransferase in the Patients with Nonalcoholic Steatohepatitis. Int. J. Hepatol. 2016, 2016, 4030476. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for Health and Wellbeing: A Potent Bioactive Metabolite of an Entomopathogenic Medicinal Fungus Cordyceps with Its Nutraceutical and Therapeutic Potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione Dysregulation and the Etiology and Progression of Human Diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef]
- Pizzorno, J. Glutathione! Integr. Med. 2014, 13, 8–12. [Google Scholar]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Protective Effect of Sulforaphane against Oxidative Stress: Recent Advances. Exp. Toxicol. Pathol. 2012, 64, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.; Schwarz, Y.; Li, S.S.; Li, L.; King, I.B.; Chen, C.; Eaton, D.L.; Potter, J.D.; Lampe, J.W. CYP1A2, GSTM1, and GSTT1 Polymorphisms and Diet Effects on CYP1A2 Activity in a Crossover Feeding Trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).