Low Serum Creatinine Levels in Early Pregnancy Are Associated with a Higher Incidence of Postpartum Abnormal Glucose Metabolism among Women with Gestational Diabetes Mellitus: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
2.2. Study Design, Sites, and Participants
2.3. Data Collection
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association between Serum Creatinine Quartiles in Early Pregnancy and Postpartum AGM
3.3. Association of Serum Creatinine and Postpartum AGM Incidence: Subgroup Analyses
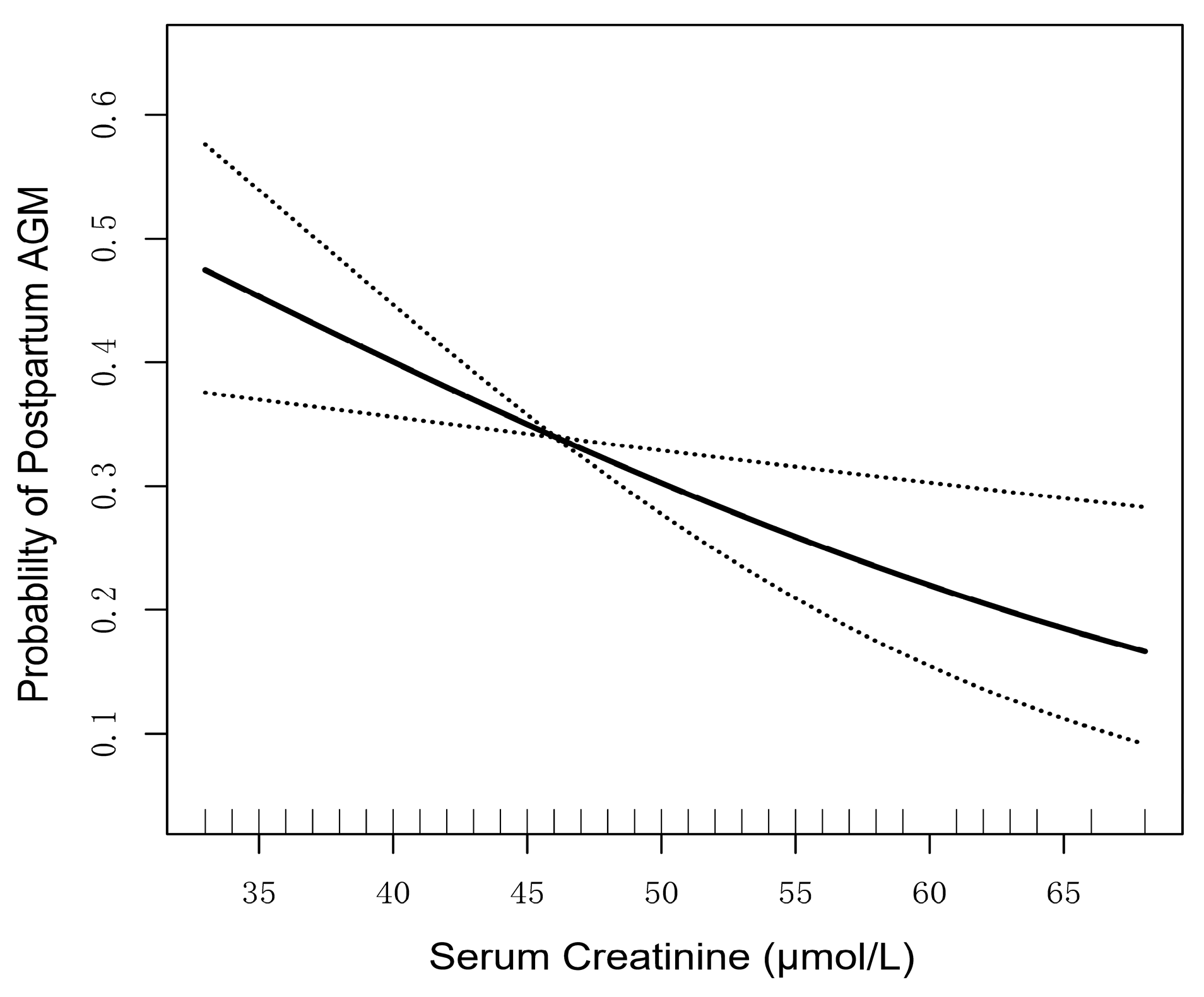
3.4. Association of Continuous Serum Creatinine Levels and Postpartum AGM Incidence
3.5. Continuous Serum Creatinine Levels and Postpartum Glucose Level, β-Cell Function and HOMA-IR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Catalano, P.M.; Tyzbir, E.D.; Wolfe, R.R.; Calles, J.; Roman, N.M.; Amini, S.B.; Sims, E.A. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am. J. Physiol. 1993, 264, E60–E67. [Google Scholar] [CrossRef]
- Catalano, P.M. Trying to understand gestational diabetes. Diabet. Med. 2014, 31, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.; Hingorani, A.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Kim, C.; Newton, K.; Knopp, R. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef]
- Draznin, B.; Aroda, V.R.; Bakris, G.; Benson, G.; Brown, F.M.; Freeman, R.; Green, J.; Huang, E.; Isaacs, D.; Kahan, S.; et al. 15. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S232–S243. [Google Scholar] [CrossRef]
- Rayanagoudar, G.; Hashi, A.A.; Zamora, J.; Khan, K.S.; Hitman, G.A.; Thangaratinam, S. Quantification of the type 2 diabetes risk in women with gestational diabetes: A systematic review and meta-analysis of 95,750 women. Diabetologia 2016, 59, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Brydon, P.; Taylor, R.S.; Hollins, A.; Munro, A.; Jenkins, D.; Dunne, F. Maternal ante-natal parameters as predictors of persistent postnatal glucose intolerance: A comparative study between Afro-Caribbeans, Asians and Caucasians. Diabet. Med. 2003, 20, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Kousta, E.; Efstathiadou, Z.; Lawrence, N.J.; Jeffs, J.A.; Godsland, I.F.; Barrett, S.C.; Doré, C.J.; Penny, A.; Anyaoku, V.; Millauer, B.A.; et al. The impact of ethnicity on glucose regulation and the metabolic syndrome following gestational diabetes. Diabetologia 2006, 49, 36–40. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Stotland, N.E.; Cheng, Y.W.; Ramos, G.A.; Caughey, A.B. The association between body mass index and gestational diabetes mellitus varies by race/ethnicity. Am. J. Perinatol. 2011, 28, 515–520. [Google Scholar] [CrossRef]
- Hedderson, M.; Ehrlich, S.; Sridhar, S.; Darbinian, J.; Moore, S.; Ferrara, A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 2012, 35, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Ishikawa, K.; Takeda, K.; Kobayashi, A.; Kurita, K.; Kumagai, J.; Yokoh, H.; Yokote, K. Postpartum risk of diabetes and predictive factors for glucose intolerance in East Asian women with gestational diabetes. Diabetes Res. Clin. Pract. 2018, 140, 1–8. [Google Scholar] [CrossRef]
- Sudasinghe, B.H.; Wijeyaratne, C.N.; Ginige, P.S. Long and short-term outcomes of Gestational Diabetes Mellitus (GDM) among South Asian women—A community-based study. Diabetes Res. Clin. Pract. 2018, 145, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ding, W.; Xu, S.; Chen, H.; Liu, B.; Wang, Z. The relationship between total cholesterol and postpartum impaired glucose tolerance in women with gestational diabetes mellitus. Lipids Health Dis. 2020, 19, 142. [Google Scholar] [CrossRef]
- Ferrannini, E.; Smith, J.D.; Cobelli, C.; Toffolo, G.; Pilo, A.; DeFronzo, R.A. Effect of insulin on the distribution and disposition of glucose in man. J. Clin. Investig. 1985, 76, 357–364. [Google Scholar] [CrossRef]
- Wulan, S.N.; Westerterp, K.R.; Plasqui, G. Ethnic differences in body composition and the associated metabolic profile: A comparative study between Asians and Caucasians. Maturitas 2010, 65, 315–319. [Google Scholar] [CrossRef]
- Rush, E.C.; Freitas, I.; Plank, L.D. Body size, body composition and fat distribution: Comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br. J. Nutr. 2009, 102, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Kawanabe, S.; Nagai, Y.; Nakamura, Y.; Nishine, A.; Nakagawa, T.; Tanaka, Y. Association of the muscle/fat mass ratio with insulin resistance in gestational diabetes mellitus. Endocr. J. 2019, 66, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Molnar, M.Z.; Tayek, J.A.; Ix, J.H.; Noori, N.; Benner, D.; Heymsfield, S.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine as a marker of muscle mass in chronic kidney disease: Results of a cross-sectional study and review of literature. J. Cachexia Sarcopenia Muscle 2013, 4, 19–29. [Google Scholar] [CrossRef]
- Canaud, B.; Ye, X.; Usvyat, L.; Kooman, J.; van der Sande, F.; Raimann, J.; Wang, Y.; Kotanko, P. Clinical and predictive value of simplified creatinine index used as muscle mass surrogate in end-stage kidney disease haemodialysis patients-results from the international MONitoring Dialysis Outcome initiative. Nephrol. Dial. Transplant. 2020, 35, 2161–2171. [Google Scholar] [CrossRef]
- Park, J.; Mehrotra, R.; Rhee, C.M.; Molnar, M.Z.; Lukowsky, L.R.; Patel, S.S.; Nissenson, A.R.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrol. Dial. Transplant. 2013, 28, 2146–2155. [Google Scholar] [CrossRef]
- Harita, N.; Hayashi, T.; Sato, K.K.; Nakamura, Y.; Yoneda, T.; Endo, G.; Kambe, H. Lower serum creatinine is a new risk factor of type 2 diabetes: The Kansai healthcare study. Diabetes Care 2009, 32, 424–426. [Google Scholar] [CrossRef]
- Bao, X.; Gu, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Shi, H.; Wang, H.; Sun, S.; et al. Low serum creatinine predicts risk for type 2 diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3011. [Google Scholar] [CrossRef]
- Kashima, S.; Inoue, K.; Matsumoto, M.; Akimoto, K. Low serum creatinine is a type 2 diabetes risk factor in men and women: The Yuport Health Checkup Center cohort study. Diabetes Metab. 2017, 43, 460–464. [Google Scholar] [CrossRef]
- Hu, H.; Nakagawa, T.; Honda, T.; Yamamoto, S.; Okazaki, H.; Yamamoto, M.; Miyamoto, T.; Eguchi, M.; Kochi, T.; Shimizu, M.; et al. Low serum creatinine and risk of diabetes: The Japan Epidemiology Collaboration on Occupational Health Study. J. Diabetes Investig. 2019, 10, 1209–1214. [Google Scholar] [CrossRef]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Seltzer, H.S.; Allen, E.W.; Herron, A.L., Jr.; Brennan, M.T. Insulin secretion in response to glycemic stimulus: Relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J. Clin. Investig. 1967, 46, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kong, L.; Yang, Y.; Wei, Y.; Zhu, W.; Su, R.; Lin, L.; Yang, H. Recommended reference values for serum lipids during early and middle pregnancy: A retrospective study from China. Lipids Health Dis. 2018, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Hjelmesæth, J.; Røislien, J.; Nordstrand, N.; Hofsø, D.; Hager, H.; Hartmann, A. Low serum creatinine is associated with type 2 diabetes in morbidly obese women and men: A cross-sectional study. BMC Endocr. Disord. 2010, 10, 6. [Google Scholar] [CrossRef]
- Bentley-Lewis, R.; Huynh, J.; Xiong, G.; Lee, H.; Wenger, J.; Clish, C.; Nathan, D.; Thadhani, R.; Gerszten, R. Metabolomic profiling in the prediction of gestational diabetes mellitus. Diabetologia 2015, 58, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. Prediction of Glucose Intolerance in Early Postpartum in Women with Gestational Diabetes Mellitus Based on the 2013 WHO Criteria. J. Clin. Med. 2019, 8, 383. [Google Scholar] [CrossRef]
- Meyer, C.; Pimenta, W.; Woerle, H.J.; Van Haeften, T.; Szoke, E.; Mitrakou, A.; Gerich, J. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 2006, 29, 1909–1914. [Google Scholar] [CrossRef]
- Bock, G.; Dalla Man, C.; Campioni, M.; Chittilapilly, E.; Basu, R.; Toffolo, G.; Cobelli, C.; Rizza, R. Pathogenesis of pre-diabetes: Mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 2006, 55, 3536–3549. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Jenkinson, C.P.; Richardson, D.K.; Tripathy, D.; DeFronzo, R.A. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: Results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006, 55, 1430–1435. [Google Scholar] [CrossRef]
- Dragsted, L.O. Biomarkers of meat intake and the application of nutrigenomics. Meat Sci. 2010, 84, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Sanchez-Rodriguez, D.; Locquet, M.; Reginster, J.Y.; Lengelé, L.; Bruyère, O. Malnutrition as a Strong Predictor of the Onset of Sarcopenia. Nutrients 2019, 11, 2883. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.Y.N.; Perez-Cornago, A.; Bradbury, K.E.; Key, T.J. Biomarker Concentrations in White and British Indian Vegetarians and Nonvegetarians in the UK Biobank. J. Nutr. 2021, 151, 3168–3179. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cui, X.; Wang, B.; Tang, Q.; Cai, J.; Shen, X. Healthy adult vegetarians have better renal function than matched omnivores: A cross-sectional study in China. BMC Nephrol. 2020, 21, 268. [Google Scholar] [CrossRef]
- Limones, M.; Sevillano, J.; Sanchez-Alonso, M.G.; Herrera, E.; Ramos-Alvarez, M.D.P. Metabolic alterations associated with maternal undernutrition during the first half of gestation lead to a diabetogenic state in the rat. Eur. J. Nutr. 2019, 58, 2521–2533. [Google Scholar] [CrossRef]
- Lorenzo, C.; Nath, S.D.; Hanley, A.J.; Abboud, H.E.; Gelfond, J.A.; Haffner, S.M. Risk of type 2 diabetes among individuals with high and low glomerular filtration rates. Diabetologia 2009, 52, 1290–1297. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Charchar, F.J.; Maric, C.; McClure, J.; Crawford, L.; Grzeszczak, W.; Sattar, N.; Zukowska-Szczechowska, E.; Dominiczak, A.F. Glomerular hyperfiltration: A new marker of metabolic risk. Kidney Int. 2007, 71, 816–821. [Google Scholar] [CrossRef]
- Eid, A.; Bodin, S.; Ferrier, B.; Delage, H.; Boghossian, M.; Martin, M.; Baverel, G.; Conjard, A. Intrinsic gluconeogenesis is enhanced in renal proximal tubules of Zucker diabetic fatty rats. J. Am. Soc. Nephrol. 2006, 17, 398–405. [Google Scholar] [CrossRef]
- Meigs, J.B.; Hu, F.B.; Rifai, N.; Manson, J.E. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 2004, 291, 1978–1986. [Google Scholar] [CrossRef]
- Harel, Z.; McArthur, E.; Hladunewich, M.; Dirk, J.S.; Wald, R.; Garg, A.X.; Ray, J.G. Serum Creatinine Levels before, during, and after Pregnancy. JAMA 2019, 321, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.M.; Park, J.S.; Hong, J.S.; Chin, H.J.; Na, K.Y.; Kim, D.K.; Oh, K.H.; Joo, K.W.; Kim, Y.S.; et al. Midterm eGFR and Adverse Pregnancy Outcomes: The Clinical Significance of Gestational Hyperfiltration. Clin. J. Am. Soc. Nephrol. 2017, 12, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hwang, S.; Lee, T.S.; Cho, J.; Seo, D.M.; Choi, S.J.; Uh, Y. Gestational age-specific serum creatinine can predict adverse pregnancy outcomes. Sci. Rep. 2022, 12, 11224. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.M.; Park, J.S.; Hong, J.S.; Chin, H.J.; Na, K.Y.; Kim, D.K.; Oh, K.H.; Joo, K.W.; Kim, Y.S.; et al. Gestational Estimated Glomerular Filtration Rate and Adverse Maternofetal Outcomes. Kidney Blood Press. Res. 2018, 43, 1688–1698. [Google Scholar] [CrossRef]
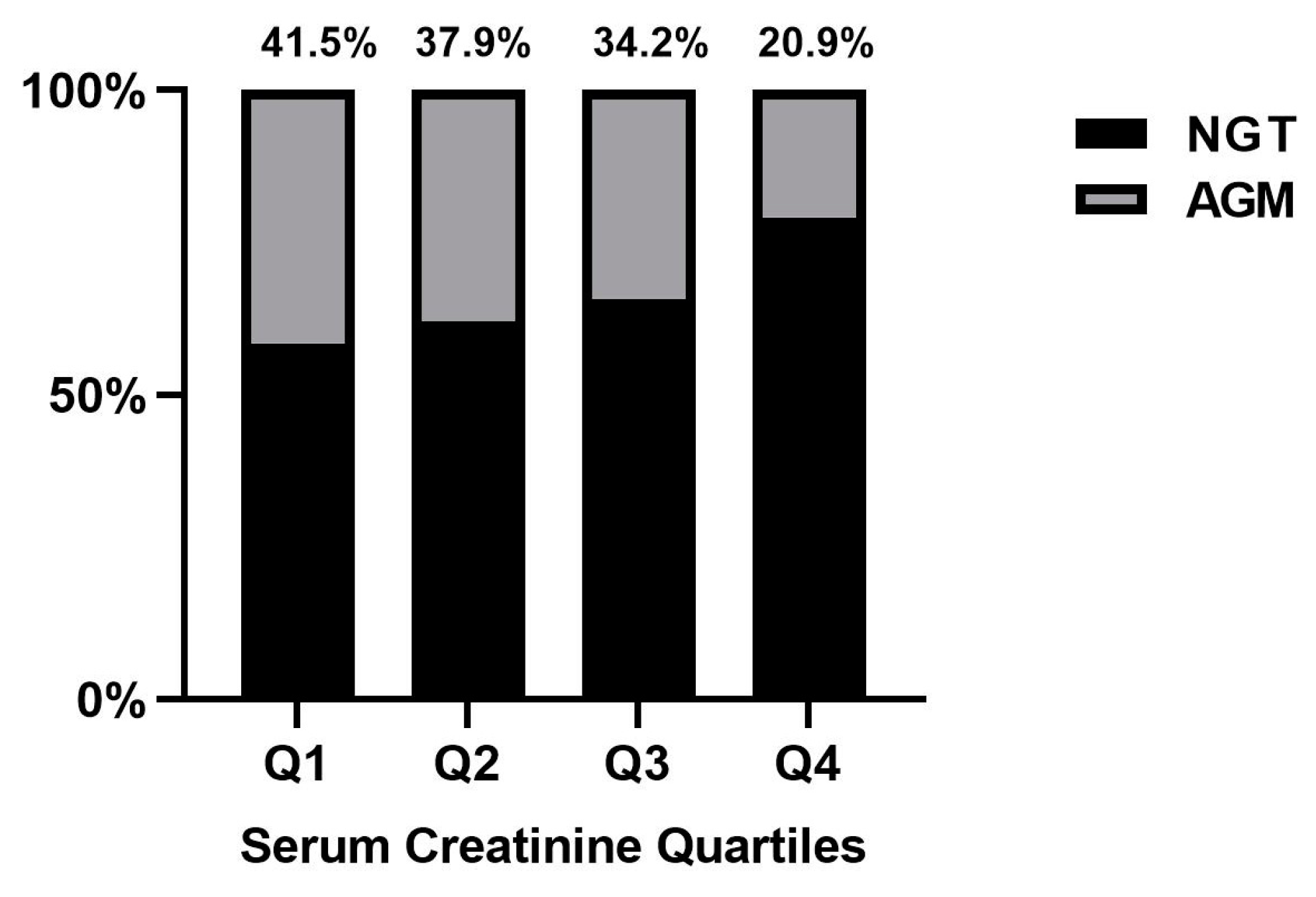
| Total Sample | Serum Creatinine | p Value | ||||
|---|---|---|---|---|---|---|
| Characteristics | Q1 (33–41 µmol/L) | Q2 (42–45 µmol/L) | Q3 (46–50 µmol/L) | Q4 (51–68 µmol/L) | ||
| n | 501 | 118 | 124 | 149 | 110 | |
| Maternal age (years) | 33 (30–37) | 33 (29–35) | 33 (30–37) | 33 (30–37) | 35 (31–38.3) | 0.009 |
| Height (cm) | 159 (156–163) | 158 (155–161) | 158 (155–161) | 160 (156–164) | 160 (157–164) | 0.005 |
| Weight before pregnancy (kg) | 54 (50–60) | 53 (48–58) | 54 (50–60) | 54 (50–61) | 56 (52–62) | 0.030 |
| Pre-pregnancy BMI (kg/m2) | 21.5 (19.8–23.4) | 21.2 (19.3–23.2) | 21.7 (20.0–23.5) | 21.1 (20.0–23.4) | 22.7 (20.2–23.8) | 0.155 |
| Weight delivery | 65.0 (60.0–70.7) | 63.0 (59.7–69) | 65.5 (60–70) | 65.6 (60.1–73.2) | 65.5 (61–71.1) | 0.090 |
| Gestational weight gain (kg) | 10.0 (7.5–13.2) | 10.5 (7.5–13.4) | 10.0 (7.4–12.7) | 11.0 (8.3–14.5) | 9.5 (7–12.2) | 0.095 |
| Previous GDM, n (%) | 20 (4) | 5 (4.2) | 6 (4.8) | 6 (4) | 3 (2.7) | 0.871 |
| Family history of T2DM, n (%) | 84 (16.8) | 6 (11.8) | 2 (4.7) | 9 (19.6) | 3 (7.3) | 0.403 |
| Insulin-requiring GDM, n (%) | 7 (1.4) | 2 (1.7) | 1 (0.8) | 3 (2) | 1 (0.9) | 0.808 |
| FPG (mg/dL) | 77.4 (72–82.8) | 76.5 (72–82.8) | 77.4 (72–82.8) | 77.4 (72–82.8) | 77.4 (71.6–82.8) | 0.982 |
| ALT (U/L) | 13.0 (10.0–18.0) | 12.0 (9.0–18.3) | 13.0 (10.0–18.0) | 13.0 (10.0–18.0) | 13 (10–19) | 0.512 |
| AST (U/L) | 17.0 (15.0–20.0) | 17.0 (15.0–21.0) | 17.0 (16.0–20.0) | 18.0 (15.0–21.0) | 17.0 (15.0–20.0) | 0.758 |
| GGT(U/L) | 13.0 (11.0–16.3) | 13.0 (10.0–18.0) | 13.0 (10.0–17.0) | 13.0 (11.0–16.0) | 12.0 (10.0–16.0) | 0.611 |
| BUN (mmol/L) | 2.7 (2.2–3.2) | 2.5 (2.1–2.9) | 2.7 (2.3–3.1) | 2.7 (2.4–3.1) | 3.1 (2.7–3.5) | <0.001 |
| 24–28 w FPG (mg/dL) | 81.0 (75.6–86.4) | 81.0 (75.6–86.4) | 81.0 (75.6–86.4) | 81.0 (75.6–86.4) | 79.2 (75.6–88.2) | 0.963 |
| 24–28 w 1-h glucose (mg/dL) | 180 (165.6–190.8) | 180 (163.8–194.4) | 180 (169.2–192.6) | 178.2 (163.8–192.6) | 180 (169.2–189) | 0.680 |
| 24–28 w 2-h glucose (mg/dL) | 158.4 (151.2–171) | 160.2 (147.6–171) | 160.2 (151.2–172.8) | 158.4 (149.4–169.2) | 158.4 (153–171) | 0.615 |
| 24–28 w TG (mmol/L) | 2.1 (1.8–2.7) | 2.1 (1.9–2.7) | 2.1 (1.8–2.6) | 2.1 (1.7–2.7) | 2.2 (1.8–2.7) | 0.802 |
| 24–28 w LDL-C (mmol/L) | 3.4 (3.0–4.0) | 3.4 (2.9–4.0) | 3.5 (3.1–4.1) | 3.4 (3.0–3.9) | 3.5 (3.0–4.1) | 0.486 |
| 24–28 w HDL-C (mmol/L) | 2.0 (1.7–2.3) | 1.9 (1.6–2.2) | 2.0 (1.7–2.2) | 2.0 (1.7–2.3) | 2.0 (1.8–2.2) | 0.441 |
| Unadjusted Model | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95 % CI) | p | OR (95 % CI) | p | OR (95 % CI) | p | OR (95 % CI) | p | |
| Q1 (33–41μmol/L) | 2.69 (1.49–4.83) | 0.001 | 3.18 (1.74–5.82) | <0.001 | 2.98 (1.61–5.50) | 0.001 | 3.37 (1.77–6.42) | <0.001 |
| Q2 (42–45μmol/L) | 2.31 (1.29–4.15) | 0.005 | 2.55 (1.41–4.63) | 0.002 | 2.45 (1.34–4.49) | 0.004 | 2.42 (1.29–4.51) | 0.006 |
| Q3 (46–50μmol/L) | 1.97 (1.11–3.48) | 0.020 | 2.18 (1.74–5.82) | 0.008 | 2.04 (1.13–3.68) | 0.018 | 2.27 (1.23–4.18) | 0.008 |
| Q4 (51–68μmol/L) | Reference | Reference | Reference | Reference | ||||
| Serum Creatinine | |||||||
|---|---|---|---|---|---|---|---|
| Q1 (33–41 µmol/L) | Q2 (42–45 µmol/L) | Q3 (46–50 µmol/L) | Q4 (51–68 µmol/L) | ||||
| OR (95%CI) | p Value | OR (95%CI) | p Value | OR (95%CI) | p Value | ||
| Maternal age (years) | |||||||
| <median | 3.29 (1.19–9.13) | 0.022 | 1.44 (0.51–4.04) | 0.493 | 2.56 (0.96–6.81) | 0.060 | Reference |
| ≥median | 3.96 (1.69–9.28) | 0.002 | 3.41 (1.52–7.66) | 0.003 | 2.07 (0.93–4.60) | 0.076 | Reference |
| BMI (kg/m2) | |||||||
| <23 | 2.53 (1.18–5.43) | 0.017 | 2.28 (1.08–4.80) | 0.030 | 2.26 (1.11–4.62) | 0.025 | Reference |
| ≥23 | 7.44 (2.10–26.32) | 0.002 | 2.86 (0.86–9.51) | 0.087 | 2.23 (0.63–7.92) | 0.216 | Reference |
| Dyslipidemia | |||||||
| Yes | 6.15 (1.49–25.33) | 0.012 | 6.16 (1.61–23.55) | 0.008 | 4.13 (1.02–16.74) | 0.047 | Reference |
| No | 3.02 (1.39–6.55) | 0.005 | 1.58 (0.74–3.37) | 0.240 | 1.84 (0.89–3.81) | 0.099 | Reference |
| Crude | Adjusted For age | Multiple Adjusted | ||||
|---|---|---|---|---|---|---|
| OR (95 % CI) | p Value | OR (95 % CI) | p Value | OR (95 % CI) | p Value | |
| Serum creatinine (Per 2 μmol/L increase) | 0.92 (0.86–0.98) | 0.007 | 0.90 (0.85–0.96) | 0.001 | 0.90 (0.84–0.96) | 0.003 |
| Crude | Adjusted For Age | Multiple Adjusted | ||||
|---|---|---|---|---|---|---|
| β (95 % CI) | p Value | β (95 % CI) | p Value | β (95 % CI) | p Value | |
| FPG (mmol/L) | 0.07 (0. 001–0.14) | 0.049 | 0.05 (0.97–0.96) | 0.154 | 0.05 (−0.01 −0.12) | 0.104 |
| 2-h glucose (mmol/L) | −0.17 (−0.34–0.01) | 0.040 | −0.22 (−0.39–0.06) | 0.009 | −0.23 (−0.40–0.07) | 0.007 |
| Insulinogenic index | 0.54 (0.05–1.03) | 0.030 | 0.62 (0.13–1.11) | 0.014 | 0.55 (0.06–1.04) | 0.027 |
| HOMA-IR | 0.33 (−0.08–0.75) | 0.117 | 0.04 (−0.02–0.10) | 0.180 | 0.24 (−0.15–0.63) | 0.235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Zeng, R.; Xu, C.; Lai, F.; Chen, L.; Wang, C.; Pei, L.; Li, Z.; Li, Y.; Xiao, H.; et al. Low Serum Creatinine Levels in Early Pregnancy Are Associated with a Higher Incidence of Postpartum Abnormal Glucose Metabolism among Women with Gestational Diabetes Mellitus: A Retrospective Cohort Study. Nutrients 2023, 15, 2193. https://doi.org/10.3390/nu15092193
Chen N, Zeng R, Xu C, Lai F, Chen L, Wang C, Pei L, Li Z, Li Y, Xiao H, et al. Low Serum Creatinine Levels in Early Pregnancy Are Associated with a Higher Incidence of Postpartum Abnormal Glucose Metabolism among Women with Gestational Diabetes Mellitus: A Retrospective Cohort Study. Nutrients. 2023; 15(9):2193. https://doi.org/10.3390/nu15092193
Chicago/Turabian StyleChen, Nan, Rui Zeng, Changliu Xu, Fenghua Lai, Li Chen, Chenxue Wang, Ling Pei, Zhuyu Li, Yanbing Li, Haipeng Xiao, and et al. 2023. "Low Serum Creatinine Levels in Early Pregnancy Are Associated with a Higher Incidence of Postpartum Abnormal Glucose Metabolism among Women with Gestational Diabetes Mellitus: A Retrospective Cohort Study" Nutrients 15, no. 9: 2193. https://doi.org/10.3390/nu15092193
APA StyleChen, N., Zeng, R., Xu, C., Lai, F., Chen, L., Wang, C., Pei, L., Li, Z., Li, Y., Xiao, H., & Cao, X. (2023). Low Serum Creatinine Levels in Early Pregnancy Are Associated with a Higher Incidence of Postpartum Abnormal Glucose Metabolism among Women with Gestational Diabetes Mellitus: A Retrospective Cohort Study. Nutrients, 15(9), 2193. https://doi.org/10.3390/nu15092193






