Association of Vitamin D Level and Maternal Gut Microbiome during Pregnancy: Findings from a Randomized Controlled Trial of Antenatal Vitamin D Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Vitamin D Antenatal Asthma Reduction Trial (VDAART)
2.2. Stool Sample Collection and Processing
2.3. Blood Sample Collection and Processing
2.4. Serum Vitamin D Level
2.5. Mothers’ Characteristics
2.6. Statistical Analyses
2.6.1. Alpha Diversity
2.6.2. Beta Diversity
2.6.3. Abundance Association Analysis
3. Results
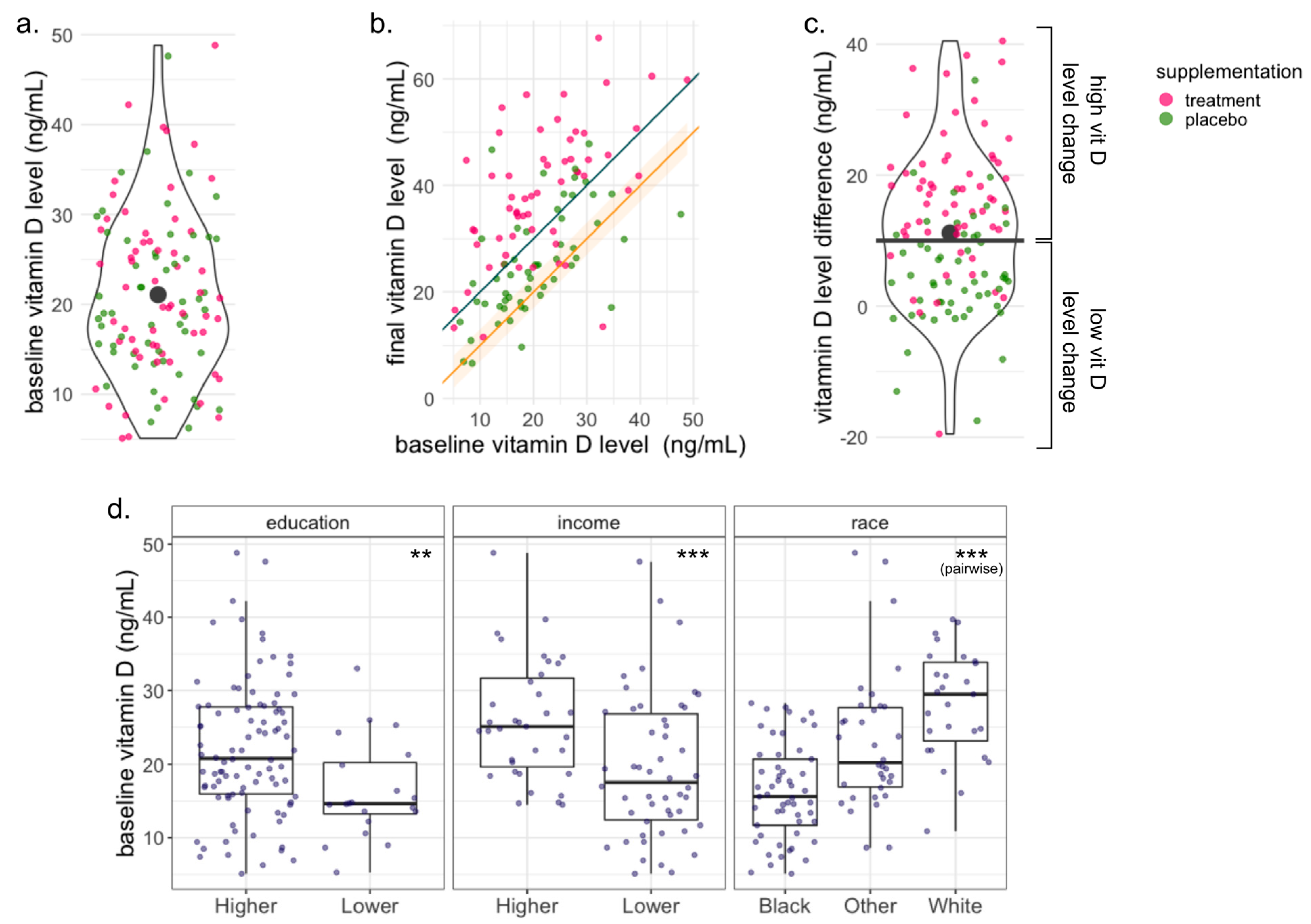
3.1. Subject Characteristics
3.2. Microbiome Composition Is Associated with Baseline Vitamin D Level
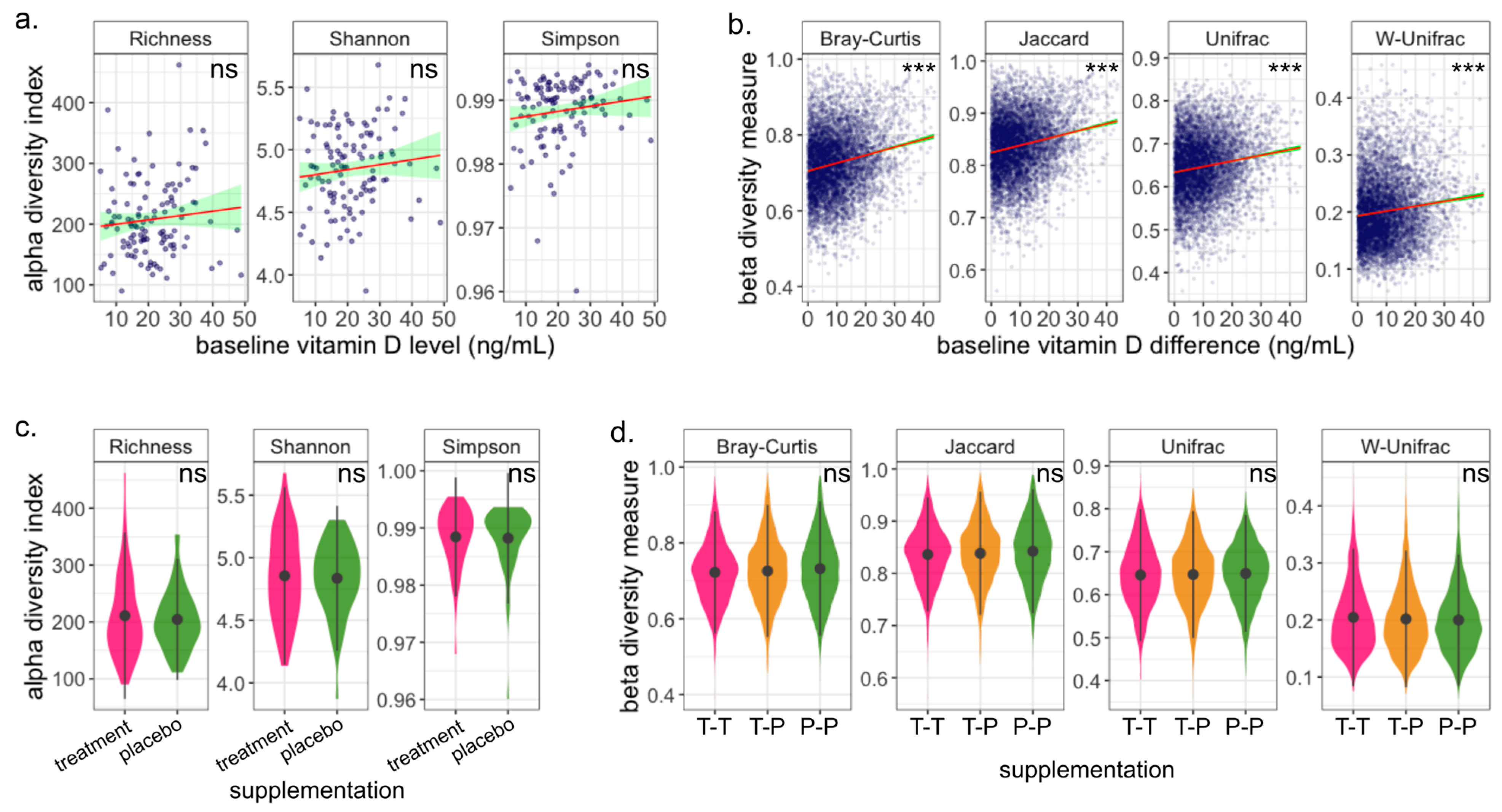
3.3. Gut Microbiome Is Robust to Vitamin D Supplementation during Pregnancy
3.4. Change in Vitamin D Level Does Not Impact Microbiome Diversity
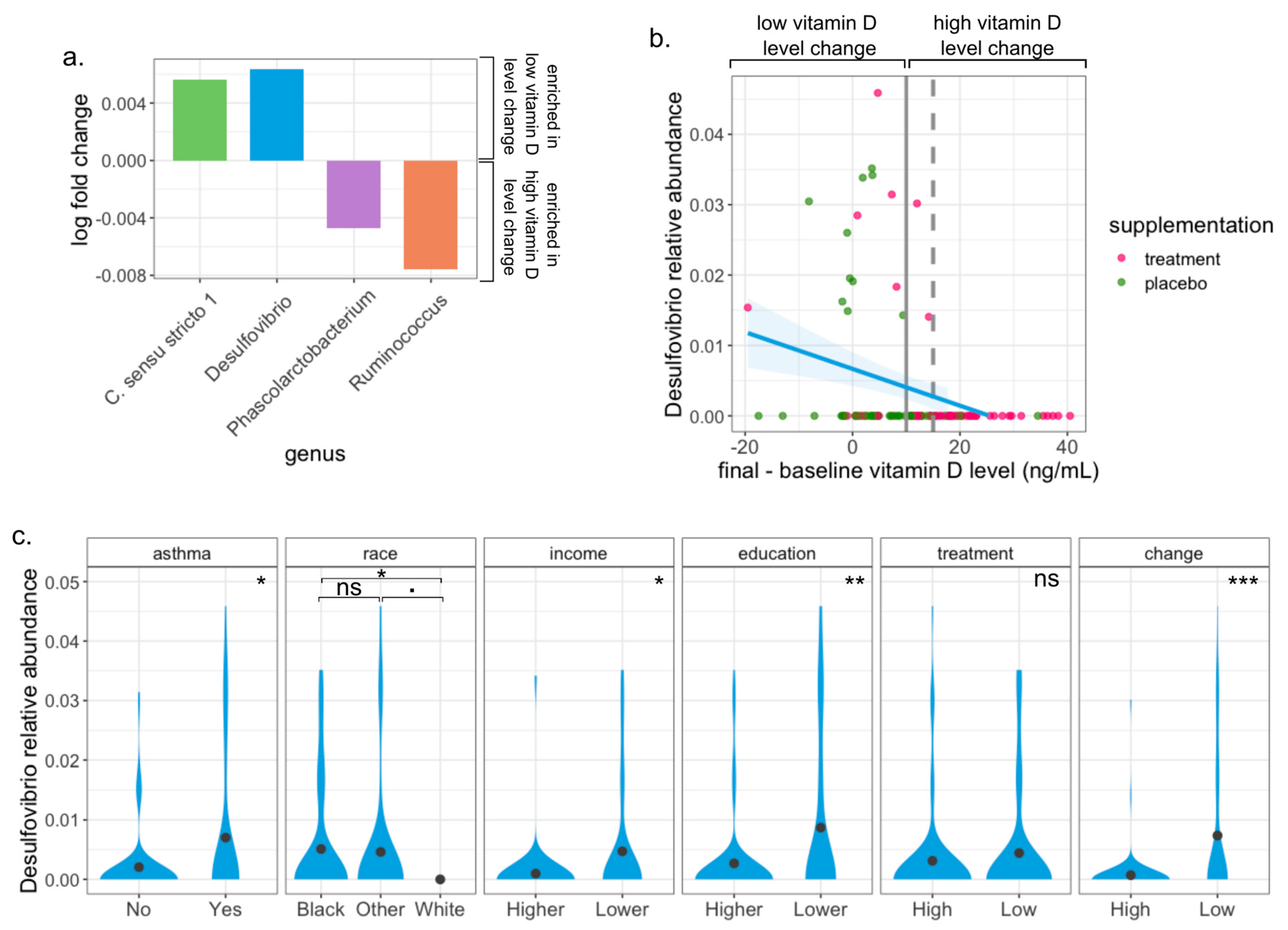
3.5. Desulfovibrio Is Enriched in Pregnant Women with Low Change of Vitamin D Level
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holick, M.F. Vitamin D and Bone Health. J. Nutr. 1996, 126, 1159S. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and Bone Health; Potential Mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of General Physiologic Features and Functions of Vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.-C. Vitamin D and Inflammation. Jt. Bone Spine 2010, 77, 552. [Google Scholar] [CrossRef]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and Intestinal Homeostasis: Barrier, Microbiota, and Immune Modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where Is the Vitamin D Receptor? Arch. Biochem. Biophys. 2012, 523, 123. [Google Scholar] [CrossRef]
- Ardesia, M.; Ferlazzo, G.; Fries, W. Vitamin D and Inflammatory Bowel Disease. Biomed. Res. Int. 2015, 2015, 470805. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837. [Google Scholar] [CrossRef]
- Boirivant, M.; Amendola, A.; Butera, A. Intestinal Microflora and Immunoregulation. Mucosal Immunol. 2008, 1 (Suppl. S1), S47. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Vieira, A.T.; Vinolo, M.A.R.; Oliveira, F.A.; Curi, R.; Martins, F.d.S. The Central Role of the Gut Microbiota in Chronic Inflammatory Diseases. J. Immunol. Res. 2014, 2014, 689492. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable Bowel Syndrome: A Microbiome-Gut-Brain Axis Disorder? World J. Gastroenterol. 2014, 20, 14105. [Google Scholar] [CrossRef] [PubMed]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef] [PubMed]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Lutfor, A.B.; Razzaque, M.S. Vitamin D and the Host-Gut Microbiome: A Brief Overview. Acta Histochem. Cytochem. 2020, 53, 33. [Google Scholar] [CrossRef]
- Waterhouse, M. Vitamin D and the Gut Microbiome: A Systematic Review of in Vivo Studies. Eur. J. Nutr. 2019, 16, 2895–2910. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470. [Google Scholar] [CrossRef]
- Fuhler, G.M. The Immune System and Microbiome in Pregnancy. Best Pract. Res. Clin. Gastroenterol. 2020, 44–45, 101671. [Google Scholar] [CrossRef]
- Sordillo, J.E.; Zhou, Y.; McGeachie, M.J.; Ziniti, J.; Lange, N.; Laranjo, N.; Savage, J.R.; Carey, V.; O’Connor, G.; Sandel, M.; et al. Factors Influencing the Infant Gut Microbiome at Age 3–6 Months: Findings from the Ethnically Diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J. Allergy Clin. Immunol. 2017, 139, 482. [Google Scholar] [CrossRef]
- Drall, K.M.; Field, C.J.; Haqq, A.M.; de Souza, R.J.; Tun, H.M.; Morales-Lizcano, N.P.; Konya, T.B.; Guttman, D.S.; Azad, M.B.; Becker, A.B.; et al. Vitamin D Supplementation in Pregnancy and Early Infancy in Relation to Gut Microbiota Composition and C. Difficile Colonization: Implications for Viral Respiratory Infections. Gut Microbes 2020, 12, 1799734. [Google Scholar] [CrossRef]
- Kassem, Z.; Sitarik, A.; Levin, A.M.; Lynch, S.V.; Havstad, S.; Fujimura, K.; Kozyrskyj, A.; Ownby, D.R.; Johnson, C.C.; Yong, G.J.; et al. Maternal and Cord Blood Vitamin D Level and the Infant Gut Microbiota in a Birth Cohort Study. Matern. Health Neonatol. Perinatol. 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Godfrey, K.M.; McDonald, D.; Treuren, W.V.; Bjørnholt, J.V.; Midtvedt, T.; Moen, B.; Rudi, K.; Knight, R.; Brantsæter, A.L.; et al. Fat and Vitamin Intakes during Pregnancy Have Stronger Relations with a Pro-Inflammatory Maternal Microbiota than Does Carbohydrate Intake. Microbiome 2016, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, K.; Obuchowska, A.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Changes in the Gut Microbiome and Pathologies in Pregnancy. Int. J. Environ. Res. Public Health 2022, 19, 9961. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D.; et al. Maternal Gut Microbiota in Pregnancy Influences Offspring Metabolic Phenotype in Mice. Science 2020, 367, eaaw8429. [Google Scholar] [CrossRef]
- Vuillermin, P.J.; Macia, L.; Nanan, R.; Tang, M.L.; Collier, F.; Brix, S. The Maternal Microbiome during Pregnancy and Allergic Disease in the Offspring. Semin. Immunopathol. 2017, 39, 669. [Google Scholar] [CrossRef] [PubMed]
- Ishimwe, J.A. Maternal Microbiome in Preeclampsia Pathophysiology and Implications on Offspring Health. Physiol. Rep. 2021, 9, e14875. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.A.; Chen, Y.C.; Chen, Y.Y.; Kozyrskyj, A.L.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Bisgaard, H.; Stokholm, J.; Chawes, B.; et al. The Maternal Prenatal and Offspring Early-Life Gut Microbiome of Childhood Asthma Phenotypes. Allergy 2023, 78, 418. [Google Scholar] [CrossRef]
- Dunlop, A.L.; Mulle, J.G.; Ferranti, E.P.; Edwards, S.; Dunn, A.B.; Corwin, E.J. The Maternal Microbiome and Pregnancy Outcomes That Impact Infant Health: A Review. Adv. Neonatal. Care 2015, 15, 377. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Lange, N.E.; Carey, V.J.; Brown, S.; Laranjo, N.; Harshfield, B.J.; O’Connor, G.T.; Sandel, M.; Strunk, R.C.; Bacharier, L.B.; et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): Rationale, Design, and Methods of a Randomized, Controlled Trial of Vitamin D Supplementation in Pregnancy for the Primary Prevention of Asthma and Allergies in Children. Contemp. Clin. Trials 2014, 38, 37. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’hara, R.; Solymos, P.; Stevens, M.; Szoecs, E. Vegan: Community Ecology Package, Package Version 2.6-4. unpublished. 2022. [Google Scholar]
- Lin, H.; Peddada, S.D. Analysis of Compositions of Microbiomes with Bias Correction. Nat. Commun. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-Scale Meta-Omics Studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson, R.E.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA 2016, 315, 362. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-Year Follow-up of a Trial of Antenatal Vitamin D for Asthma Reduction. New Engl. J. Med. 2020, 382, 525. [Google Scholar] [CrossRef] [PubMed]
- Zadshir, A.; Tareen, N.; Pan, D.; Norris, K.; Martins, D. The Prevalence of Hypovitaminosis D Among US Adults: Data from the NHANES III. Ethn. Dis. 2005, 15, 97. [Google Scholar]
- Ginde, A.A.; Liu, M.C.; Camargo, C.A. Demographic Differences and Trends of Vitamin D Insufficiency in the US Population, 1988–2004. Arch. Intern. Med. 2009, 169, 626. [Google Scholar] [CrossRef]
- Peroni, D.G.; Nuzzi, G.; Trambusti, I.; Di Cicco, M.E.; Comberiati, P. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front. Immunol. 2020, 11, 700. [Google Scholar] [CrossRef]
- Ali, N.S.; Nanji, K. A Review on the Role of Vitamin D in Asthma. Cureus 2017, 9, e1288. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the Immune System. Curr. Opin. Pharmacol. 2010, 10, 482. [Google Scholar] [CrossRef]
- Gale, C.R.; Robinson, S.M.; Harvey, N.C.; Javaid, M.K.; Jiang, B.; Martyn, C.N.; Godfrey, K.M.; Cooper, C. Maternal Vitamin D Status during Pregnancy and Child Outcomes. Eur. J. Clin. Nutr. 2008, 62, 68. [Google Scholar] [CrossRef]
- Tsiaras, W.; Weinstock, M. Factors Influencing Vitamin D Status. Acta Derm. Venerol. 2011, 91, 115. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, J.; Valente, F.M.; Pereira, I.A.; Costa, A.; Grimont, P.A.; Le Faou, A.E. Reclassification of the Only Species of the Genus Desulfomonas, Desulfomonas Pigra, as Desulfovibrio Piger Comb. Nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 1305. [Google Scholar] [PubMed]
- Muyzer, G.; Stams, A.J.M. The Ecology and Biotechnology of Sulphate-Reducing Bacteria. Nat. Rev. Microbiol. 2008, 6, 6. [Google Scholar]
- Kushkevych, I.; Dordević, D.; Kollar, P.; Vítězová, M.; Drago, L. Hydrogen Sulfide as a Toxic Product in the Small–Large Intestine Axis and Its Role in IBD Development. J. Clin. Med. 2019, 8, 1054. [Google Scholar] [CrossRef]
- Loubinoux, J.; Bronowicki, J.-P.; Pereira, I.A.C.; Mougenel, J.-L.; Faou, A.E. Sulfate-Reducing Bacteria in Human Feces and Their Association with Inflammatory Bowel Diseases. FEMS Microbiol. Ecol. 2002, 40, 107. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kos, J.; Kollar, P.; Kralova, K.; Jampilek, J. Activity of Ring-Substituted 8-Hydroxyquinoline-2-Carboxanilides against Intestinal Sulfate-Reducing Bacteria Desulfovibrio Piger. Med. Chem. Res. 2018, 27, 278. [Google Scholar] [CrossRef]
- Kushkevych, I.; Leščanová, O.; Dordević, D.; Jančíková, S.; Hošek, J.; Vítězová, M.; Buňková, L.; Drago, L. The Sulfate-Reducing Microbial Communities and Meta-Analysis of Their Occurrence during Diseases of Small–Large Intestine Axis. J. Clin. Med. 2019, 8, 1656. [Google Scholar] [CrossRef]
- Weng, Y.J.; Gan, H.Y.; Li, X.; Huang, Y.; Li, Z.C.; Deng, H.M.; Chen, S.Z.; Zhou, Y.; Wang, L.S.; Han, Y.P.; et al. Correlation of Diet, Microbiota and Metabolite Networks in Inflammatory Bowel Disease. J. Dig. Dis. 2019, 20, 447. [Google Scholar] [CrossRef]
- Pham, V.T.; Fehlbaum, S.; Seifert, N.; Richard, N.; Bruins, M.J.; Sybesma, W.; Rehman, A.; Steinert, R.E. Effects of Colon-Targeted Vitamins on the Composition and Metabolic Activity of the Human Gut Microbiome—A Pilot Study. Gut Microbes 2021, 13, 1. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D Regulates the Gut Microbiome and Protects Mice from Dextran Sodium Sulfate–Induced Colitis. J. Nutr. 2013, 143, 1679. [Google Scholar] [CrossRef]
- Garg, M.; Hendy, P.; Ding, J.N.; Shaw, S.; Hold, G.; Hart, A. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J. Crohn’s Colitis 2018, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, K.L.; Rehman, S.F.; Vaughan, A.; Lachner, N.; Budden, K.F.; Kim, R.Y.; Wood, D.L.; Gellatly, S.L.; Shukla, S.D.; Wood, L.G.; et al. Disease-Associated Gut Microbiome and Metabolome Changes in Patients with Chronic Obstructive Pulmonary Disease. Nat. Commun. 2020, 11, 1. [Google Scholar] [CrossRef]
- Sohn, K.-H.; Baek, M.; Choi, S.-M.; Bae, B.; Kim, R.Y.; Kim, Y.-C.; Kim, H.-Y.; Yi, H.; Kang, H.-R. Alteration of Lung and Gut Microbiota in IL-13-Transgenic Mice Simulating Chronic Asthma. J. Microbiol. Biotechnol. 2020, 30, 1819. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell Longev. 2009, 2, 270. [Google Scholar] [CrossRef]
- Singh, A.K.; Cabral, C.; Kumar, R.; Ganguly, R.; Rana, H.K.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Perna, S. Is Vitamin D Supplementation Useful for Weight Loss Programs? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2019, 55, 368. [Google Scholar] [CrossRef]
| Baseline Vitamin D Level | |||||
|---|---|---|---|---|---|
| Size (%) | Mean | SD | p Value | ||
| 114 (100) | 21.08 | 9.07 | |||
| Race | Black | 53 (46.5) | 16.21 | 6.49 | 3.6 × 10−9 |
| Other | 34 (29.8) | 23.03 | 9.47 | ||
| White | 27 (23.7) | 28.18 | 7.33 | ||
| Household income | 50k or more | 35 (30.7) | 26.01 | 8.11 | 2.8 ×10 −4 |
| Less than 50k | 50 (43.9) | 19.45 | 9.73 | ||
| NA | 29 (25.4) | 17.93 | 6.41 | ||
| Education | College or more | 94 (82.5) | 22.08 | 9.21 | 0.010 |
| Highschool, technical school or less | 20 (17.5) | 16.36 | 6.77 | ||
| Site name | Boston | 51 (44.7) | 21.01 | 8.57 | 0.001 |
| San Diego | 15 (13.2) | 28.74 | 8.61 | ||
| Saint Louis | 48 (42.1) | 18.76 | 8.56 | ||
| Maternal asthma | No | 75 (65.8) | 21.24 | 9.25 | 0.796 |
| Yes | 39 (34.2) | 20.77 | 8.82 | ||
| Maternal hay fever | No | 49 (43.0) | 21.04 | 10.01 | 0.971 |
| Yes | 65 (57.0) | 21.11 | 8.37 | ||
| Maternal age | 27.56 (5.85) | 27.56 | 5.85 | 0.326 | |
| Difference vitamin D level | 11.18 (11.30) | 11.18 | 11.3 | 0.796 | |
| Treatment | High dose | 59 (51.8) | 21.64 | 9.61 | 0.929 |
| Low dose | 55 (48.2) | 20.48 | 8.49 | ||
| Treatment Assignment | |||||
|---|---|---|---|---|---|
| Overall | Treatment | Placebo | p Value | ||
| 114 | 59 | 55 | |||
| Race | Black | 53 (46.5) | 26 (44.1) | 27 (49.1) | 0.614 |
| Other | 34 (29.8) | 20 (33.9) | 14 (25.5) | ||
| White | 27 (23.7) | 13 (22.0) | 14 (25.5) | ||
| Household income (%) | 50k or more | 35 (30.7) | 19 (32.2) | 16 (29.1) | 0.927 |
| Less than 50k | 50 (43.9) | 25 (42.4) | 25 (45.5) | ||
| NA | 29 (25.4) | 15 (25.4) | 14 (25.5) | ||
| Education (%) | College or more | 94 (82.5) | 48 (81.4) | 46 (83.6) | 0.941 |
| Highschool, technical school or less | 20 (17.5) | 11 (18.6) | 9 (16.4) | ||
| Baseline vitamin D level (mean (SD)) | 21.08 (9.07) | 21.64 (9.61) | 20.48 (8.49) | 0.496 | |
| Site name (%) | Boston | 51 (44.7) | 29 (49.2) | 22 (40.0) | 0.543 |
| San Diego | 15 (13.2) | 8 (13.6) | 7 (12.7) | ||
| Saint Louis | 48 (42.1) | 22 (37.3) | 26 (47.3) | ||
| Maternal asthma (%) | No | 75 (65.8) | 39 (66.1) | 36 (65.5) | 1.000 |
| Yes | 39 (34.2) | 20 (33.9) | 19 (34.5) | ||
| Maternal hay fever (%) | No | 49 (43.0) | 26 (44.1) | 23 (41.8) | 0.958 |
| Yes | 65 (57.0) | 33 (55.9) | 32 (58.2) | ||
| Maternal age (mean (SD)) | 27.56 (5.85) | 27.47 (5.55) | 27.65 (6.19) | 0.871 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparicio, A.; Gold, D.R.; Weiss, S.T.; Litonjua, A.A.; Lee-Sarwar, K.; Liu, Y.-Y. Association of Vitamin D Level and Maternal Gut Microbiome during Pregnancy: Findings from a Randomized Controlled Trial of Antenatal Vitamin D Supplementation. Nutrients 2023, 15, 2059. https://doi.org/10.3390/nu15092059
Aparicio A, Gold DR, Weiss ST, Litonjua AA, Lee-Sarwar K, Liu Y-Y. Association of Vitamin D Level and Maternal Gut Microbiome during Pregnancy: Findings from a Randomized Controlled Trial of Antenatal Vitamin D Supplementation. Nutrients. 2023; 15(9):2059. https://doi.org/10.3390/nu15092059
Chicago/Turabian StyleAparicio, Andrea, Diane R. Gold, Scott T. Weiss, Augusto A. Litonjua, Kathleen Lee-Sarwar, and Yang-Yu Liu. 2023. "Association of Vitamin D Level and Maternal Gut Microbiome during Pregnancy: Findings from a Randomized Controlled Trial of Antenatal Vitamin D Supplementation" Nutrients 15, no. 9: 2059. https://doi.org/10.3390/nu15092059
APA StyleAparicio, A., Gold, D. R., Weiss, S. T., Litonjua, A. A., Lee-Sarwar, K., & Liu, Y.-Y. (2023). Association of Vitamin D Level and Maternal Gut Microbiome during Pregnancy: Findings from a Randomized Controlled Trial of Antenatal Vitamin D Supplementation. Nutrients, 15(9), 2059. https://doi.org/10.3390/nu15092059






